Copyright
©The Author(s) 2016.
World J Gastroenterol. Jul 21, 2016; 22(27): 6287-6295
Published online Jul 21, 2016. doi: 10.3748/wjg.v22.i27.6287
Published online Jul 21, 2016. doi: 10.3748/wjg.v22.i27.6287
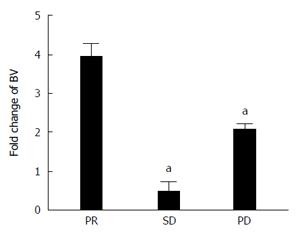
Figure 1 Increase of bevacizumab level in serum.
Increase of the serum concentration of the drug in function of time; the serum was withdrawn before the 2nd and the 5th cycle of therapy. Results are divided in three histograms, representative of the fold change of bevacizumab (BV) in patients who showed partial response (PR), stable disease (SD) and progression disease (PD). aP < 0.05, BV of patients with SD or PD vs with PR.
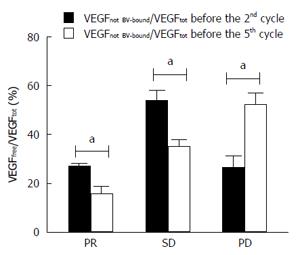
Figure 2 Residual not bevacizumab-bound vascular endothelial growth factor in plasma in function of time.
Histograms represent the percentage of the ratio of not bevacizumab (BV)-bound vascular endothelial growth factor (VEGF) to total VEGF in plasma before the 2nd and the 5th cycle of therapy, respectively. aP < 0.05, the 2nd cycle vs the 5th cycle.
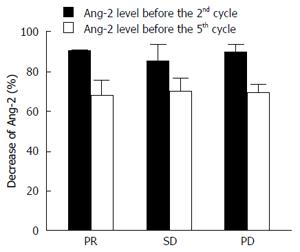
Figure 3 Decrease of angiopoietin-2 in plasma in function of time.
The percentage of the decrease of angiopoietin-2 (Ang-2) level in plasma as respect the baseline level (before the beginning of therapy) is reported in function of timing after therapy, before the 2nd and the 5th cycle.
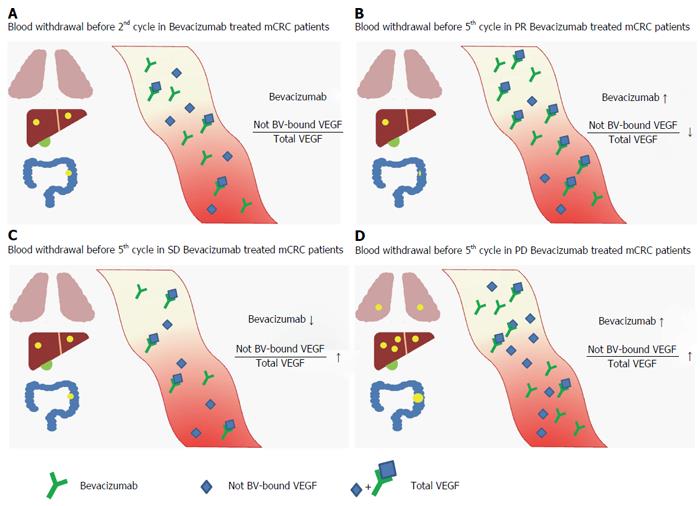
Figure 4 Grafical representation of bevacizumab not bevacizumab-bound vascular endothelial growth factor/total vascular endothelial growth factor before 2nd cycle in bevacizumab treated metastatic colorectal cancer patients (A) and before 5th cycle in partial response (B), stable disease (C) and progression disease (D) groups.
BV: Bevacizumab; VEGF: Vascular endothelial growth factor; PR: Partial response; SD: Stable disease; PD: Progression disease; mCRC: Metastatic colorectal cancer.
- Citation: Azzariti A, Porcelli L, Brunetti O, Del Re M, Longo V, Nardulli P, Signorile M, Xu JM, Calabrese A, Quatrale AE, Maiello E, Lorusso V, Silvestris N. Total and not bevacizumab-bound vascular endothelial growth factor as potential predictive factors to bevacizumab-based chemotherapy in colorectal cancer. World J Gastroenterol 2016; 22(27): 6287-6295
- URL: https://www.wjgnet.com/1007-9327/full/v22/i27/6287.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i27.6287