Copyright
©The Author(s) 2016.
World J Gastroenterol. Mar 28, 2016; 22(12): 3418-3431
Published online Mar 28, 2016. doi: 10.3748/wjg.v22.i12.3418
Published online Mar 28, 2016. doi: 10.3748/wjg.v22.i12.3418
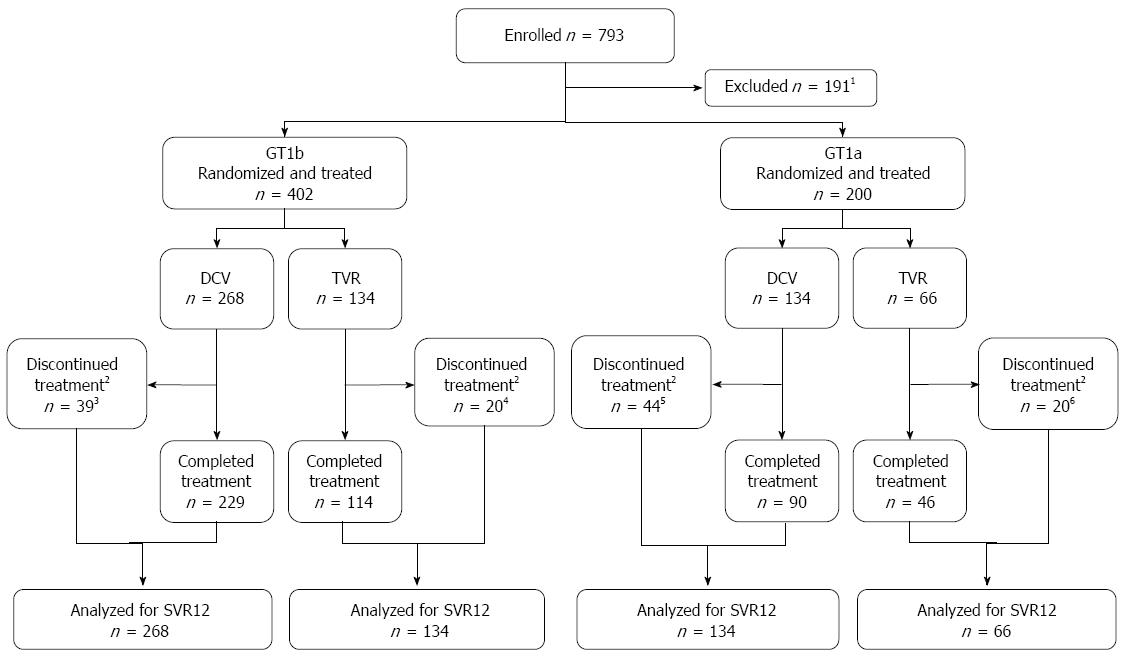
Figure 1 Patient disposition.
Efficacy analyses were based on a modified intent-to-treat analysis (included all randomized and treated patients who had received ≥ 1 dose of study medication; patients with missing HCV RNA measurements were considered failures). 1No longer met study entry criteria during the screening period (n = 139), withdrew consent (n = 28), administrative reason by sponsor (n = 5), lost to follow-up (n = 3), pretreatment AE (n = 2), or other reasons (n = 11); 2All 3 study drugs discontinued; 3Lack of efficacy (n = 15; 11 virologic breakthroughs, 3 futility, and 1 other), AE (n = 14), patient request (n = 6), lost to follow-up (n = 3), and withdrew consent (n = 1); 4AE (n = 17), withdrew consent (n = 2), and lost to follow-up (n = 1); 5Lack of efficacy (n = 23; 11 virologic breakthroughs, 4 futility, 8 other), adverse event (n = 11), lost to follow-up (n = 6), patient request (n = 1), and other (n = 3); 6Lack of efficacy (n = 5; 3 futility, 2 other), AE (n = 8), patient request (n = 3), lost to follow-up (n = 3), and death (n = 1; the death occurred in a patient who had discontinued treatment at week 16 due to bacteremia and died at posttreatment week 4 due to sepsis secondary to HCV-related cirrhosis). DCV: Daclatasvir; GT: Genotype; SVR12: Sustained virologic response (HCV-RNA < LLOQ) at posttreatment week 12; TVR: Telaprevir.
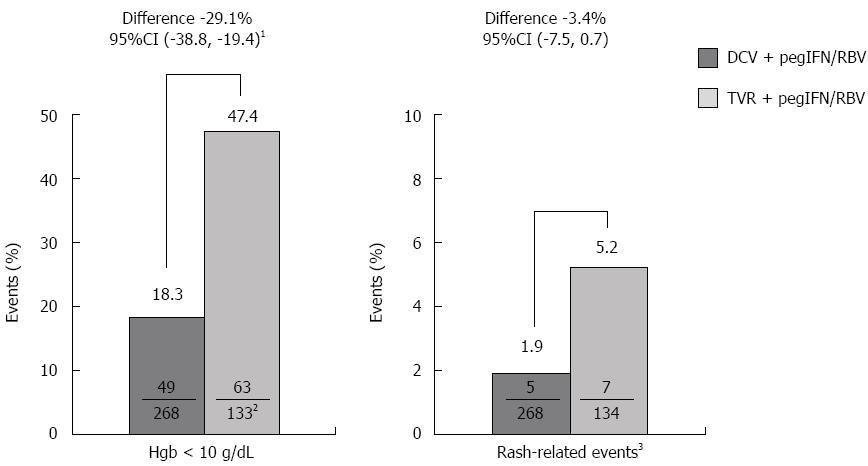
Figure 2 Safety secondary endpoints in GT1b-infected patients (first 12 wk).
mITT analysis (patients with missing data at posttreatment week 12 were considered failures). 1Superior at the 5% significance level; upper bound of the 95%CI was 0%; 2One patient in the TVR arm had no measurement due to discontinuation prior to on-treatment laboratory assessment; 3Includes rash-related SAEs, AEs leading to discontinuation, and grade 3 or 4 AEs. AE: Adverse event; CI: Confidence interval; DCV: Daclatasvir; GT: Genotype; Hgb: Hemoglobin; pegIFN: Peginterferon alfa-2a; RBV: Ribavirin; SAE: Serious adverse event; TVR: Telaprevir.
- Citation: Jacobson I, Zeuzem S, Flisiak R, Knysz B, Lueth S, Zarebska-Michaluk D, Janczewska E, Ferenci P, Diago M, Zignego AL, Safadi R, Baruch Y, Abdurakhmanov D, Shafran S, Thabut D, Bruck R, Gadano A, Thompson AJ, Kopit J, McPhee F, Michener T, Hughes EA, Yin PD, Noviello S. Daclatasvir vs telaprevir plus peginterferon alfa/ribavirin for hepatitis C virus genotype 1. World J Gastroenterol 2016; 22(12): 3418-3431
- URL: https://www.wjgnet.com/1007-9327/full/v22/i12/3418.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i12.3418