Copyright
©14 Baishideng Publishing Group Inc.
World J Gastroenterol. Nov 21, 2014; 20(43): 15992-16013
Published online Nov 21, 2014. doi: 10.3748/wjg.v20.i43.15992
Published online Nov 21, 2014. doi: 10.3748/wjg.v20.i43.15992
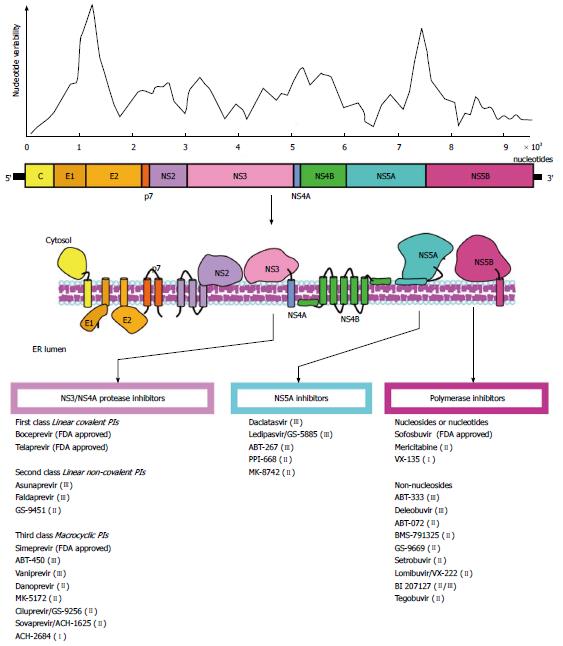
Figure 1 Hepatitis C virus genome and nucleotide variability.
A schematic representation of the viral genome is depicted. The degree of nucleotide variability along the viral genome is also shown. The target molecules for anti-HCV therapy are noted, and the antiviral agents are indicated. A selection of FDA approved and in development compounds are shown. Roman numerals in brackets indicate the current clinical phase of development. HCV: Hepatitis C virus; FDA: Food and Drug Administration; PI: Protease inhibitors.
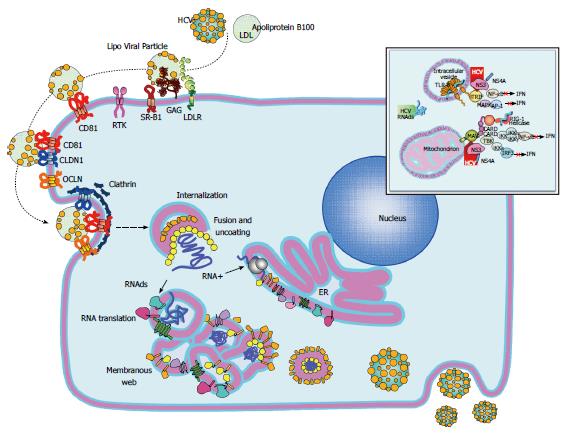
Figure 2 Hepatitis C virus replication cycle.
The replicative cycle of hepatitis C virus (HCV) is displayed. HCV interaction with its cell receptors is shown. Upon entry, the HCV genome is released into the cytoplasm and subsequently translated and translocate into the RE. The membranous web is used as scaffold for viral replication. Interferon and TLR3 signaling pathways are disrupted by the HCV NS3/4A protease by cleaving MAVS and TRIF (upper right window). Assembled virions are released via the constitutive secretory pathway. HCV: Hepatitis C virus; MAVS: Mitochondrial antiviral signaling protein; TRIF: Toll-like receptor-domain-containing adapter-inducing interferon-β.
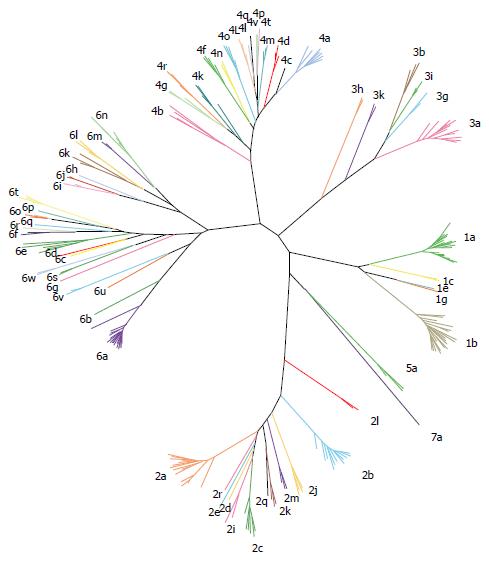
Figure 3 Hepatitis C virus genotypes and subtypes.
Representative strains belonging to all seven genotype and all different subtypes are presented.
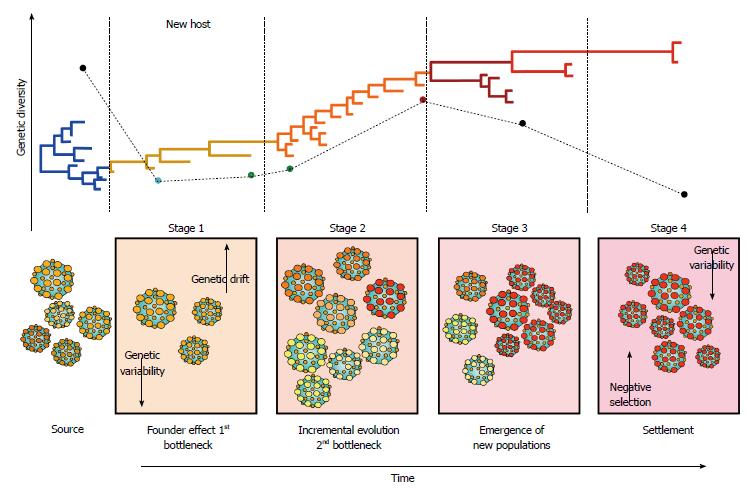
Figure 4 Hepatitis C virus molecular evolution.
The staging of molecular evolution intrahost is illustrated. All four stages of infection are portrayed. The phylogenetic relatedness between different populations throughout time is also represented. The main molecular processed driving the molecular evolution are shown in their respective stage.
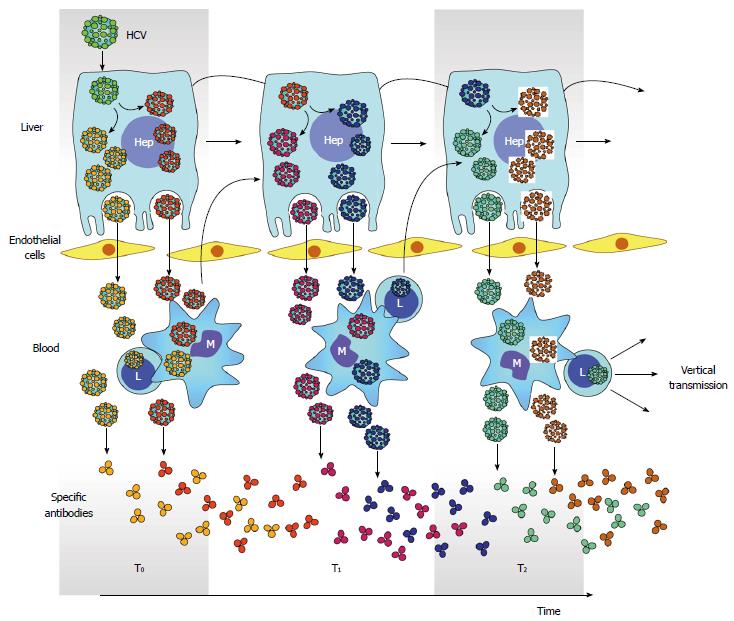
Figure 5 Hepatitis C virus compartmentalization.
Generation of multiple HCV subpopulations is color coded. Releasing of different subpopulation to circulation and infection of PBMC is also depicted. Antibodies elicited against different HCV subpopulation is also shown throughout time. However, the generation of neutralizing antibodies is delayed and gives opportunity for arisen of new viral subpopulations and subsequent infection of PBMC. In turn, infection in PBMC facilitates vertical transmission of HCV. PBMC: Peripheral blood mononuclear cells; HCV: Hepatitis C virus.
- Citation: Preciado MV, Valva P, Escobar-Gutierrez A, Rahal P, Ruiz-Tovar K, Yamasaki L, Vazquez-Chacon C, Martinez-Guarneros A, Carpio-Pedroza JC, Fonseca-Coronado S, Cruz-Rivera M. Hepatitis C virus molecular evolution: Transmission, disease progression and antiviral therapy. World J Gastroenterol 2014; 20(43): 15992-16013
- URL: https://www.wjgnet.com/1007-9327/full/v20/i43/15992.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i43.15992