Copyright
©2014 Baishideng Publishing Group Inc.
World J Gastroenterol. May 28, 2014; 20(20): 6252-6261
Published online May 28, 2014. doi: 10.3748/wjg.v20.i20.6252
Published online May 28, 2014. doi: 10.3748/wjg.v20.i20.6252
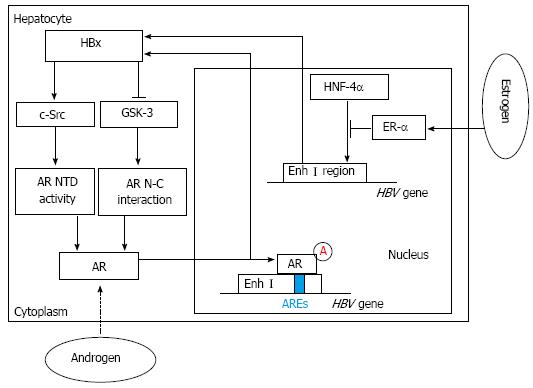
Figure 1 Hepatitis B virus X viral protein expression and sex hormone signaling pathways.
By activating c-Src and inactivating glycogen synthase kinase-3 (GSK-3), hepatitis B virus X viral protein (HBx) can enhance androgen receptor (AR) N-terminal transactivation domain (NTD) activity and AR N-C interaction, which contribute to full activation of AR. Then through binding directly to the cognate androgen-responsive element (ARE) in enhancer I (Enh I) of the hepatitis B virus (HBV) genome, androgen can cooperate with the androgen-signaling pathway to increase the transcription and replication of HBV genes and HBx expression. In contrast, estrogen can cooperate with estrogen receptor-α (ER-α) to decrease HBV RNA transcription by suppressing the activity of the HBV Enh I through preventing hepatocyte nuclear factor 4α (HNF-4α) from binding to Enh I.
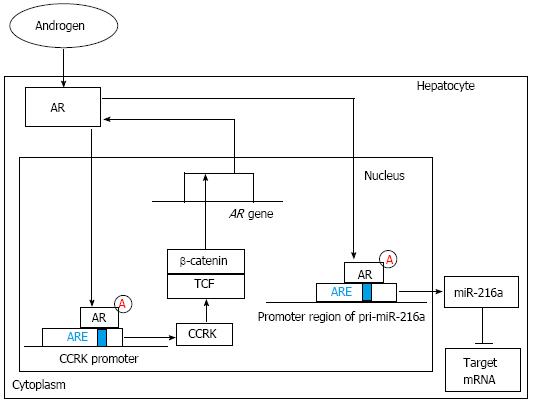
Figure 2 The regulation and function of androgen receptor signaling pathway.
On one hand, ligand-activated androgen receptor (AR) can increase cell cycle-related kinase (CCRK) expression through directly binding to androgen-responsive element (ARE) of the CCRK promoter region and stimulate its transcription and expression. Ectopic expression of CCRK in immortalized human liver cells activated β-catenin/T cell factor (TCF) signaling to stimulate cell cycle progression and to induce AR expression. On the other hand, ligand-stimulated AR can increase miR-216a transcription by directly binding to the ARE site within the promoter region of pri-miR-216a, which can lead to the elevation of the miR-216a level and the subsequent suppression of its target genes including some tumor suppressors. Finally, the early stage of hepatocarcinogenesis, including increased cell proliferation and enhanced migration and invasive activities of hepatocytes, is partly initiated.
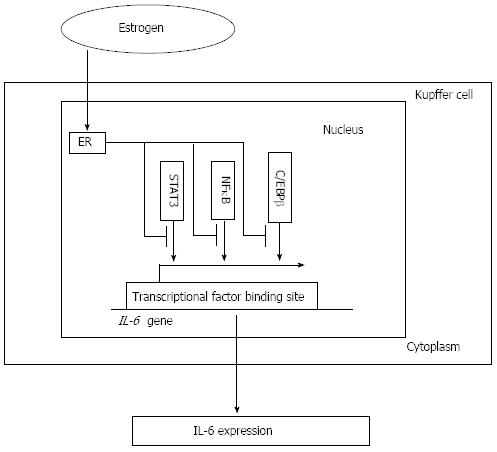
Figure 3 Interleukin-6 expression and estrogen receptor signaling pathway.
By decreasing the activity of core factors such as NFκB, STAT3 and C/EBPβ, which is associated with the interleukin-6 (IL-6) promoter activity and required for the production of IL-6 in vivo and in vitro, estrogen combines with the estrogen receptor (ER) signaling pathway to decrease the IL-6 level and attenuate its induction of liver injury. NFκB: Nuclear factor κB; STAT3: Signal transducer and activator of transcription 3; C/EBPβ: CCAAT/enhancer-binding protein β.
- Citation: Liu WC, Liu QY. Molecular mechanisms of gender disparity in hepatitis B virus-associated hepatocellular carcinoma. World J Gastroenterol 2014; 20(20): 6252-6261
- URL: https://www.wjgnet.com/1007-9327/full/v20/i20/6252.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i20.6252