Copyright
©2014 Baishideng Publishing Group Co.
World J Gastroenterol. Apr 21, 2014; 20(15): 4128-4140
Published online Apr 21, 2014. doi: 10.3748/wjg.v20.i15.4128
Published online Apr 21, 2014. doi: 10.3748/wjg.v20.i15.4128
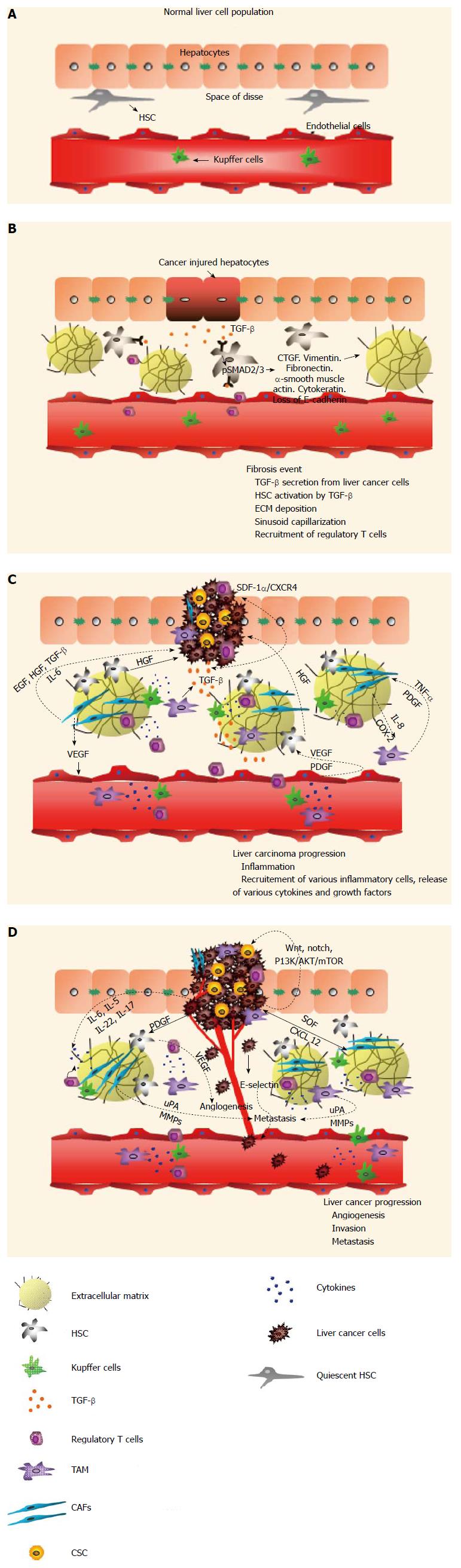
Figure 1 Progression of hepatocellular carcinoma: Crosstalk between hepatocellular carcinoma and its milieu.
A: Healthy liver cell population. The normal liver cell population consists of quiescent hepatic stellate cell (HSC), Kupffer cells, and fenestrated endothelial cells which allow the exchange of blood and substrates between the space of Disse and hepatocytes; B: Fibrotic liver and its microenvironment. Fibrotic liver features cancer-injured hepatocytes, which trigger the release of transforming growth factor-β (TGF-β), defenestration of endothelial cells and recruitment of regulatory T-cells. Binding of TGF-β to its receptor on HSC triggers phosphorylation of SMAD2/3 signaling, which activates HSC to secrete extracellular matrix (ECM) components such as vimentin, CTGF, cytokeratin and muscle actin; C: Progression of liver cancer and its interaction with the milieu. Malignant hepatocytes proliferate in an uncontrolled manner. Infiltration of immune cells causes inflammation. Malignant hepatocytes secrete TGF-β which binds to, and activates, HSC. Activated HSC deposit more ECM. Recruitment of immune cells and cancer-associated cells elicits a signaling cascade. Compressed air foam system (CAFs) secrete vascular endothelial growth factor (VEGF) to stimulate endothelial cells to induce angiogenesis. In turn, endothelial cells secrete VEGF and platelet-derived growth factor (PDGF), which triggers the release of hepatocyte growth factor (HGF) from HSC. HGF secreted by HSC promotes malignant hepatocyte proliferation. Also, PDGF induces the differentiation of HSC into myofibroblasts, which cause fibrosis and the development of HCC. Activated CAFs also secrete EGF, HGF, TGF-β and interleukin-6 (IL-6) to aid cancer cell proliferation. CAFs produce cyclo-oxygen-ase-2 (COX-2) and IL-6 to induce tumor-associated macrophages (TAMs) production. Activated TAMs release TNF-α and PDGF to reinforce CAFs activation. Stromal cell-derived factor-1α (SDF-1/CXCL12) and its receptor CXCR4 are crucial in cancer stem cell (CSC) interactions with their surroundings. TGF-β upregulates CXCR4 expression in liver cancer cells and allows them to migrate to SDF-1α enriched niches. D: Progression and growth of liver carcinoma. Angiogenesis, Invasion and Metastasis are the crucial hallmarks of cancer. In HCC, HSC secrete VEGF to promote angiogenesis. CAFs and TAMs secrete various uPAs and matrix metalloproteinases (MMPs) to induce metastasis. Cancer cells secrete E- selectin to induce metastasis. Cancer stem cells activate the Wnt, Notch, phosphoinositide 3-kinase/Protein Kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) pathway and thus contribute to the molecular heterogeneity of liver cancer. CAFs secrete SDF/CXCL12 to induce proliferation and invasion of liver tumor cells. Cytokines such as IL-6, IL-5, IL-22 and IL-17, produced by T regs or other immune cells, aid in liver cancer proliferation, angiogenesis and metastasis.
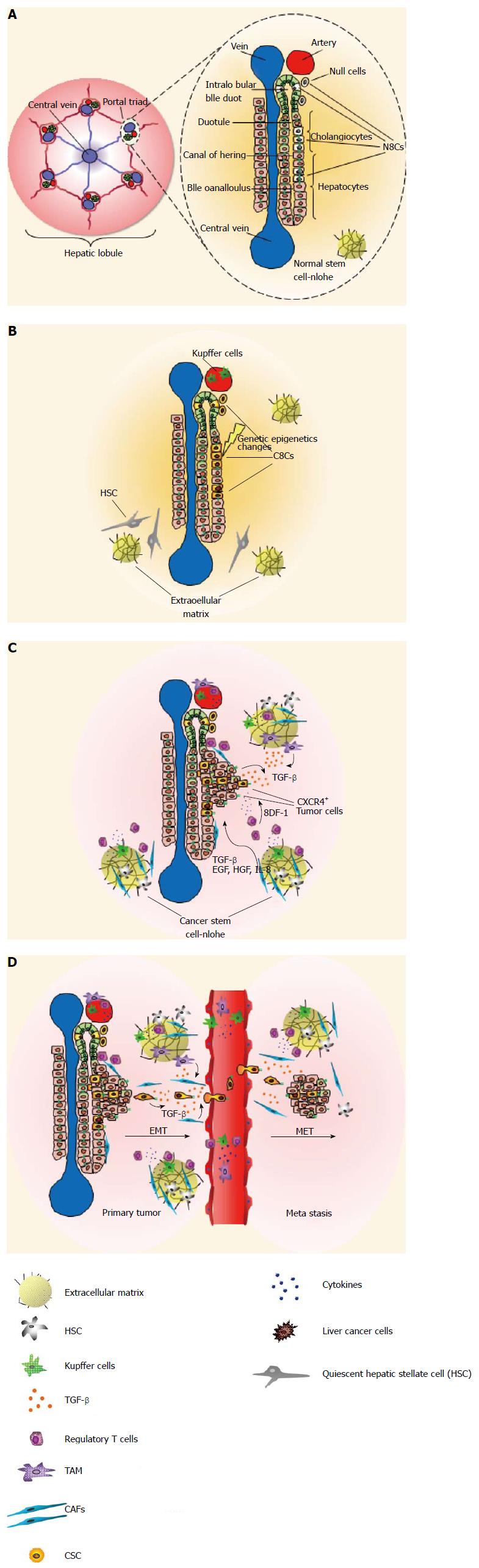
Figure 2 Role of the stem cell niche in liver tumors.
A: A hepatic lobule consists of one central vein and six surrounding portal triads, each of which has bile ducts, a hepatic artery and portal vein. BrdU-retaining cells (with black nuclei) represent putative stem cells (NSC) and are located in cholangiocytes of the intralobular bile duct, together with less-characterized null cells, small hepatocytes at the interface of cholangiocytes, and hepatocytes in the canals of Hering; B: A series of genetic and epigenetic changes in NSC (or committed progenitor cells) leads to the generation of cancer stem cell (CSC) and then to expansion of cells within the stem cell niche. The niche adapts to the presence of CSCs by recruiting cells that would not normally be present; C: The signaling pathways for the activation, expansion and differentiation of stem/progenitor cells are good candidates for contributing, under suitable conditions, to a pro-tumorigenic role. Migration and metastasis of CSCs is a multistep process, in which SDF-1 plays a crucial role by chemoattracting CXCR4+ tumor cells; D: Transforming growth factor-β (TGF-β)-induced transdifferentiation of hepatocytes and liver tumor cells from an epithelial to a mesenchymal phenotype (EMT) increases a population of cells with putative liver progenitor properties. The EMT plays a fundamental role in tumor progression and metastasis and the TGF-β-induced EMT can guide cancer cells to delaminate from primary tumors, migrate along the extracellular matrix network, and arrive at the site of metastasis via the peripheral blood. HSC: Hepatic stellate cell; TAM: Tumor-associated macrophage; CAFs: Compressed air foam system; EGF: Endothelial growth factor; HGF: Hepatocyte growth factor; IL: Interleukin.
- Citation: Rani B, Cao Y, Malfettone A, Tomuleasa C, Fabregat I, Giannelli G. Role of the tissue microenvironment as a therapeutic target in hepatocellular carcinoma. World J Gastroenterol 2014; 20(15): 4128-4140
- URL: https://www.wjgnet.com/1007-9327/full/v20/i15/4128.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i15.4128