Copyright
©The Author(s) 2018.
World J Gastroenterol. May 14, 2018; 24(18): 1962-1977
Published online May 14, 2018. doi: 10.3748/wjg.v24.i18.1962
Published online May 14, 2018. doi: 10.3748/wjg.v24.i18.1962
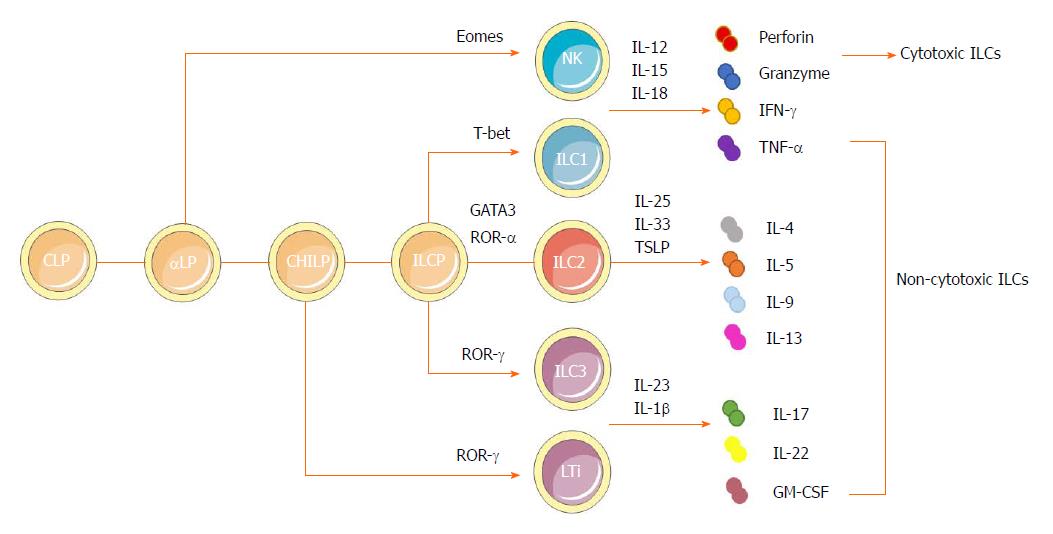
Figure 1 Developmental pathways and classification of innate lymphoid cells.
ILCs are derived from a CLP. With the same phenotype as CLP as well as expressing α4β7 integrin, αLP gives rises to cytotoxic NK cells and differentiates into a CHILP, which gives rise to all noncytotoxic ILCs. The transcription factor PLZF further divides the progeny of CHILPs into a PLZF+ ILCPs that are restricted to ILCs except LTi cells and PLZF-independent LTi cells. Group 1 ILCs comprise both Eomes-dependent NK cells and T-bet-dependent ILC1s. They could produce IFN-γ and TNF-α in response to the stimulation by IL-12, IL-15 and IL-18. NK cells can also secrete granzymes and perforin to exert cytotoxic functions. Dependent on GATA3 and ROR-α as well as respondent to cytokines IL-25, IL-33 and TSLP, group 2 ILCs could produce Th2 effector cytokines (IL-4, IL-5, IL-9 and IL-13 and amphiregulin). Group 3 ILCs encompass both RORγ-dependent LTi cells and ILC3s. They can produce IL-22, IL-17 and GM-CSF, mainly in response to IL-1β and IL-23. αLP: α-lymphoid progenitor; CHILP: Common helper-like innate lymphoid progenitor; CLP: Common lymphoid progenitor; GM-CSF: Granulocyte macrophage colony-stimulating factor; IL: Interleukin; ILC: Innate lymphoid cell; ILCP: Innate lymphoid cell precursor; INF: Interferon; LTi: Lymphoid tissue-like; NK: Natural killer; PLZF: Promyeloid leukemia zinc finger; Th: T helper; TNF: Tumor necrosis factor; TSLP: Thymic stroma lymphopoietin.
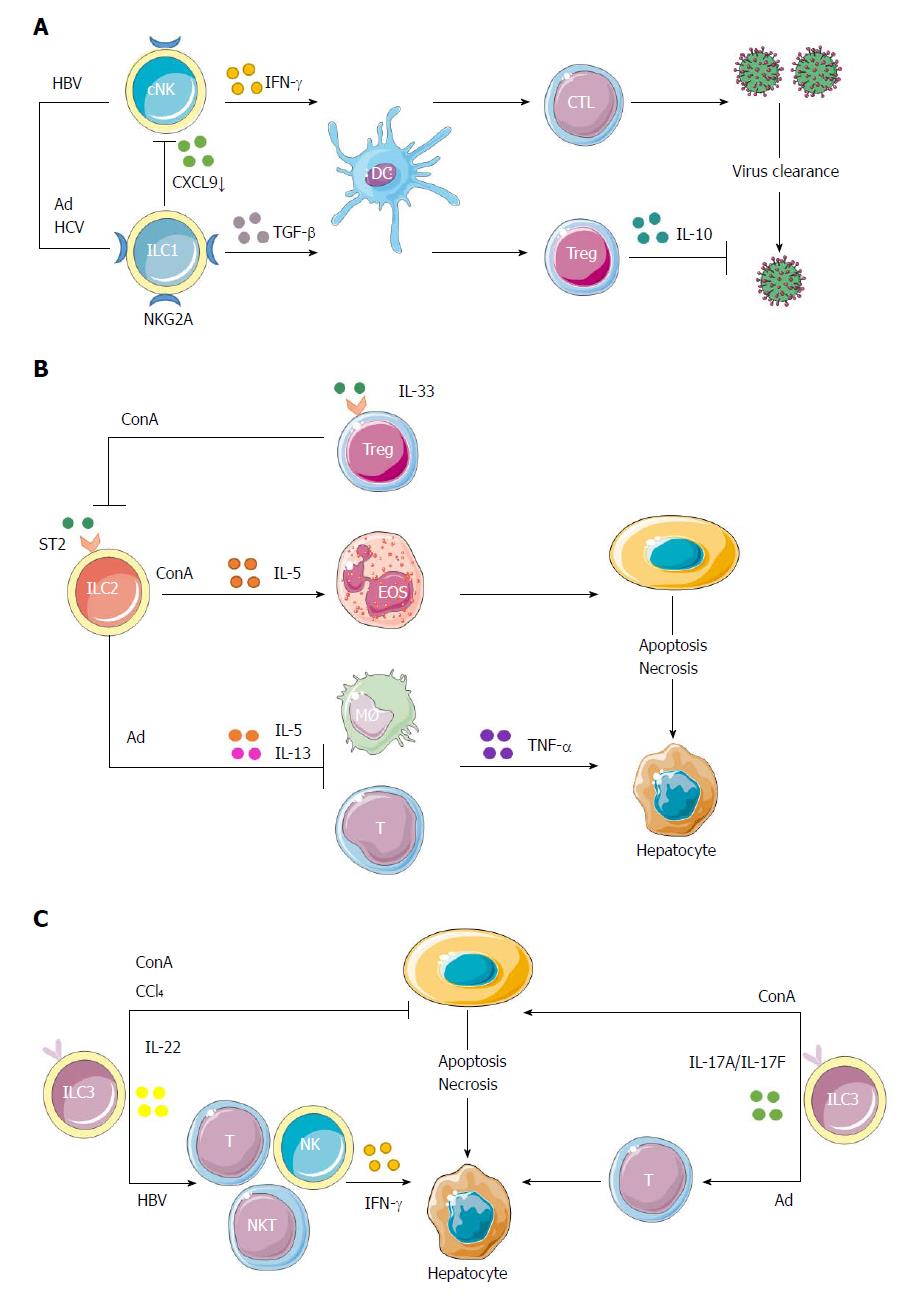
Figure 2 Protective and pathogenic roles of innate lymphoid cells in hepatic inflammation.
A: cNK cells could produce IFN-γ to enhance the priming of CD8+ T cells to clear HBV. The interactions of NK cells with hepatocytes via NKG2A inhibitory receptor could prime DCs to induce CD4+CD25+ Tregs, which would in turn up-regulate the expression of NKG2A on NK cells via IL-10 production, thus impairing the antiviral ability of NK cells. Because of increased expression of NKG2A on ILC1s in hepatic Ad as well as hepatitis C virus infection, ILC1s play a role in maintaining the liver as a tolerogenic site by inhibiting CXCL9 expression, which is required for the accumulation of cNK cells. This would further impair the activation of liver CD103+ DCs, thus interrupting the proliferation of virus-specific CD8+ T cells and the clearance of virus; B: In ConA-induced immune hepatitis, hepatic ILC2s could amplify inflammation through the expression of IL-5 to recruit eosinophils in response to IL-33 released upon liver tissue damage. The inflammatory activity of endogenous ILC2s in immune-mediated hepatitis might be regulated by IL-33-elicited ST2+ Tregs. Besides, in Ad-induced viral hepatitis, a strong expression of ILC2s was induced by IL-33 to exert a protective role through down-regulation of the hepatotoxic cytokine TNF-α in T cells and macrophages. Both the proinflammatory and protective roles of ILC2s in hepatitis are part of IL-33 action; C: In immune hepatitis, ILC3-derived IL-22 has a protective role in ConA- and carbon tetrachloride-induced hepatitis, while IL-17 plays a pathological role in ConA-induced hepatitis. Besides, Notch-mediated IL-22 is an important mediator of the inflammatory response in HBV infection, being responsible for the recruitment of antigen-nonspecific inflammatory cells into the liver and subsequent liver injury. In Ad-induced acute hepatitis, the IL-17A/F signaling is critical for adaptive T response and is responsible for affected lymphocyte infiltration and hepatic inflammation. Ad: Adenovirus; cNK: Conventional natural killer; ConA: Concanavalin A; DC: Dendritic cell; HBV: Hepatitis B virus; IL: Interleukin; ILC: Innate lymphoid cell; NK: Natural killer; Tregs: T regulatory cells.
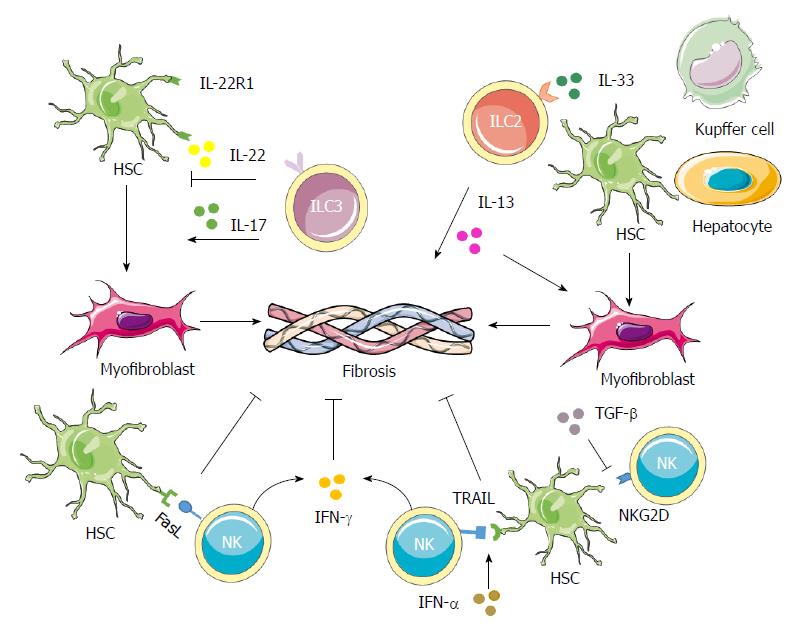
Figure 3 Contributions of innate lymphoid cells in liver fibrosis.
NK cells can decrease the proliferation and activation as well as induce cell cycle arrest of HSCs through IFN-γ. They can also induce the apoptosis of activated HSCs through the TRAIL and Fas ligand pathways. The expression of RAE-1, which is the ligand for the NKG2D activating receptor, is increased on activated HSCs, thus promoting killing by NK cells. IFN-α could increase the surface expression of TRAIL on NK cells to enhance HSCs killing by NK cells, while TGF-β down-regulates the surface expression of NKG2D and 2B4 to suppress the antifibrotic role of NK cells. In both human and mouse cases, IL-33 released from hepatocytes, HSCs and Kupffer cells in response to chronic hepatocellular stress leads to the accumulation and activation of IL-13-producing liver resident ILC2s via ST2-dependent signaling. IL-13 further triggers the activation and transdifferentiation of HSCs into myofibroblasts, to induce potent fibrogenic responses. ILC3s play more complicated roles in fibrosis. On one hand, ILC3s exert an antifibrotic effect by inducing fibroblasts senescence through IL-22 signaling and by down-regulating the expressions of collagen and CTGF through IL-17 signaling. On the other hand, IL-17 can promote inflammation and induce activation of fibroblasts, indicating a profibrotic role for the ILC3s. CTGF: Connective tissue growth factor; HSC: Hepatic stellate cell; IL: Interleukin; IFN: Interferon; NK: Natural killer; TGF: Tumor growth factor; TRAIL: TNF-related apoptosis-inducing ligand.
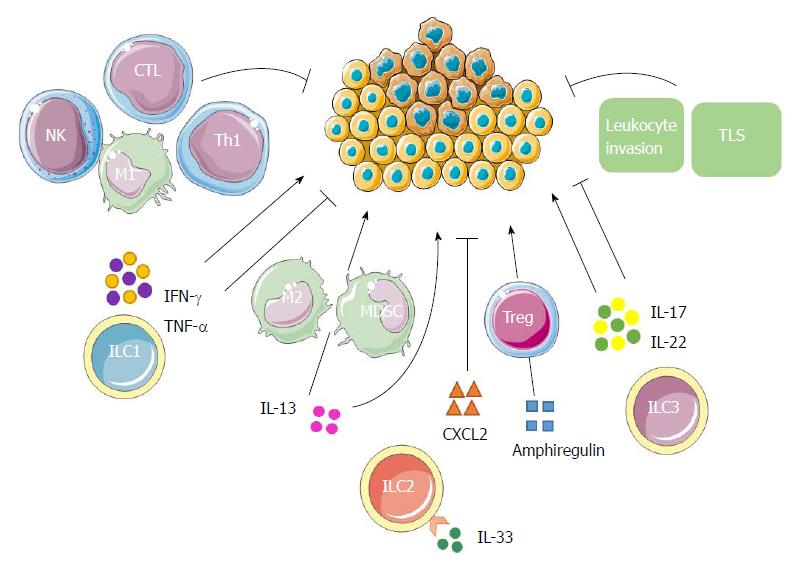
Figure 4 Possible innate lymphoid cell interactions in liver tumor immunity.
ILC1s have both antitumor and protumor effects according to the properties of cytokines secreted. IFN-γ and TNF-α have antiproliferative, antiangiogenic and proapoptotic effects against cancer cells. In addition to promoting the polarization of CD4+ T cells into Th1 cells, they can also boost the responses of macrophages, NK cells and CTLs, leading to a strong antitumor response. On the contrary, their ambiguous roles enable them to enhance tumor formation, growth and spread. ILC2s may contribute to tumor progression either directly through the tumorigenic effects of IL-13, or indirectly by stimulating M2 macrophages and MDSCs through IL-13. The production of amphiregulin suggests that ILC2s may further inhibit antitumor immune responses either directly or via stimulation of Tregs. IL-33 could also induce the secretion of a massive amount of CXCR2 ligands from ILC2s as well as create a tumor microenvironment wherein tumor cells express CXCR2, leading to apoptosis of active tumor cells. ILC3s might promote antitumor responses by enhancing leukocyte invasion, promoting TLSs’ induction and through the antitumor effects of IL-17 and IL-22. Conversely, ILC3s may promote tumor formation and progression by IL-17 and IL-22 as well according to the phase of responses and specific tumor microenvironment. CTL: Cytotoxic T lymphocyte; IL: Interleukin; ILC: Innate lymphoid cell; IFN: Interferon; MDSC: Myeloid-derived suppressor cell; NK: Natural killer; Th: T helper; TLS: Tertiary lymphoid structure; TNF: Tumor necrosis factor; Tregs: T regulatory cells.
- Citation: Shen Y, Li J, Wang SQ, Jiang W. Ambiguous roles of innate lymphoid cells in chronic development of liver diseases. World J Gastroenterol 2018; 24(18): 1962-1977
- URL: https://www.wjgnet.com/1007-9327/full/v24/i18/1962.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i18.1962