Copyright
©2014 Baishideng Publishing Group Inc.
World J Gastroenterol. Nov 21, 2014; 20(43): 16318-16322
Published online Nov 21, 2014. doi: 10.3748/wjg.v20.i43.16318
Published online Nov 21, 2014. doi: 10.3748/wjg.v20.i43.16318
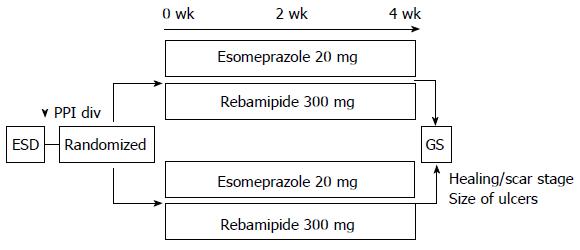
Figure 1 Study design.
After endoscopic submucosal dissection, all patients were randomized to 2 wk (2W) or 4 wk (4W) group. They were administered an intravenous infusion of omeprazole 20 mg every 12 h for 1 d, and received oral esomeprazole (20 mg/d) for 2 wk or 4 wk and rebamipide at a dose of 300 mg orally, 3 times a day for 4 wk. ESD: Endoscopic submucosal dissection; PPI div: Drip intravenous infusion of proton pump inhibitor (omeprazole 40 mg/d); GS: Gastroendoscopy.
- Citation: Arai M, Matsumura T, Okimoto K, Oyamada A, Saito K, Minemura S, Maruoka D, Tanaka T, Nakagawa T, Katsuno T, Yokosuka O. Two-week treatment with proton pump inhibitor is sufficient for healing post endoscopic submucosal dissection ulcers. World J Gastroenterol 2014; 20(43): 16318-16322
- URL: https://www.wjgnet.com/1007-9327/full/v20/i43/16318.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i43.16318