Copyright
©2014 Baishideng Publishing Group Co.
World J Gastroenterol. Apr 21, 2014; 20(15): 4128-4140
Published online Apr 21, 2014. doi: 10.3748/wjg.v20.i15.4128
Published online Apr 21, 2014. doi: 10.3748/wjg.v20.i15.4128
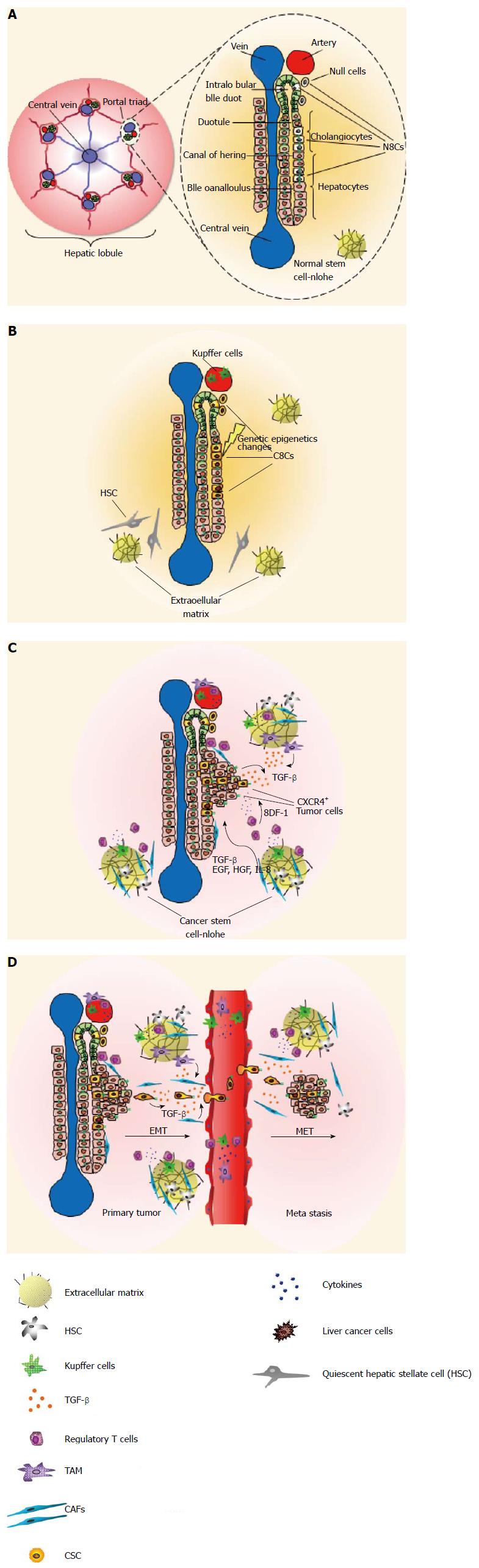
Figure 2 Role of the stem cell niche in liver tumors.
A: A hepatic lobule consists of one central vein and six surrounding portal triads, each of which has bile ducts, a hepatic artery and portal vein. BrdU-retaining cells (with black nuclei) represent putative stem cells (NSC) and are located in cholangiocytes of the intralobular bile duct, together with less-characterized null cells, small hepatocytes at the interface of cholangiocytes, and hepatocytes in the canals of Hering; B: A series of genetic and epigenetic changes in NSC (or committed progenitor cells) leads to the generation of cancer stem cell (CSC) and then to expansion of cells within the stem cell niche. The niche adapts to the presence of CSCs by recruiting cells that would not normally be present; C: The signaling pathways for the activation, expansion and differentiation of stem/progenitor cells are good candidates for contributing, under suitable conditions, to a pro-tumorigenic role. Migration and metastasis of CSCs is a multistep process, in which SDF-1 plays a crucial role by chemoattracting CXCR4+ tumor cells; D: Transforming growth factor-β (TGF-β)-induced transdifferentiation of hepatocytes and liver tumor cells from an epithelial to a mesenchymal phenotype (EMT) increases a population of cells with putative liver progenitor properties. The EMT plays a fundamental role in tumor progression and metastasis and the TGF-β-induced EMT can guide cancer cells to delaminate from primary tumors, migrate along the extracellular matrix network, and arrive at the site of metastasis via the peripheral blood. HSC: Hepatic stellate cell; TAM: Tumor-associated macrophage; CAFs: Compressed air foam system; EGF: Endothelial growth factor; HGF: Hepatocyte growth factor; IL: Interleukin.
- Citation: Rani B, Cao Y, Malfettone A, Tomuleasa C, Fabregat I, Giannelli G. Role of the tissue microenvironment as a therapeutic target in hepatocellular carcinoma. World J Gastroenterol 2014; 20(15): 4128-4140
- URL: https://www.wjgnet.com/1007-9327/full/v20/i15/4128.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i15.4128