Copyright
©The Author(s) 2016.
World J Gastroenterol. Dec 7, 2016; 22(45): 9954-9965
Published online Dec 7, 2016. doi: 10.3748/wjg.v22.i45.9954
Published online Dec 7, 2016. doi: 10.3748/wjg.v22.i45.9954
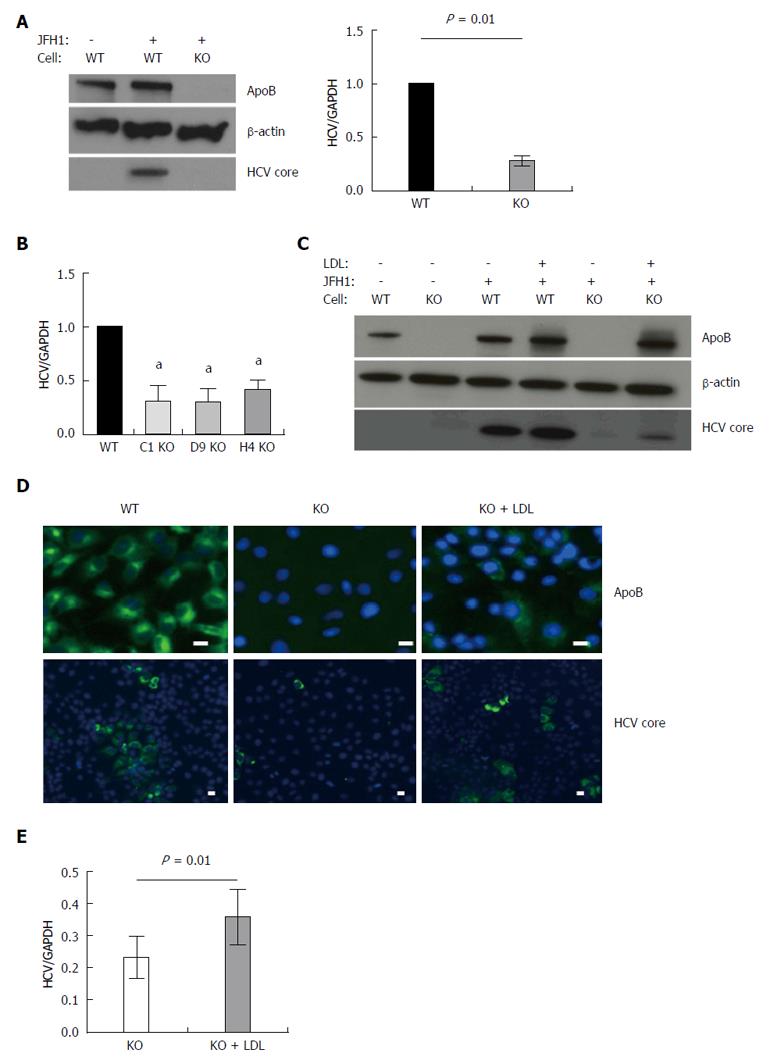
Figure 1 APOB genetic knockout Huh 7 hepatoma cells inefficiently support hepatitis C virus.
A: Using TALEN genetic deletion of APOB, knockout huh7/CD81 (high) cells were generated (APOB-/-, KO). We demonstrated that these cells inefficiently support hepatitis C virus (HCV) infection with the fully infectious tissue culture HCV virus, HCVcc (JFH1). There was markedly decreased JFH1 72 h following infection both at the level of HCV core protein and RNA. Data is shown as mean with 95% confidence interval, normalized to WT; B: To confirm this was not a clone-specific effect, three additional clones were infected with the JFH1 virus and intracellular HCV RNA assessed 72 h following infection. We again found a decrease in HCV RNA in the APOB KO clones (aP < 0.05); C, D: Rescue experiments were performed by treating cells with human LDL at the same time as infection with the JFH1 virus. We found partial restoration of intracellular apoB expression at 72 h following exposure (D), and there was a partial restoration of HCV infection, with increased expression of HCV core protein (C), HCV RNA (E), and IF demonstrating increased HCV core expression in the LDL-treated cells (D). Scale bar: 20 μm.
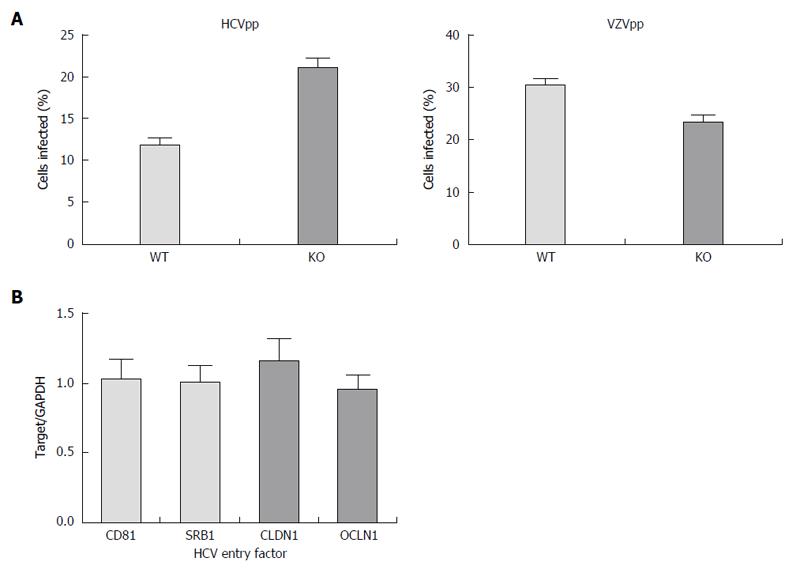
Figure 2 Hepatitis C virus entry is not impaired in APOB KO cells.
A: Using an hepatitis C virus (HCV) genotype 1b pseudoparticle (HCVpp), cells were exposed to the pseudoparticle and incubated for 72 h at which time the degree of HCV entry was assessed using GFP expression; a control VZV pseudoparticle (VZVpp) was also assessed. We that entry of neither HCV nor VZV was impaired in the APOB KO cells; B: We confirmed that the expression levels of known HCV entry factors CD81, SRB1, CLDN1, OCLN1 were preserved in the KO cells (normalized to WT cell expression level, 1).
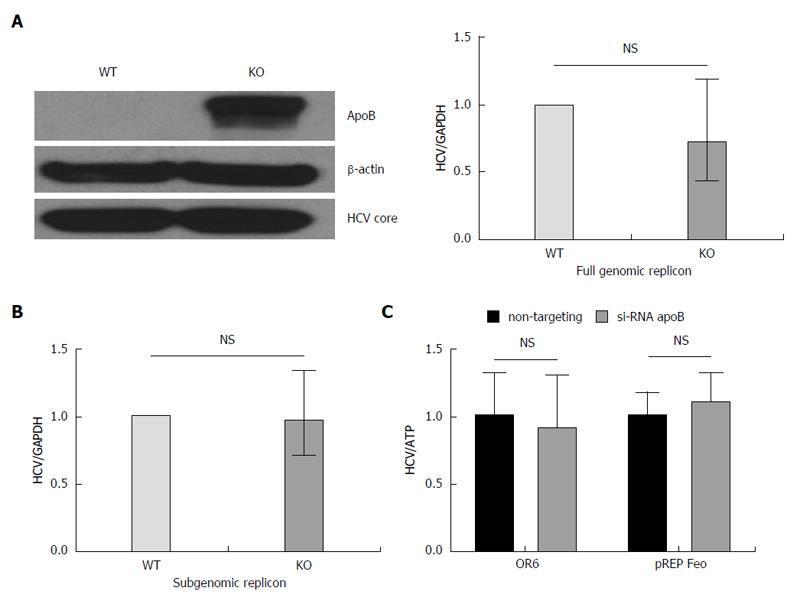
Figure 3 Loss of apoB does not impair replication in APOB KO cells.
A: A full genomic replicon derived from the JFH1 hepatitis C virus (HCV)cc strain was electroporated into WT and KO cells which were then selected in culture based on antibiotic resistance. We demonstrated that there was no decrease in intracellular HCV core or RNA in the KO compared to WT cells; B: A subgenomic replicon, which lacks expression of the E1, E2 and core proteins, was also electroporated into the cells and again there was no difference in HCV RNA. Data is shown as mean with 95%CI, normalized to WT; C: To confirm these findings, we used replicon cells with a full genome of a different viral genotype (OR6, 1b and pREP-Feo, 1b) and subsequently knocked down apoB expression using RNAi. There was no difference in HCV replication in the setting of apoB knockdown. Data is shown as mean ± SD.
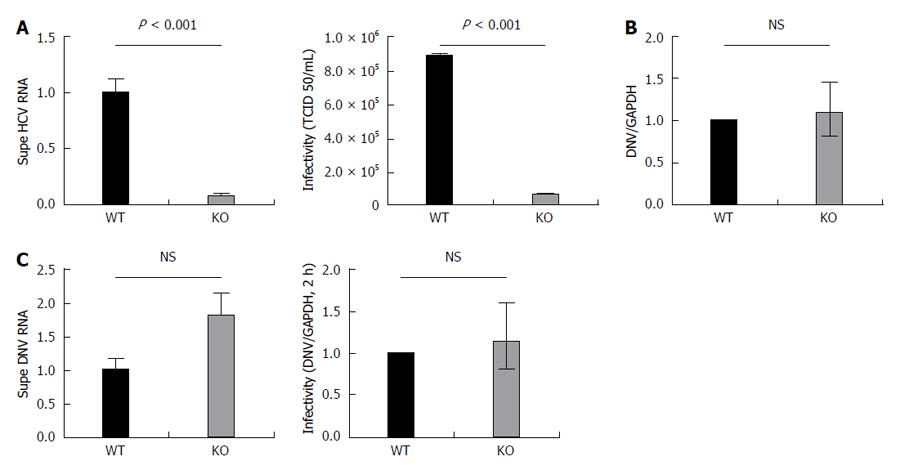
Figure 4 APOB KO cells have diminished hepatitis C virus production and KO virus has impaired infectivity, but APOB KO has no effect on dengue viral infection.
A: 72 h following infection with JFH1 hepatitis C virus (HCV)cc, supernatant was harvested and viral RNA was isolated and quantified using qPCR. The supernatant harvested from infected KO cells had significantly lower levels of HCV RNA compared to WT supernatant. Data are normalized to WT RNA titer and standard error of the mean shown. After normalizing for the differences in HCV RNA by diluting the WT virus, the infectivity of the two viruses in highly HCV-permissive Huh 7.5.1 cells was then determined using the TCID50 method. The KO generated virus had significantly reduced infectivity in the permissive Huh 7.5.1 cells; B: Dengue replication: WT and KO cells were infected with Dengue virus, and the level of intracellular RNA was assessed at 72 h. No difference was observed between WT and KO cells. Data is shown as mean with 95% confidence interval, normalized to WT; C: Supernatant of dengue-infected cells was harvested at 72 h and dengue viral RNA quantified. RNA levels were slightly higher in the supernatant of the KO cells, but the difference was not significant. A similar trend was observed with the infectivity of the KO generated dengue virus, assessed at DNV RNA at 2 h following infection.
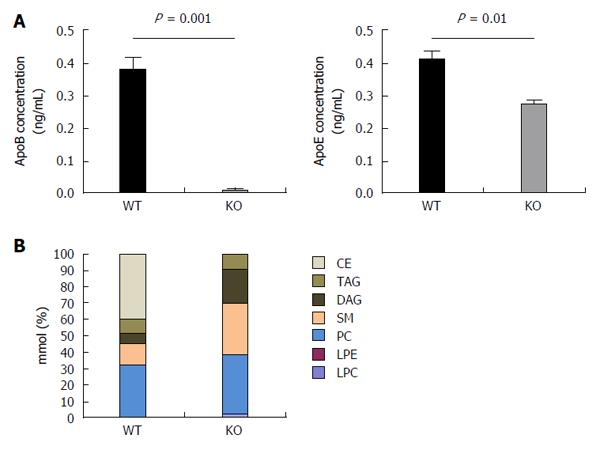
Figure 5 Hepatitis C virus produced by APOB KO cells has a fundamentally altered lipidome.
A: The concentrations of apoB and apoE were determined of the viral supernatant using ELISA. ApoB levels were barely detected in the viral supernatant of the KO cells. ApoE concentrations were also found to be lower in KO compared to the WT cells; B: Hepatitis C virus (HCV)cc generated in APOB KO cells has an altered lipidome. Using a Jc1E2FLAG HCV tissue culture fully infectious virus, viral particles were purified using affinity purification particles were extracted and the lipidome was purified using liquid chromatography - mass spectrometry (LC-MS). LC-MS demonstrated that the viral particles generated in the APOB knockout cells are completely depleted in cholesterol esters. CE: Cholesterol and cholesterol esters; TAG: Triacylglycerols; DAG: Diacylglycerols; SM: Sphingomyelins; PC: Phosphatidylcholines; LPE: Lysophosphatidylethanolamines; LPC: Lysophosphatidylcholines.
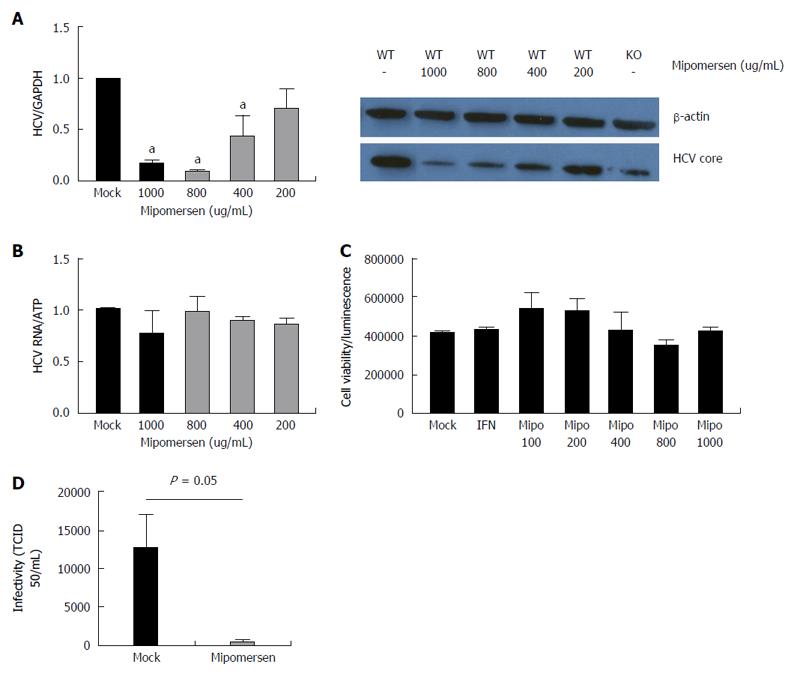
Figure 6 Mipomersen exerts a dose-dependent and potent anti-hepatitis C virus effect.
A: Huh7/CD81 high cells were treated with escalating doses of mipomersen from 200 ug/mL to 1000 ug/mL and then infected with JFH1. At 72 h following infection, there was a dose-dependent inhibition of hepatitis C virus (HCV), demonstrated at both the RNA and protein (HCV core) level. Data are shown as means with 95%CI (aP < 0.05); B: OR6 genotype 1b replicon cells were treated with mipomersen for 72 h and no effect was observed on HCV replication; C: There was no negative impact of escalating doses of mipomersen on cell viability; D: Infectivity of the virus was assessed using the TCID50 method. Virus generated in cells treated with mipomersen had a significant reduction in infectivity compared with virus generated in mock-treated cells. Data shown as mean TCID50/mL ± SEM.
- Citation: Schaefer EAK, Meixiong J, Mark C, Deik A, Motola DL, Fusco D, Yang A, Brisac C, Salloum S, Lin W, Clish CB, Peng LF, Chung RT. Apolipoprotein B100 is required for hepatitis C infectivity and Mipomersen inhibits hepatitis C. World J Gastroenterol 2016; 22(45): 9954-9965
- URL: https://www.wjgnet.com/1007-9327/full/v22/i45/9954.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i45.9954