Copyright
©The Author(s) 2015.
World J Gastroenterol. Jan 21, 2015; 21(3): 836-853
Published online Jan 21, 2015. doi: 10.3748/wjg.v21.i3.836
Published online Jan 21, 2015. doi: 10.3748/wjg.v21.i3.836
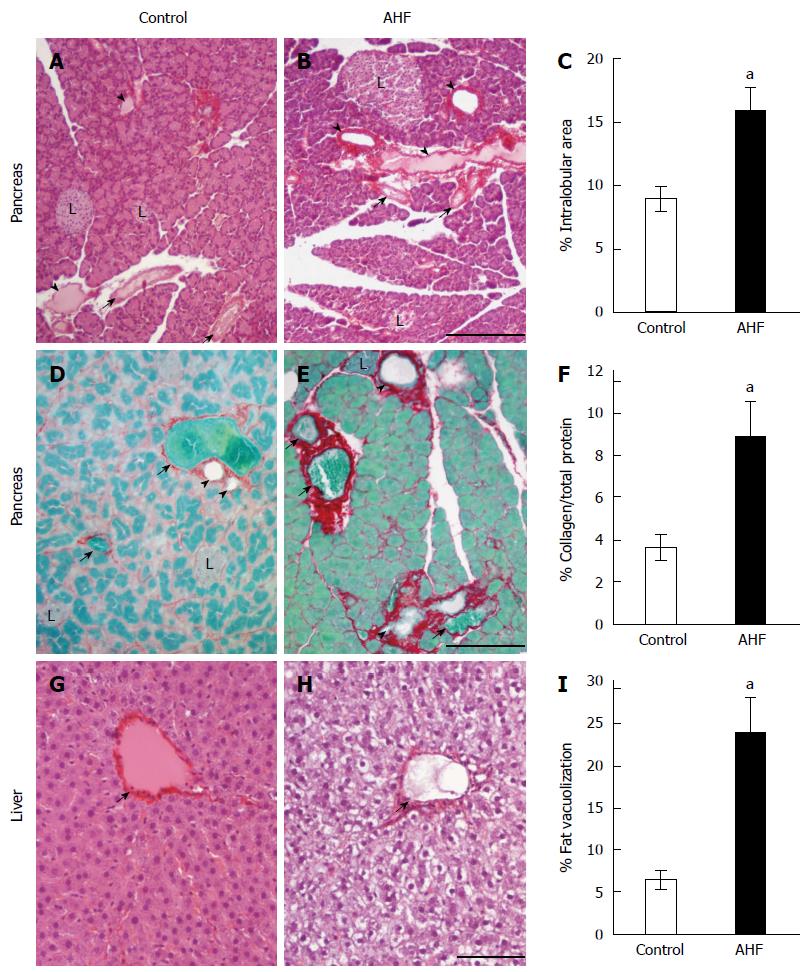
Figure 1 Histopathological analysis of the effects of alcohol and high fat diet fat diet on pancreas and liver.
A: Micrograph of pancreas from a control regular chow fed rat stained with hematoxylin and eosin (HE); B: Micrograph of pancreas from an alcohol and high fat diet (AHF) fed rat stained with HE; C: Quantification of intralobular spaces (area percentage) determined there was a significant increase in pancreata from AHF fed rats; D: Micrograph of pancreas from a control rat fed regular chow with Sirius red stained collagen fibrosis and fast green counterstain; E: Micrograph of pancreas from an AHF fed rat with Sirius red stained collagen fibrosis and fast green counterstain; F: Quantification of extracellular collagen deposits (stained red) as a percent of total tissue area. A significant increase in collagen staining was detected in pancreata of AHF fed rats; G: Micrograph of liver from a control rat fed regular chow stained with HE; H: Micrograph of liver from an AHF fed rat stained with HE; I: Quantification of intracellular fat vacuolization (area percentage). Unstained areas are fat vacuoles that were significantly increased in liver samples from AHF fed rats. aP < 0.05, AHF vs control. L: Islet of Langerhans; Solid arrow head: Pancreatic duct; Small arrow: Blood vessel. A, B: Scalebar 50 μm; D, E: Scalebar 100 μm; G, H: Scalebar 100 μm.
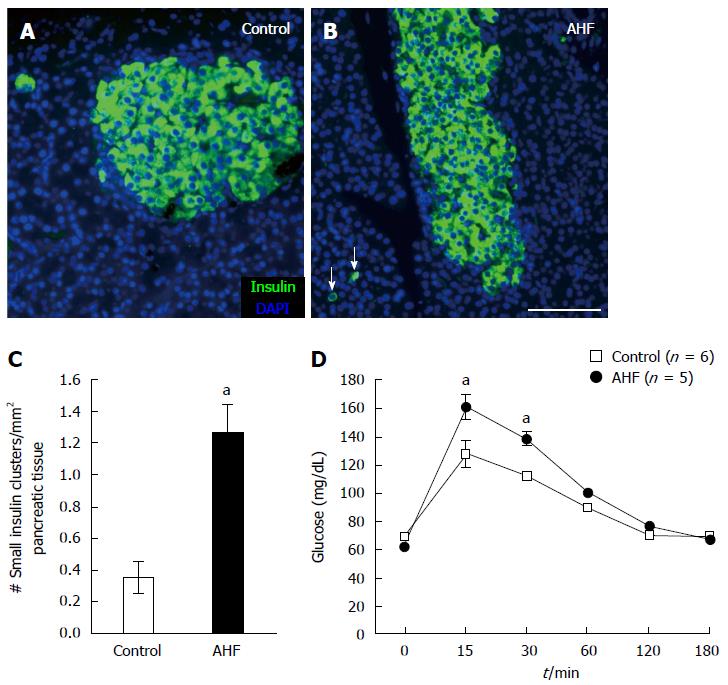
Figure 2 Chronic alcohol and high fat diet induced insulin cell proliferation and decreased glucose tolerance.
A: Micrograph of insulin immunoreactivity in pancreas from a control rat fed regular chow; B: Micrograph of insulin immunoreactivity in pancreas from a rat fed alcohol and high fat (AHF). In tissue samples from AHF fed rats insulin producing cells in the islets are atrophied and an increase of spurious proliferating insulin expressing cells (arrows) is detected in the pancreas; C: Quantification of the number of single cells or small insulin cell clusters per mm2 pancreas tissue detected a significant increase in AHF fed rats; D: Intraperitoneal glucose tolerance test at 10 wk determined that glucose tolerance was reduced in fasted AHF fed animals. Blood glucose levels of AHF fed animals peaked to significantly higher levels 15 and 30 min post injection. aP < 0.05, AHF vs control. A-B: Scalebar 100 μm.
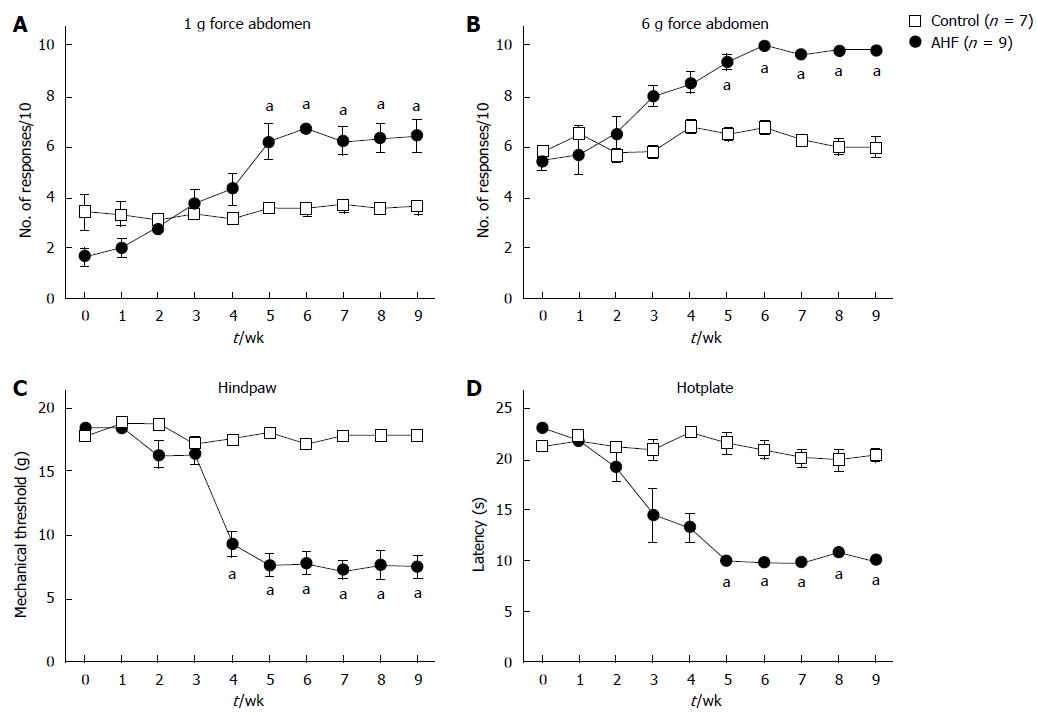
Figure 3 Time course of nociceptive behaviors in rats with chronic pancreatitis evoked by mechanical and heat stimuli.
A, B: Abdominal sensitivity is determined by the number of abdominal withdrawals in response to von Frey filament bending force stimulations (× 10). Responses to (A) low intensity (1 g) and (B) high intensity (6 g) mechanical stimulation elicited significantly more responses in alcohol and high fat (AHF) fed rats starting in week 5; C: Mechanical thresholds of the hindpaws were determined with von Frey filaments using the up-down method. In week 4 mechanical sensitivity thresholds of AHF fed rats were significantly reduced and did not recover while on the AHF diet; D: Heat sensitivity was determined by measuring the response latency (s) in the hotplate test (50 °C). In week 4, AHF fed rats demonstrated reduced response latencies in the hotplate test which did not recover during the 10 wk experimental time course. Open squares: control animals. Solid circles: AHF fed animals. aP < 0.05, AHF vs control.
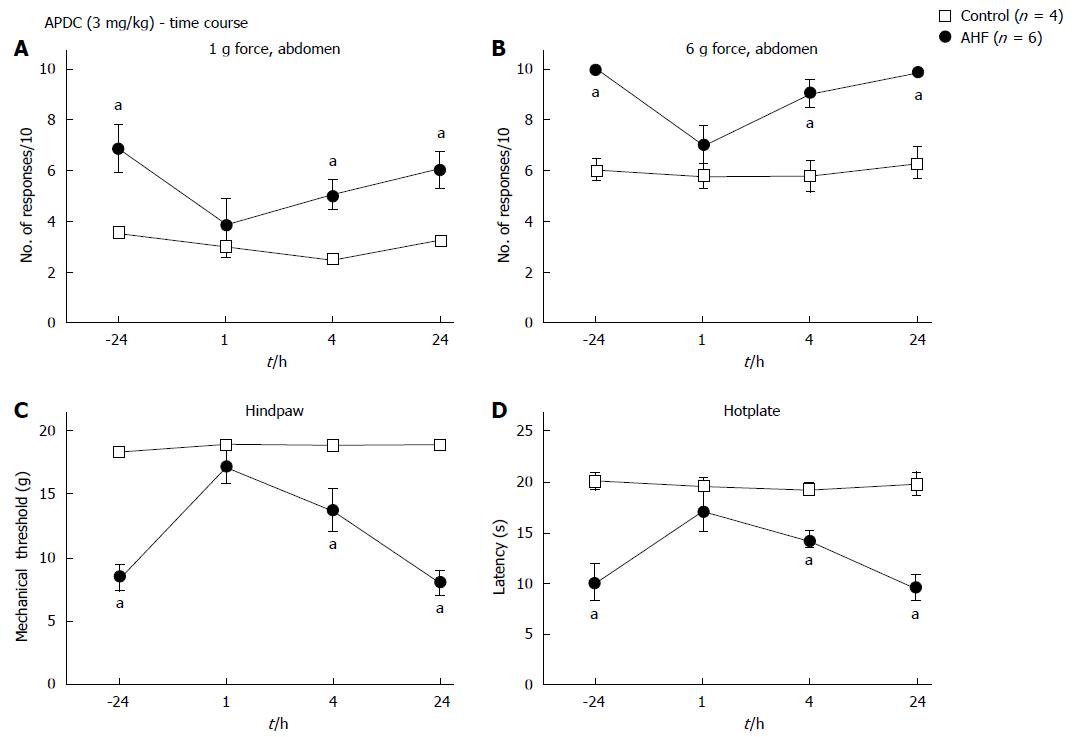
Figure 4 Time course of nociceptive responses to mechanical and heat stimuli after a single systemic treatment with (2R,4R)-4-Aminopyrrolidine-2,4-dicarboxylate.
A, B: Animals were treated with a single ip dose of (2R,4R)-4-Aminopyrrolidine-2,4-dicarboxylate (APDC) (3 mg/kg). APDC reversed the increase in nociception responses 1 h post treatment evoked by (A) low intensity (1 g) abdominal stimulation and (B) high intensity (6 g) abdominal stimulation; C: Alcohol and high fat (AHF) fed rats had increased hindpaw mechanical withdrawal thresholds; D: Response latencies of AHF fed rats increased 1 h after APDC treatment in the hotplate test, but sensitivity of the controls was not altered. aP < 0.05, AHF vs control.
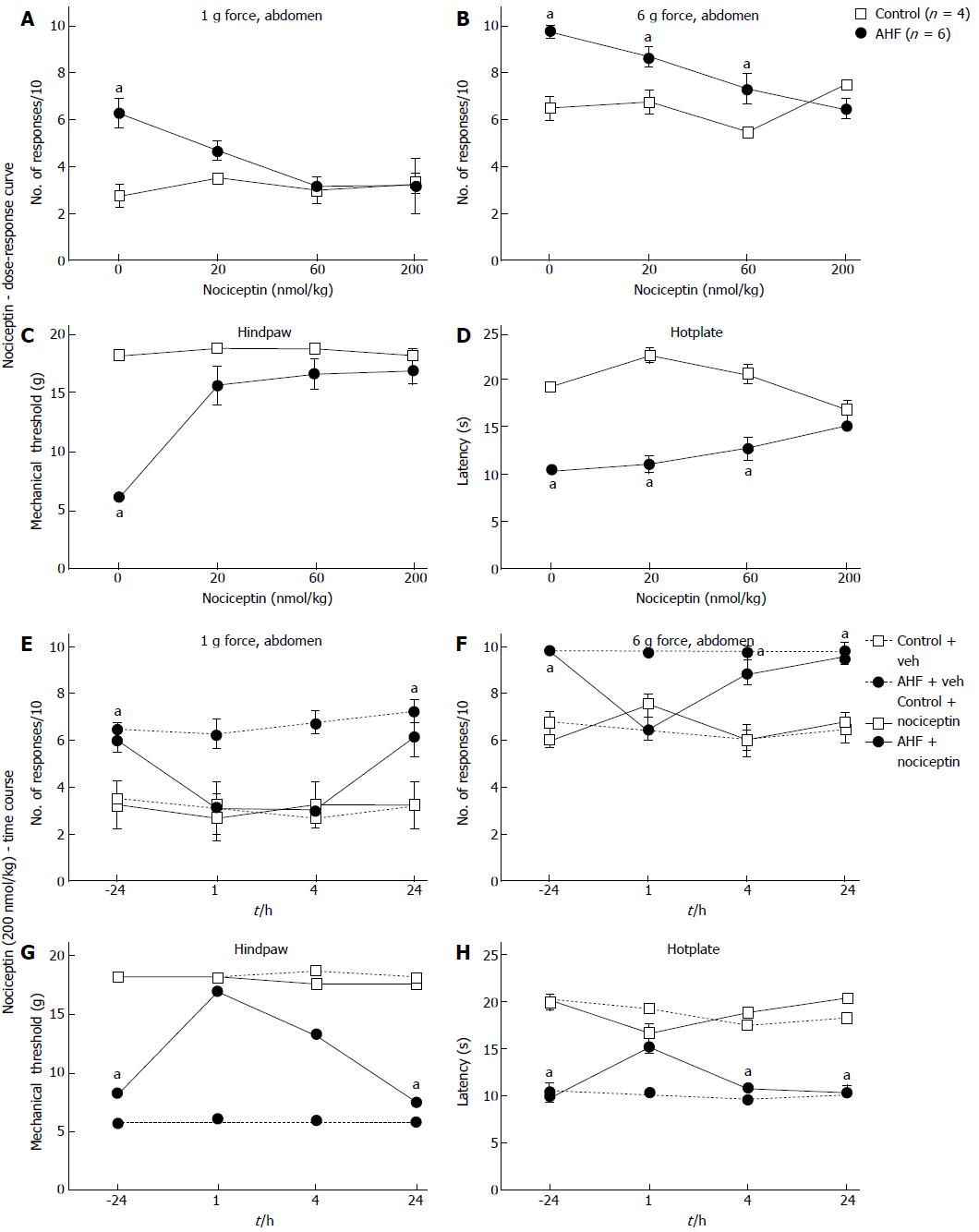
Figure 5 Dose-response curves and time course of nociceptive responses to mechanical and heat stimuli after a single systemic treatment with nociceptin.
A, B: Comparisons of the number of responses to mechanical stimulation on the abdomen skin 1 h after treatment with nociceptin (ip) for control and alcohol and high fat (AHF) fed rats. Dose-dependent responses were reduced to (A) low intensity (1 g) and (B) high intensity (6 g) mechanical stimulation of the abdomen; C: Nociceptin increased hindpaw mechanical withdrawal threshold in AHF fed rats; D: Hotplate response latencies of AHF fed animals improved with the highest dose of nociception. In control animals nociceptin did not alter mechanical and heat sensitivity. Vehicle treatments had no effect on mechanical or heat sensitivity of animals from either group; E-H: Mechanical and heat responses in animals treated with either a single systemic injection of nociceptin (200 nmol/kg in saline) or with the saline vehicle; E: Abdominal withdrawals in response to low intensity (1 g) mechanical stimulation decreased to control levels at the 1 and 4 h post injection timepoints of AHF fed rats. Mechanical hypersensivity of vehicle treated AHF diet fed rats did not improve; F: Responses of AHF fed rats to high intensity (6 g) abdominal mechanical stimulation were decreased to control values only 1 h after injection of nociception; G: Hindpaws mechanical withdrawal thresholds of AHF fed rats recovered to control values for up to 4 h post injection; H: Hotplate response latencies of AHF fed rats improved only at the 1 h time point after nociceptin treatment. aP < 0.05, AHF vs control.
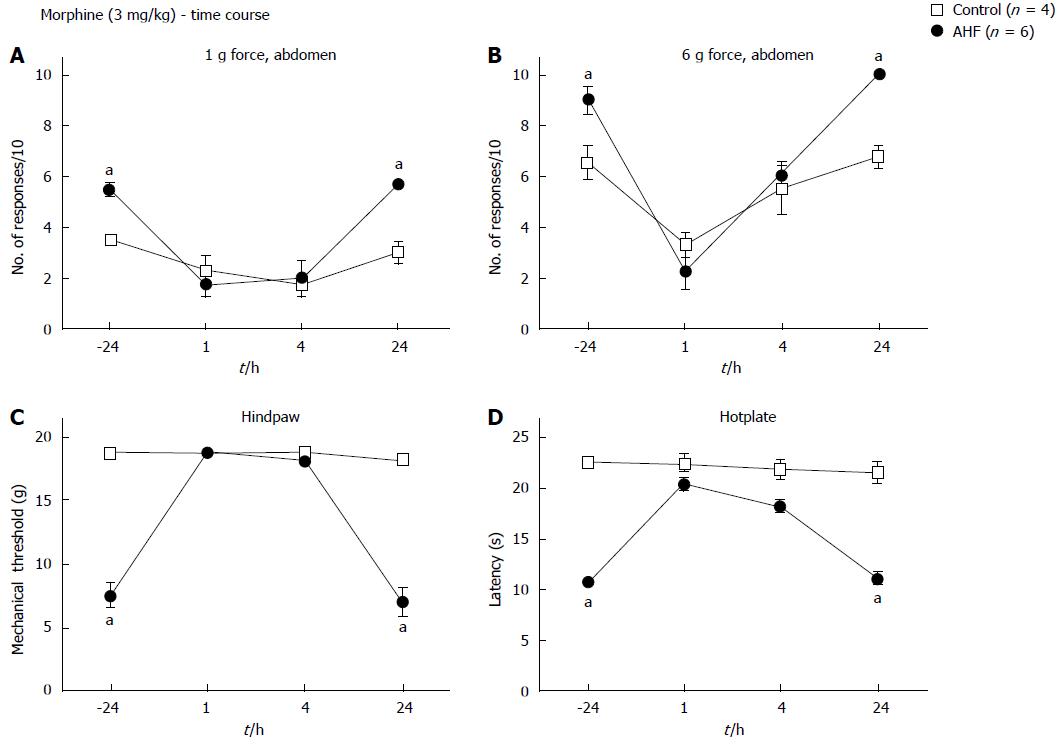
Figure 6 Time course of nociceptive responses to mechanical and heat stimuli after a single systemic treatment with morphine.
Animals were injected with morphine (3 mg/kg, subcutaneous) and responses to mechanical and heat stimuli measured. Morphine treatment of alcohol and high fat (AHF) fed rats decreased the number of responses to (A) low (1 g) and (B) high intensity (6 g) mechanical stimulation of the abdominal skin for up to 4 h. Abdominal withdrawal responses of controls evoked by high intensity (6 g) mechanical stimulation were reduced 1 h after treatment compared to before morphine. Hindpaw (C) mechanical withdrawal thresholds and (D) response latencies on the hot plate test of AHF fed rats were improved at 1 and 4 h after morphine treatment. Controls were unaffected by the low dose morphine. aP < 0.05, AHF vs control.
- Citation: McIlwrath SL, Westlund KN. Pharmacological attenuation of chronic alcoholic pancreatitis induced hypersensitivity in rats. World J Gastroenterol 2015; 21(3): 836-853
- URL: https://www.wjgnet.com/1007-9327/full/v21/i3/836.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i3.836