Copyright
©2014 Baishideng Publishing Group Inc.
World J Gastroenterol. Sep 21, 2014; 20(35): 12381-12390
Published online Sep 21, 2014. doi: 10.3748/wjg.v20.i35.12381
Published online Sep 21, 2014. doi: 10.3748/wjg.v20.i35.12381
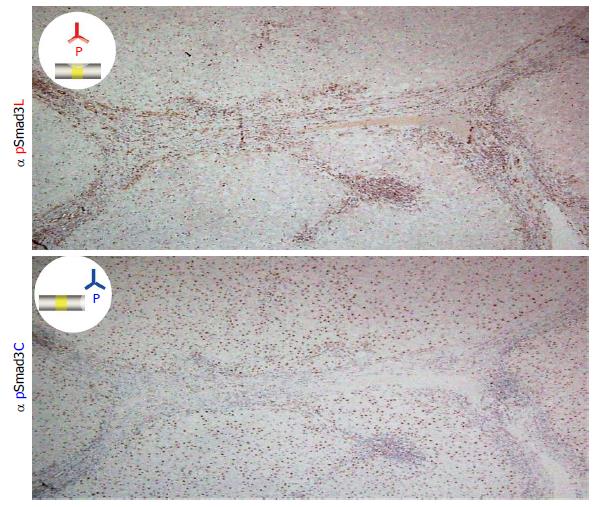
Figure 1 Two distinct hepatocytic Smad3 phospho-isoforms in chronic hepatitis C.
Liver specimens with moderate fibrosis and inflammation from a patient with chronic hepatitis C were immunostained using domain-specific antibodies (Abs) that are able to distinguish between the phosphorylated linker region and the C-terminal region of Smad3. Each section was counterstained with hematoxylin (blue). The brown color indicates specific Ab reactivity. The distribution of pSmad3C and pSmad3L showed a sharp contrast in the liver specimens. Whereas the hepatocytes adjacent to the collagen fibers of the portal tract showed nuclear localization of pSmad3L (upper column), pSmad3C was predominantly located in the hepatocytic nuclei distant from the portal tract (lower column).
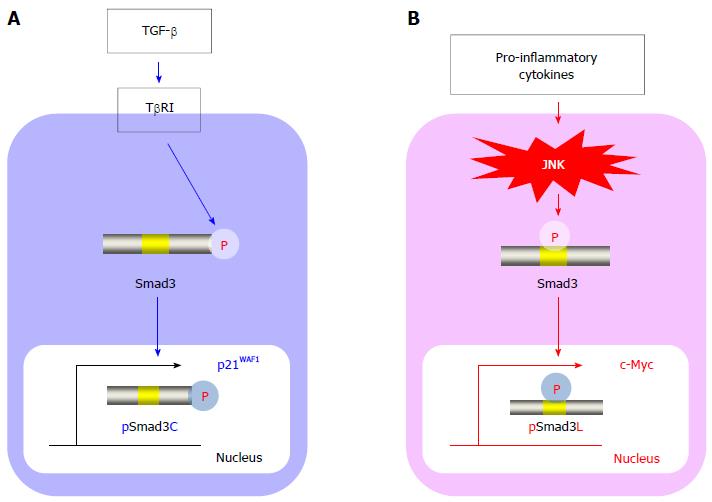
Figure 2 Smad3 phospho-isoform signaling: Tumor suppressive TβRI/pSmad3C pathway vs carcinogenic JNK/pSmad3L pathway.
A: In normal hepatocytes, transforming growth factor (TGF)-β activates the TGF-β type I receptor (TβRI), leading to the direct phosphorylation of the C-terminal region of Smad3. The C-terminal phosphorylated Smad3 (pSmad3C) moves to the nucleus and transmits cytostatic signals by up-regulating p21WAF1. The TβRI/pSmad3C pathway is crucial for homeostasis and is a tumor suppressor; B: In hepatocytes affected by mitogenic stimuli, pro-inflammatory cytokines activate c-Jun N-terminal kinase (JNK), drastically altering the Smad3 signaling to induce the phosphorylation of Smad3 at its linker region. The linker-phosphorylated Smad3 (pSmad3L) undergoes translocation to the nucleus to stimulate c-Myc transcription, resulting in a proliferative state. During hepatitis C virus-related chronic liver disease progression, the JNK/pSmad3L pathway is constitutively activated and promotes carcinogenesis.
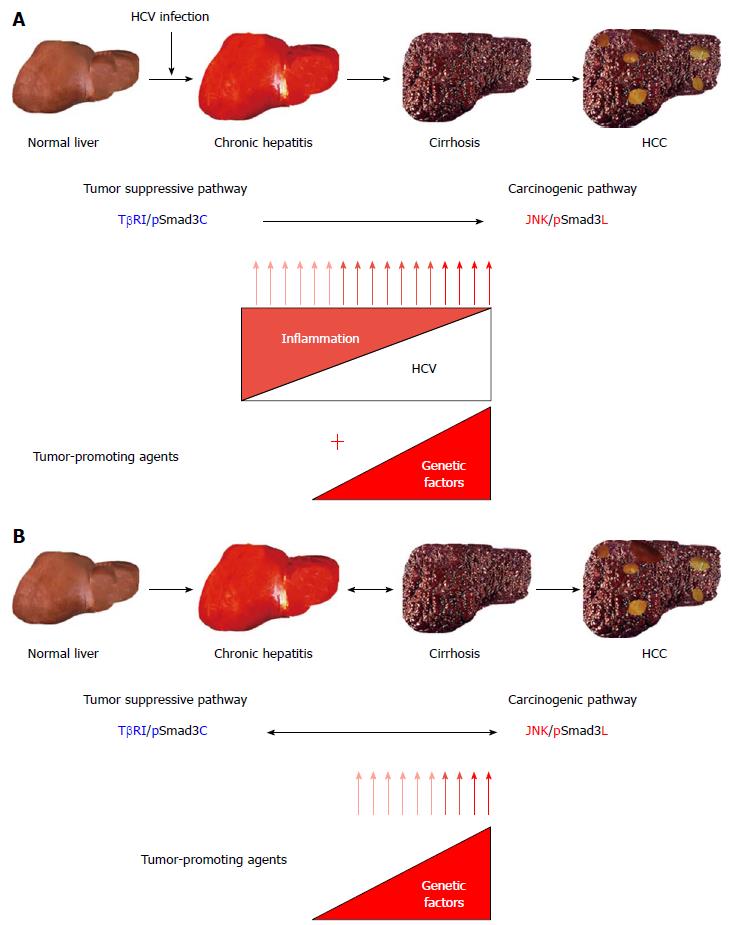
Figure 3 Reversibility of Smad3 phospho-isoform signaling between tumor suppression and carcinogenesis in human hepatitis C virus-related liver diseases.
A: In the course of hepatitis C virus (HCV)-related chronic liver diseases, chronic inflammation and host genetic/epigenetic alterations additively shift the hepatocytic Smad3 phospho-isoform signaling from tumor suppression to carcinogenesis, increasing the risk of hepatocellular carcinoma (HCC); B: When inflammation has abated in response to HCV clearance, the carcinogenic c-Jun N-terminal kinase (JNK)/linker-phosphorylated Smad3 (pSmad3L) signaling, which depends on a persistent viral infection and chronic inflammation, gradually shifts to the tumor suppressive transforming growth factor (TGF)-β type I receptor (TβRI)/C-terminal phosphorylated Smad3 (pSmad3C) signaling (Blue arrow). However, HCC could develop, especially in patients with cirrhosis, when an inflammation-independent process of hepatic carcinogenesis, possibly caused by genetic or epigenetic alterations, has already begun before the HCV clearance (red arrow). Chronic inflammation caused by persistent HCV infection represents an early fibro-carcinogenic step that provides a nonmutagenic tumor-promoting stimulus, although the livers that are chronically infected with HCV might eventually accumulate mitogenic genetic or epigenetic alterations capable of driving multistep hepatic carcinogenesis.
- Citation: Yamaguchi T, Yoshida K, Murata M, Matsuzaki K. Smad3 phospho-isoform signaling in hepatitis C virus-related chronic liver diseases. World J Gastroenterol 2014; 20(35): 12381-12390
- URL: https://www.wjgnet.com/1007-9327/full/v20/i35/12381.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i35.12381