Copyright
©2014 Baishideng Publishing Group Inc.
World J Gastroenterol. Nov 7, 2014; 20(41): 15087-15097
Published online Nov 7, 2014. doi: 10.3748/wjg.v20.i41.15087
Published online Nov 7, 2014. doi: 10.3748/wjg.v20.i41.15087
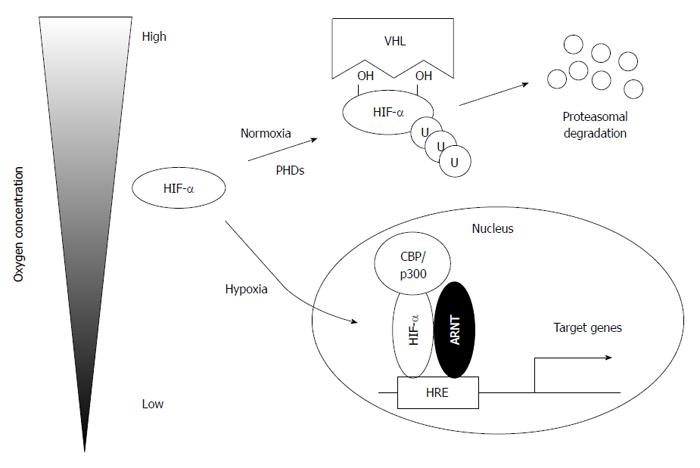
Figure 1 Regulation of hypoxia-inducible factors under normal and low oxygen concentrations.
Under normoxia conditions, hypoxia-inducible factor α (HIF-α) is hydroxylated at specific proline residues in an oxygen-dependent manner, ubiquitylated by von Hippel-Lindau (VHL) protein (pVHL), and, subsequently, degraded by the proteasome. Under hypoxia conditions, stabilized HIF-α translocates to the nucleus and binds aryl hydrocarbon receptor nuclear translocator (ARNT) to activate its target genes. PHD: Prolyl hydroxylase domain; HRE: Hypoxia-response element.
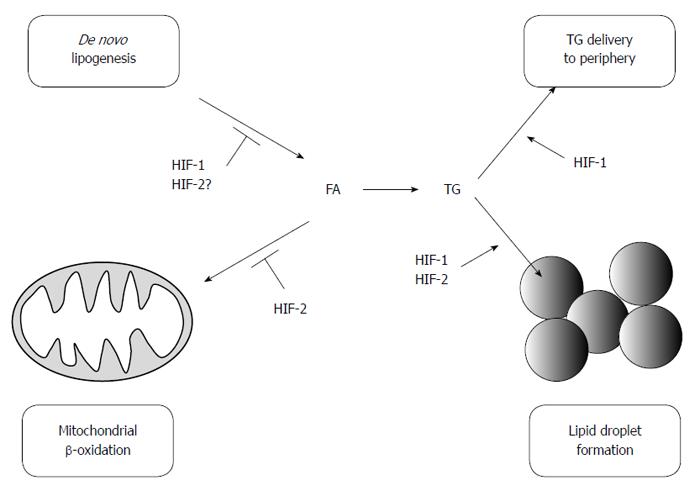
Figure 2 Hypoxia-inducible factors regulate hepatic lipid metabolism.
HIF-2 promotes lipid accumulation mainly by preventing mitochondrial β-oxidation and promoting lipid droplet formation. In contrast, HIF-1 suppresses lipid accumulation by inhibiting de novo lipogenesis. HIF-1 also promotes lipid storage and lipid export. HIF: Hypoxia-inducible factor; FA: Fatty acids; TG: Triglycerides.
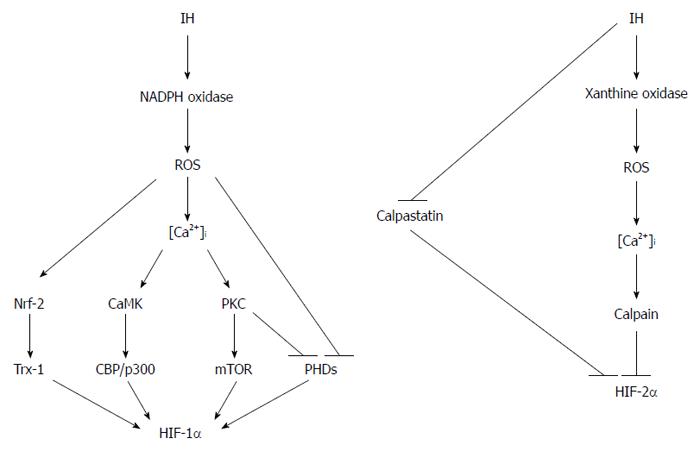
Figure 3 Distinct regulations of hypoxia-inducible factor-1α and hypoxia-inducible factor-2α under intermittent hypoxia conditions.
ROS and Ca2+ play crucial roles in the regulation of HIF activity in response to intermittent hypoxia, which results in the activation of HIF-1 and suppression of HIF-2. IH: Intermittent hypoxia; OSA: Obstructive sleep apnea; NADPH: Nicotinamide adenine dinucleotide phosphate; PKC: Protein kinase C; mTOR: Mammalian target of rapamycin; CBP: CREB-binding protein; PHD: Prolyl hydroxylase domain; HIF: Hypoxia-inducible factor.
- Citation: Suzuki T, Shinjo S, Arai T, Kanai M, Goda N. Hypoxia and fatty liver. World J Gastroenterol 2014; 20(41): 15087-15097
- URL: https://www.wjgnet.com/1007-9327/full/v20/i41/15087.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i41.15087