Copyright
©The Author(s) 2015.
World J Gastroenterol. Mar 28, 2015; 21(12): 3509-3518
Published online Mar 28, 2015. doi: 10.3748/wjg.v21.i12.3509
Published online Mar 28, 2015. doi: 10.3748/wjg.v21.i12.3509
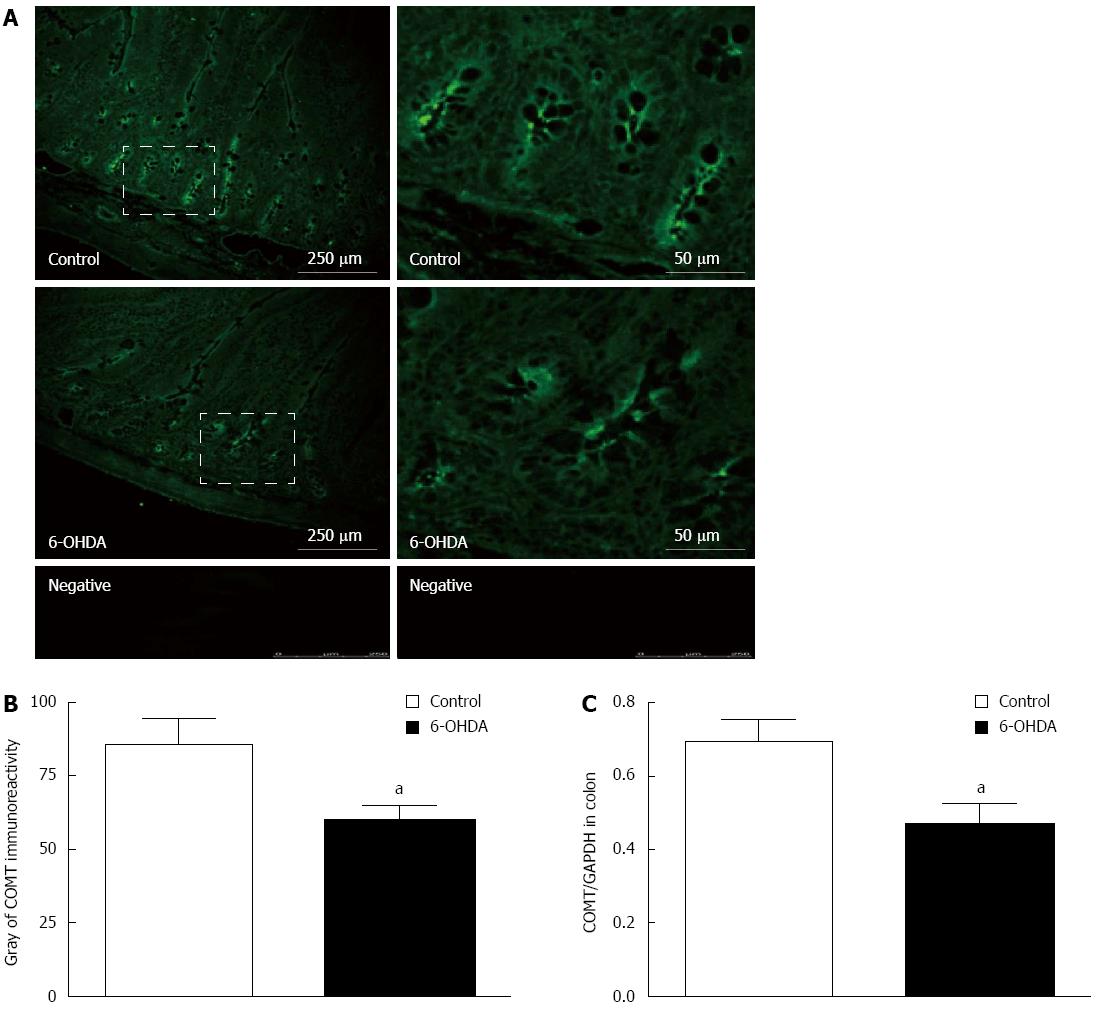
Figure 1 Characteristics of catechol-O-methyltransferase localization in normal and 6-OHDA Parkinson’s disease model rats.
A: The COMT immunoreactivity in colon; B: The gray changes of COMT immunoreactivity in colon; C: The changes of expression of COMT in colonic mucosal preparations in 6-OHDA PD rats (n = 8, aP < 0.05 vs control). COMT: Catechol-O-methyltransferase; PD: Parkinson’s disease.
Figure 2 Effect of entacapone on colon smooth muscle motility in 6-OHDA Parkinson’s disease rats.
A: Inhibitory effect of entacapone on colon longitudinal muscle contraction; B: Dose-response curve effect of entacapone on colon smooth muscle motility in 6-OHDA rats (n = 30); C: Representative tracing of strips when using entacapone (ENT) and β2 receptor antagonist ICI-118,551 (1.0 × 10-5 mol/L) in 6-OHDA rats; D: The role of β2 receptor in entacapone-induced effect of smooth muscle inhibition (n = 9, aP < 0.05 vs control).
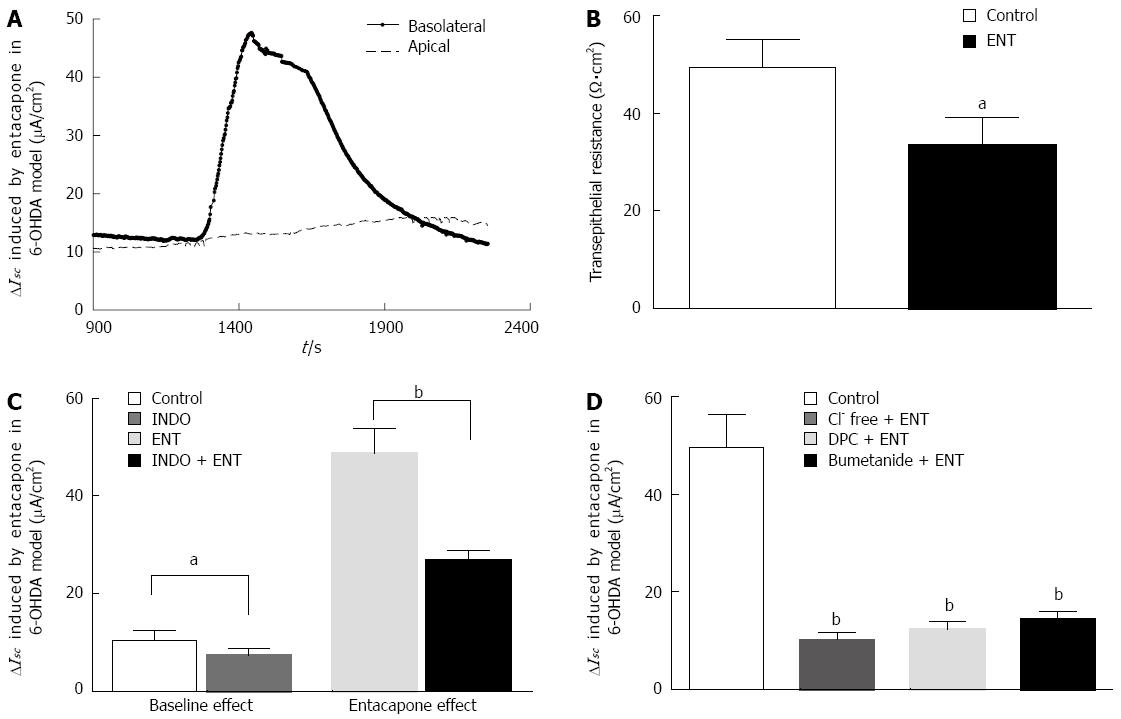
Figure 3 Entacapone-induced ISC response in colonic mucosa specimens in 6-OHDA Parkinson’s disease rats.
A: The short circuit current (ISC) changes induced by apical and basolateral using of ENT (200 μmol L-1); B: Basolateral application of ENT (200 μmol/L) induced transepithelial electric resistance (n = 13); C: Effect of indomethacin (INDO) (10 μmol/L) on the entacapone (ENT)-caused ISC changes with 6-OHDA PD rats (n = 9); D: Summary of the effects of pretreatment by removing Cl- from the extracellular fluid, the apical use of DPC (1 mmol/L) (a type of Cl-channel inhibitor), and the basolateral use of Na+-K+-2Cl- co-transporter (NKCC) antagonist bumetanide (100 μmol/L) on the ENT-caused ISC changes in mucosal preparations of colon in 6-OHDA PD rats (n = 9, aP < 0.05, bP < 0.01 vs control).
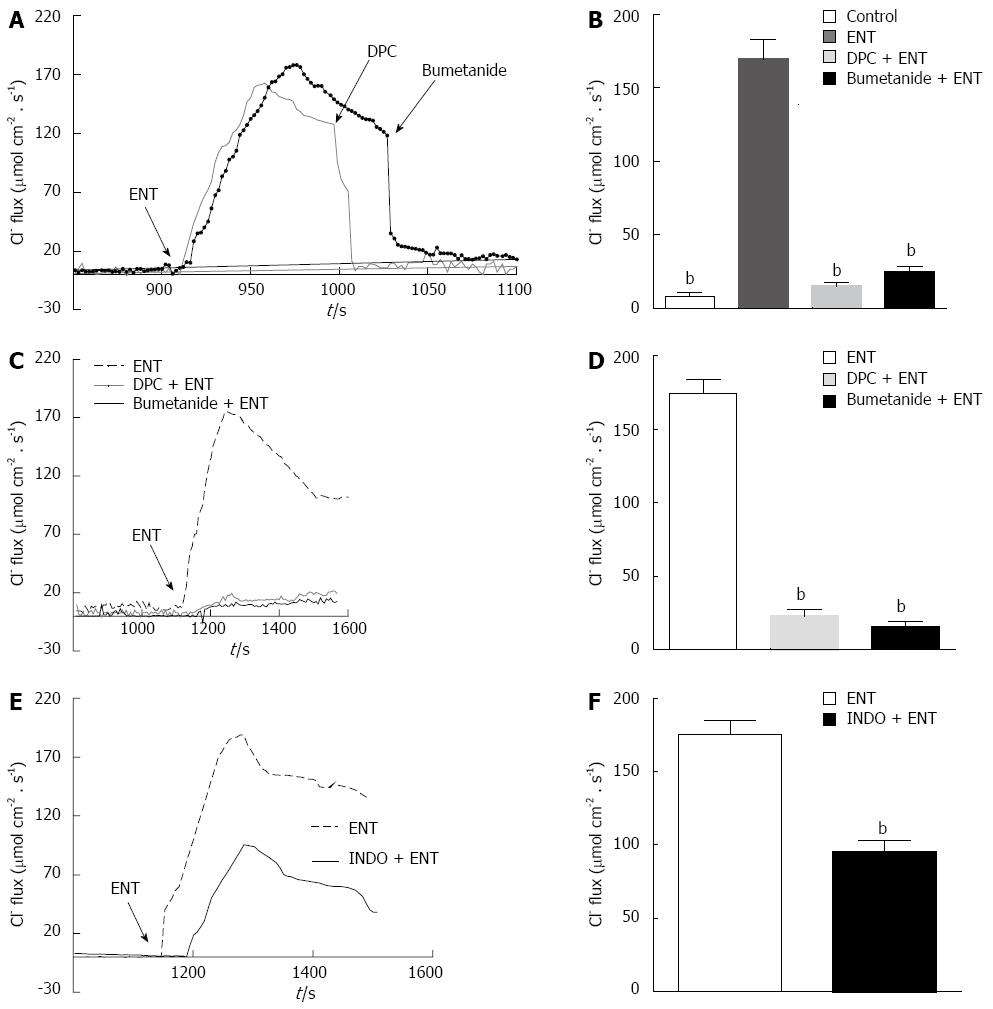
Figure 4 Utilizing scanning ion-selective electrode technique to observe the effect of entacapone on colon mucosa Cl--flux in 6-OHDA rats.
A: Application of entacapone (ENT) (200 μmol/L), diphenylamine-2, 2’-dicarboxylic acid (DPC) (1 mmol/L) and bumetanide (100 μmol/L), causing the typical performance of Cl—flux in colon specimens in 6-OHDA Parkinson’s disease (PD) rats; B: Generalization of the roles of bumetanide and DPC on the Cl--flux caused by ENT in colon specimens of 6-OHDA PD rats (n = 10); C: Application of ENT (200 μmol/L), preprocessing by bumetanide or DPC, causing the typical performance of Cl—flux in colon specimens of 6-OHDA PD rats; D: Generalization of Cl--flux caused by ENT after treatment by bumetanide and DPC in colonic specimens (n = 9); E: Application of ENT (200 μmol/L), and preprocessing by indomethacin (INDO) (10 μmol/L), causing the typical performance of Cl—flux in colon specimens in 6-OHDA PD rats; F: Generalization of the roles of INDO on the Cl--flux caused by ENT (n = 9, bP < 0.01 vs control).
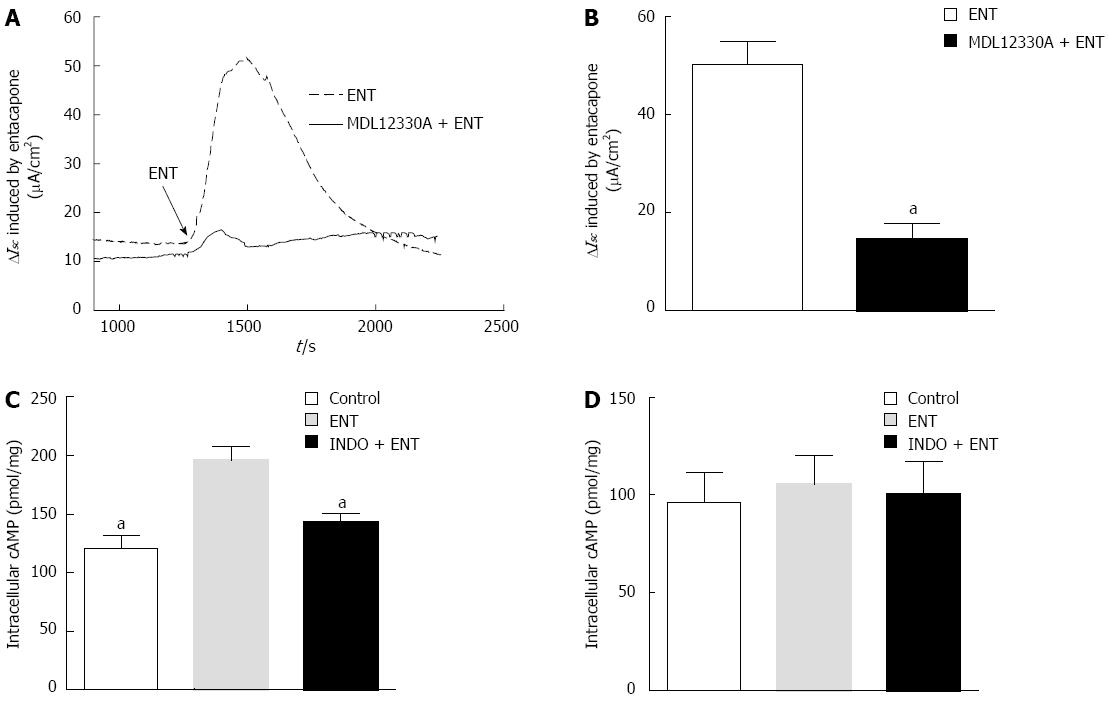
Figure 5 cAMP pathway involved in entacapone-induced Cl-secretion in 6-OHDA Parkinson’s disease rats.
A: Representation of effects of MDL 12330A (20 μmol/L) on entacapone (ENT)-induced ISC changes; B: Generalization of ISC caused by ENT after treatment by MDL 12330A; C: Generalization of the roles of indomethacin (INDO) on intracellular cAMP content caused by ENT in 6-OHDA Parkinson’s disease (PD) rats; D: Generalization of the roles of INDO on intracellular cGMP content caused by ENT in 6-OHDA PD rats (n = 6,aP < 0.05 vs control).
- Citation: Li LS, Liu CZ, Xu JD, Zheng LF, Feng XY, Zhang Y, Zhu JX. Effect of entacapone on colon motility and ion transport in a rat model of Parkinson’s disease. World J Gastroenterol 2015; 21(12): 3509-3518
- URL: https://www.wjgnet.com/1007-9327/full/v21/i12/3509.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i12.3509