Copyright
©2013 Baishideng Publishing Group Co.
World J Gastroenterol. Aug 7, 2013; 19(29): 4689-4701
Published online Aug 7, 2013. doi: 10.3748/wjg.v19.i29.4689
Published online Aug 7, 2013. doi: 10.3748/wjg.v19.i29.4689
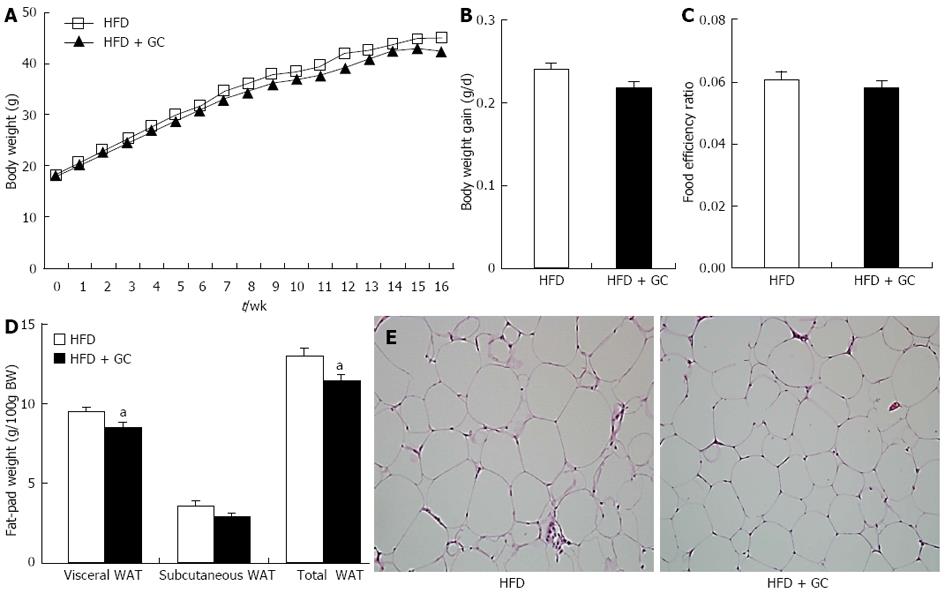
Figure 1 Effects of Garcinia Cambogia supplementation on body weight gain, food intake, fat-pad weight and adipocyte size in mice fed a high-fat diet for 16 wk.
A-D: Data are expressed as the mean ± SE (n = 10); E: HE staining is shown. Representative photographs of epididymal white adipose tissue (WAT) (original magnification × 200). High-fat diet (HFD), mice fed a high-fat diet alone; HFD + Garcinia Cambogia (GC), mice fed a high-fat diet containing GC (1%, w/w). aP < 0.05 vs control group.
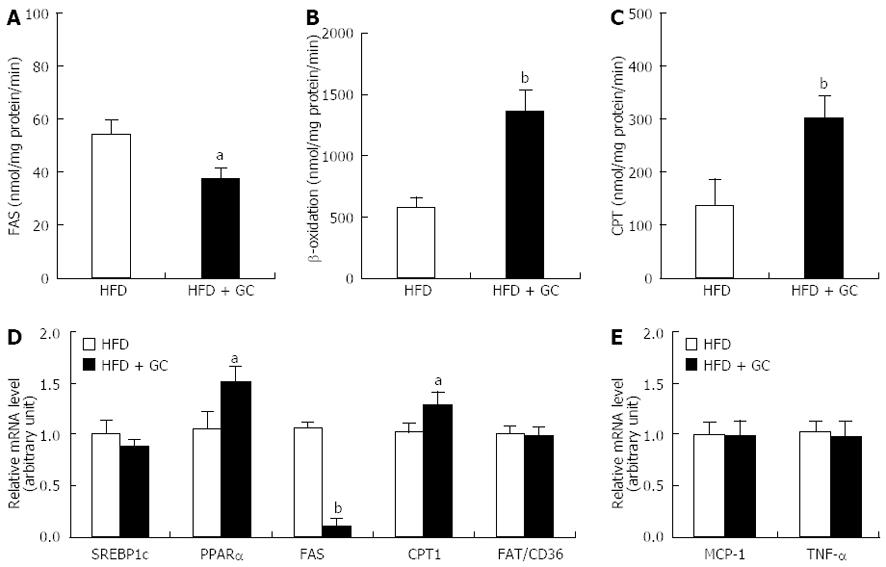
Figure 2 Effects of Garcinia Cambogia supplementation on fatty acid-regulating enzyme activity and gene expression in epididymal white adipose tissue of mice fed a high-fat diet for 16 wk.
Data are expressed as the mean ± SE (n = 10). A: Fatty acid synthase (FAS); B: β-oxidation; C: Carnitine palmitoyltransferase (CPT); D, E: Relative mRNA level. High-fat diet (HFD), mice fed a high-fat diet alone; HFD + Garcinia Cambogia (GC), mice fed a high-fat diet containing GC (1%, w/w). aP < 0.05, bP < 0.01 vs control group. WAT: White adipose tissue; PPARα: Peroxisome proliferator-activated receptor α.
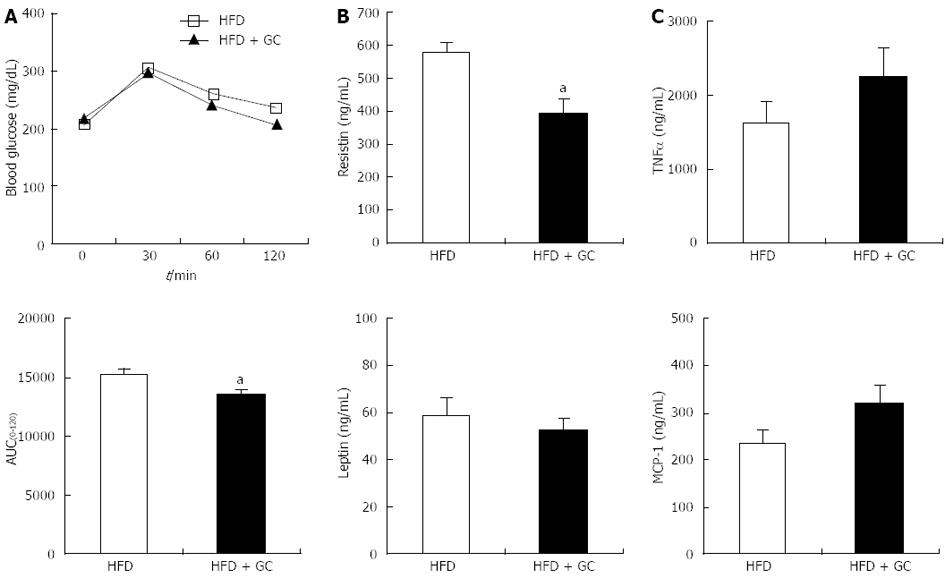
Figure 3 Effects of Garcinia Cambogia supplementation on glucose tolerance and plasma adipocytokine levels in mice fed a high-fat diet for 16 wk.
Data are expressed as the mean ± SE (n = 10). A: The intraperitoneal glucose tolerance test was performed on the 15th week of Garcinia Cambogia (GC) supplementation in high-fat diet (HFD)-fed mice. Following a 12-h fast, the mice were injected intraperitoneally with glucose (0.5 g/kg body weight). Blood glucose was then measured via the tail vein at the indicated time [above: Blood glucose values; below: Areas under the curves (AUC)]; B, C: Plasma levels of leptin, resistin, monocyte chemoattractant protein-1 (MCP-1) and tumor necrosis factor-α (TNF-α) were assayed after 16 wk of GC supplementation in HFD-fed mice. HFD, mice fed a high-fat diet alone; HFD + GC, mice fed a high-fat diet containing GC (1%, w/w). aP < 0.05 vs control group.
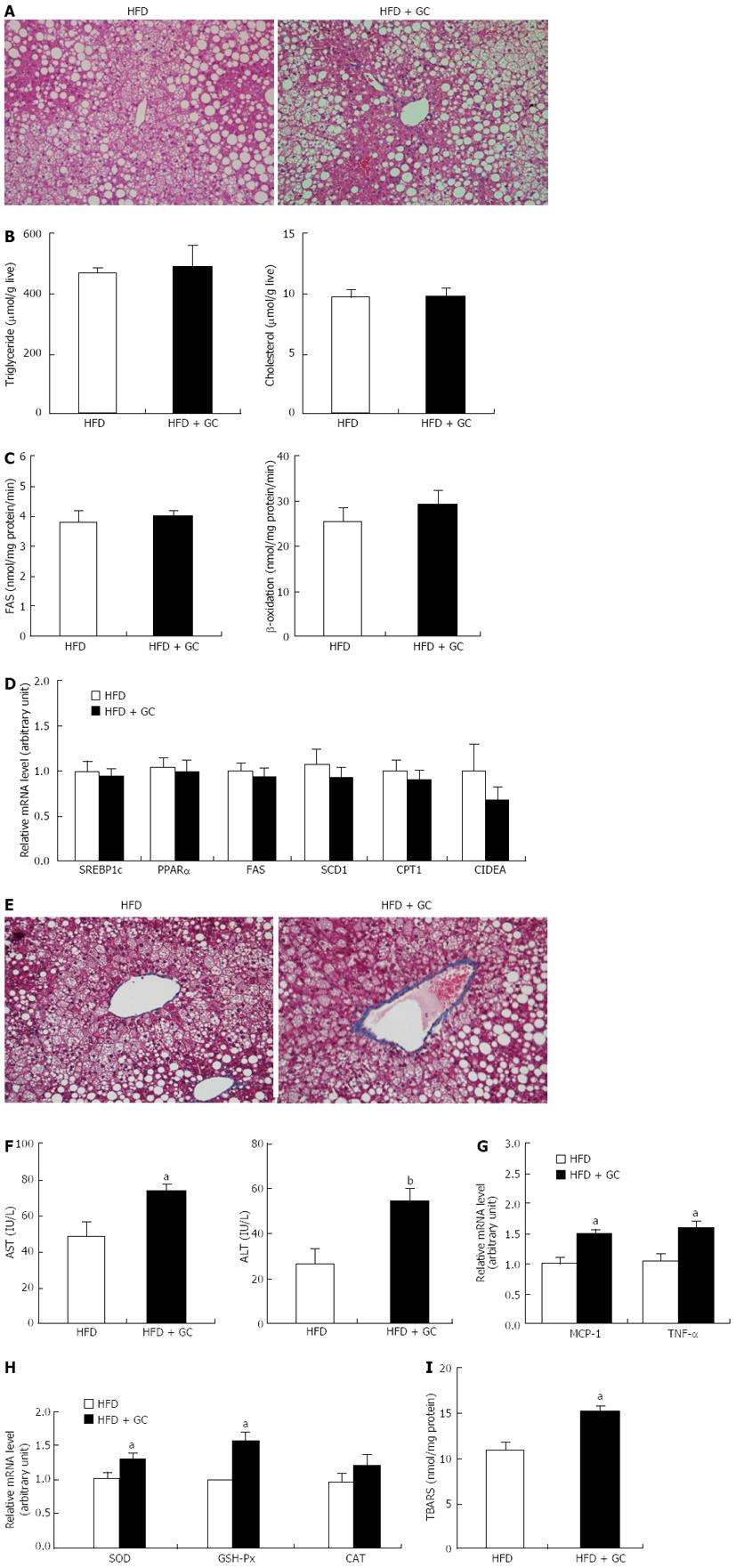
Figure 4 Long-term Garcinia Cambogia supplementation did not affect high-fat diet-induced hepatic steatosis but increased hepatic collagen accumulation, inflammation and oxidative stress.
A: The liver tissue sections were stained with hematoxylin and eosin; B: Triglyceride and cholesterol level; C: Fatty acid synthase (FAS) and β-oxidation level; D: Relative mRNA level; E: The liver tissue sections were stained with Masson’s trichrome; F: Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) level; G, H: Relative mRNA level; I: thiobarbituric acid-reactive substances (TBARS) concentration. Representative images are shown (original magnification × 200). Data are expressed as the mean ± SE (n = 10). High-fat diet (HFD), mice fed a high-fat diet alone; HFD + Garcinia Cambogia (GC), mice fed a high-fat diet containing GC (1%, w/w). aP < 0.05, bP < 0.01 vs control group. CAT: Catalase; CIDEA: Cell death-inducing DNA fragmentation factor-α-like effector A; CPT: Carnitine palmitoyltransferase; FAS: Fatty acid synthase; GSH-Px: Glutathione peroxidase; MCP-1: Monocyte chemoattractant protein-1; PPARα: Peroxisome proliferator-activated receptor α; SCD1: Stearoyl-CoA desaturase; SOD: Superoxide dismutase; SREBP1c: Sterol-regulatory-element-binding protein 1c; TNF-α: Tumor necrosis factor-α.
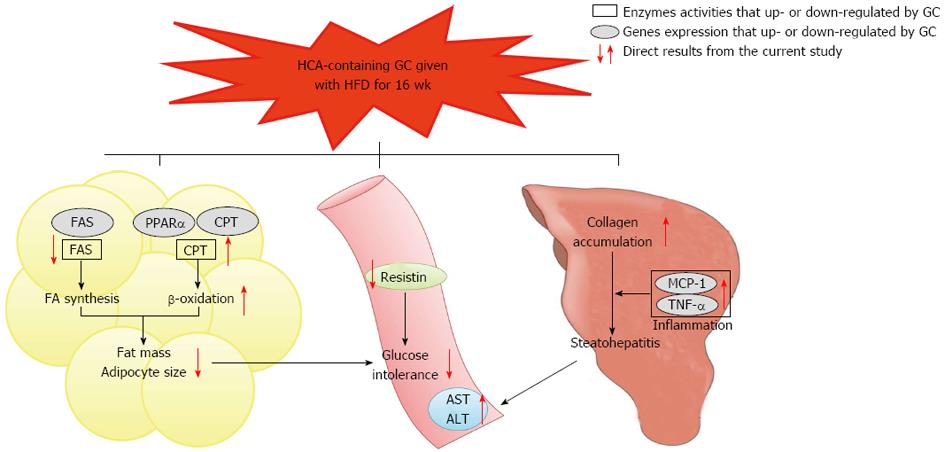
Figure 5 Summary of the long-term Garcinia Cambogia supplementation effects on adiposity, glucose tolerance and steatohepatitis in high-fat diet-induced obese mice.
The Garcinia Cambogia (GC) supplementation decreased fatty acid synthase (FAS) mRNA expression and its activity, while peroxisome proliferator-activated receptor α (PPARα) and carnitine palmitoyltransferase (CPT) mRNA expression along with the activities of CPT and β-oxidation were increased in the visceral adipose tissue, indicating that these changes may be potential mechanisms for reducing body fat accumulation and glucose intolerance induced by high-fat diet (HFD). Furthermore, GC supplementation decreased the plasma resistin level, which may be also related to improved glucose tolerance. There were no significant changes in hepatic lipid accumulation as well as in hepatic gene expression and enzymatic activity involved in fatty acid synthesis, oxidation and storage. However, GC increased pro-inflammatory monocyte chemoattractant protein-1 (MCP-1) and tumor necrosis factor-α (TNF-α) mRNA expression, lipid peroxidation and collagen accumulation in the liver. Plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were also increased by GC supplementation in HFD-induced obese mice, thus suggesting that GC may negatively affect liver function by increasing hepatic fibrosis, inflammation and oxidative stress without affecting hepatic fat accumulation. FA: Fatty acid; HCA: Hydroxycitric acid. Open square means enzymes activities that up- or down-regulated by GC. Solid circle means genes expression that up- or down-regulated by GC.
-
Citation: Kim YJ, Choi MS, Park YB, Kim SR, Lee MK, Jung UJ.
Garcinia Cambogia attenuates diet-induced adiposity but exacerbates hepatic collagen accumulation and inflammation. World J Gastroenterol 2013; 19(29): 4689-4701 - URL: https://www.wjgnet.com/1007-9327/full/v19/i29/4689.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i29.4689