Copyright
©2014 Baishideng Publishing Group Inc.
World J Gastroenterol. Sep 21, 2014; 20(35): 12381-12390
Published online Sep 21, 2014. doi: 10.3748/wjg.v20.i35.12381
Published online Sep 21, 2014. doi: 10.3748/wjg.v20.i35.12381
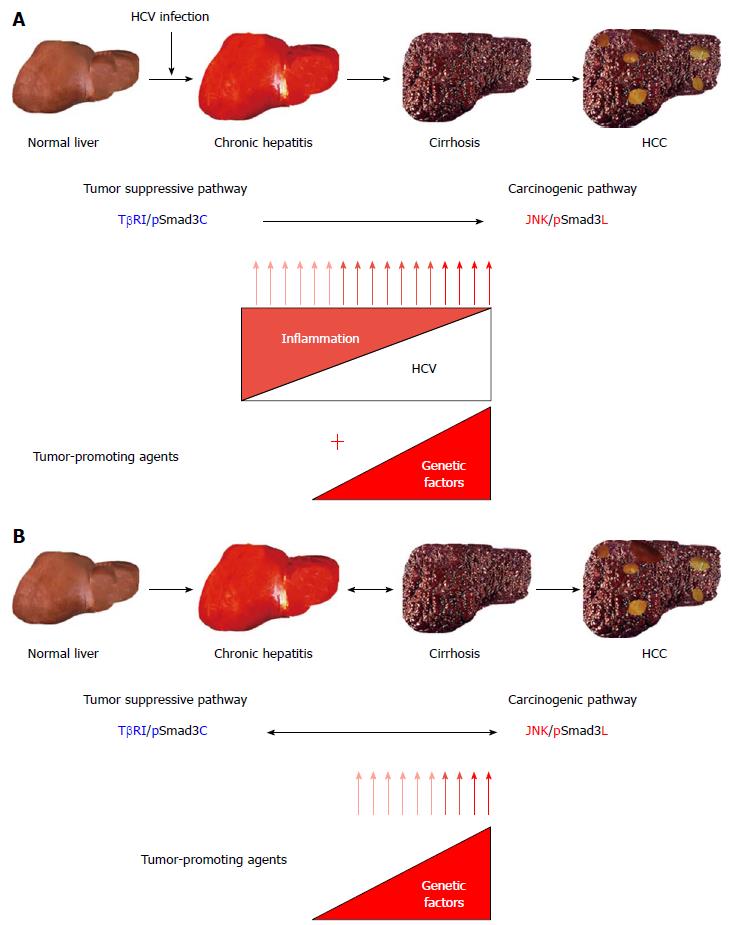
Figure 3 Reversibility of Smad3 phospho-isoform signaling between tumor suppression and carcinogenesis in human hepatitis C virus-related liver diseases.
A: In the course of hepatitis C virus (HCV)-related chronic liver diseases, chronic inflammation and host genetic/epigenetic alterations additively shift the hepatocytic Smad3 phospho-isoform signaling from tumor suppression to carcinogenesis, increasing the risk of hepatocellular carcinoma (HCC); B: When inflammation has abated in response to HCV clearance, the carcinogenic c-Jun N-terminal kinase (JNK)/linker-phosphorylated Smad3 (pSmad3L) signaling, which depends on a persistent viral infection and chronic inflammation, gradually shifts to the tumor suppressive transforming growth factor (TGF)-β type I receptor (TβRI)/C-terminal phosphorylated Smad3 (pSmad3C) signaling (Blue arrow). However, HCC could develop, especially in patients with cirrhosis, when an inflammation-independent process of hepatic carcinogenesis, possibly caused by genetic or epigenetic alterations, has already begun before the HCV clearance (red arrow). Chronic inflammation caused by persistent HCV infection represents an early fibro-carcinogenic step that provides a nonmutagenic tumor-promoting stimulus, although the livers that are chronically infected with HCV might eventually accumulate mitogenic genetic or epigenetic alterations capable of driving multistep hepatic carcinogenesis.
- Citation: Yamaguchi T, Yoshida K, Murata M, Matsuzaki K. Smad3 phospho-isoform signaling in hepatitis C virus-related chronic liver diseases. World J Gastroenterol 2014; 20(35): 12381-12390
- URL: https://www.wjgnet.com/1007-9327/full/v20/i35/12381.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i35.12381