Copyright
©2013 Baishideng Publishing Group Co.
World J Gastroenterol. Jun 21, 2013; 19(23): 3583-3595
Published online Jun 21, 2013. doi: 10.3748/wjg.v19.i23.3583
Published online Jun 21, 2013. doi: 10.3748/wjg.v19.i23.3583
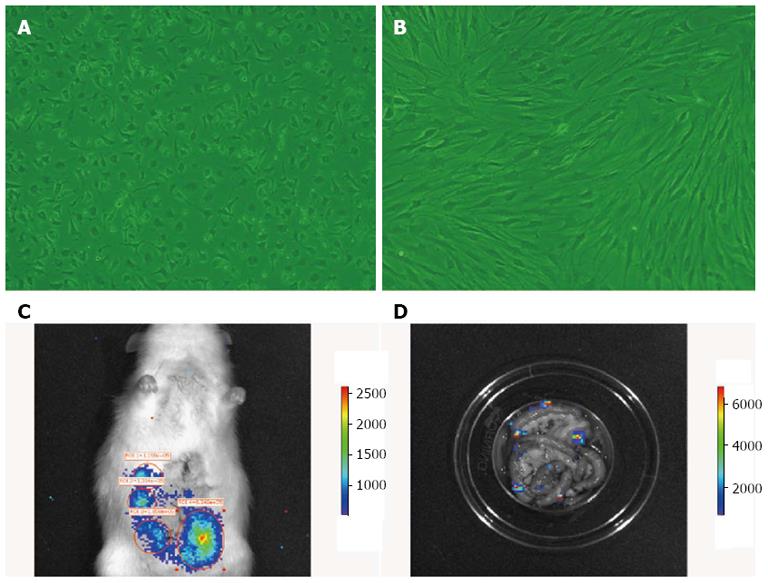
Figure 1 Morphology of bone-marrow mesenchymal stem cells in vitro, and in vivo cell tracing of bone-marrow mesenchymal stem cells colonization in rat intestine.
A: First-passage bone-marrow mesenchymal stem cells (BM MSCs); B: Third-passage BM MSCs (× 200); C: Homing of fluorescently labeled BM MSCs (B16-F10-Luc-G5) to the rat intestine 6 h after transplantation; D: After the intestine was removed and washed repeatedly, fluorescently labeled BM MSCs were still observed, confirming the cells homed to the intestine and survived.
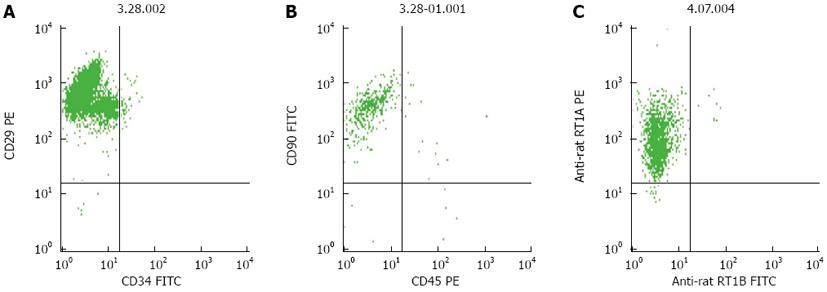
Figure 2 Flow cytometric analysis of third-passage bone-marrow mesenchymal stem cells.
A: The proportion of CD29-positive and CD34-negative cells was approximately 96%; B: The proportion of CD90-positive and CD450-negative was approximately 98%; C: The proportion of RT1A-positive and RT1B-negative cells was > 98%.
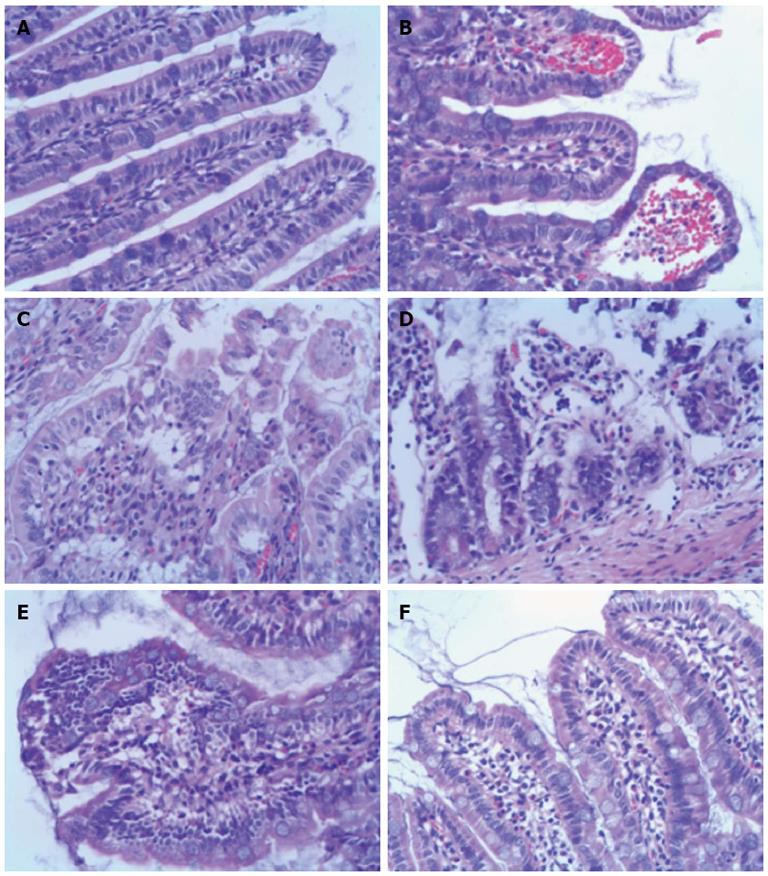
Figure 3 Histopathology of ileum sections at different time points after intestinal ischemia/reperfusion injury (hematoxylin and eosin, × 200).
A: Sham group; the intestine showed normal villous architecture and glands, with no vascular congestion; B: In the ischemia/reperfusion (I/R) injury 2 h group, the degree of intestinal mucosa injury was marked with massive epithelial lifting down the sides of the villi and ulceration at the villous tips; C: At 6 h, there was intestinal mucosa degradation and disintegration of the lamina propria, hemorrhage, and ulceration; D: At 24 h, the damaged mucosa showed denuded villi with dilated capillaries and increased cellularity of the lamina propria; E: In the I/R injury group at 72 h, there was massive epithelial lifting down the sides of the villi and ulceration at the villous tips; F: However, at 144 h, the damaged mucosa had recovered.
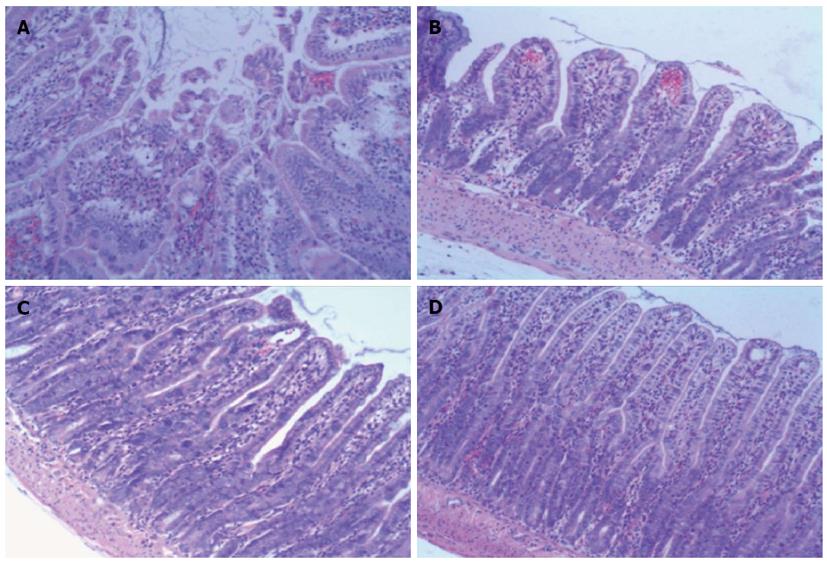
Figure 4 Histopathology of ileum sections of different groups at 6 h after ischemia/reperfusion injury (hematoxylin and eosin, × 100).
A: In the ischemia/reperfusion (I/R) injury group there was marked intestinal mucosa injury at 6 h, with intestinal mucosa degradation and disintegration of the lamina propria, hemorrhage, and ulceration. B: In the bone-marrow mesenchymal stem cells + I/R injury group at 6 h, the damaged mucosa had recovered and there was extension of the subepithelial space with moderate lifting of the epithelial layer from the lamina propria, massive epithelial lifting down the sides of the villi, and ulceration at the villous tips. C and D: In the anti-tumor necrosis factor (TNF)-α + I/R injury group and the anti-TNF-αR1-IgG + I/R injury group at 6 h, the damaged mucosa had almost recovered to resemble that in the Sham control group.
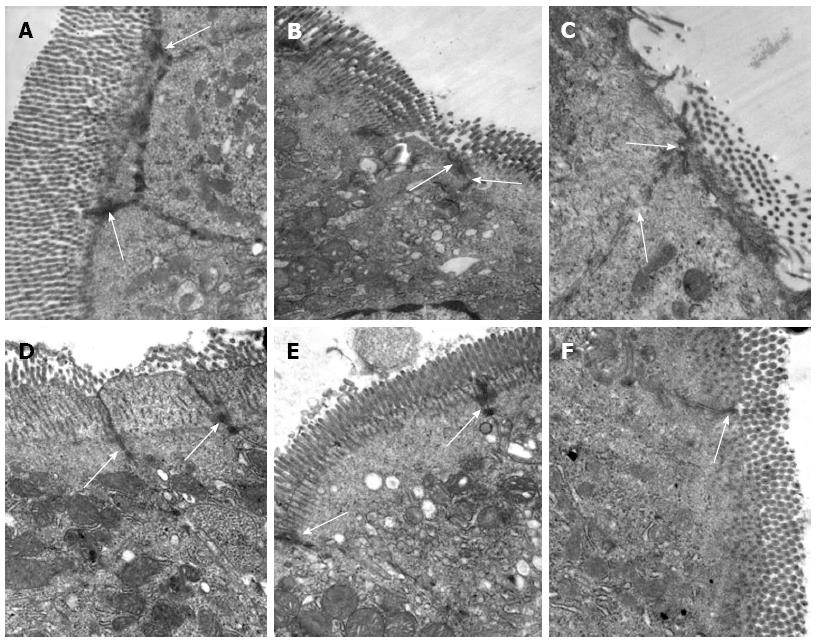
Figure 5 Bone-marrow mesenchymal stem cells and tumor necrosis factor-α blockade prevent ultrastructural pathological damage after intestinal ischemia/reperfusion injury.
Transmission electron microscopy of the rat intestine after ischemia/reperfusion (I/R) injury. A: Epithelial cells and tight junctions (TJs) (arrows) were intact in the Sham group, × 30000; B: At 2 h after I/R injury, epithelial cells were swollen and shrunken, microvilli and organelles were normal, and TJs (arrows) were disrupted in the saline (NS) + I/R injury group, × 25000; C: At 6 h after I/R injury in the NS + I/R injury group, some microvilli were loose, TJs (arrows) were disrupted, and organelles were swollen with reduced electron density, × 30000; D: At 6 h after I/R injury and administration of bone-marrow mesenchymal stem cells, the microvilli and mitochondria of the endothelial cells were almost normal and TJs (arrows) were not disrupted, × 30000; E and F: TJs (arrows) between endothelial cells were intact 6 h after I/R injury in rats that received anti-tumor necrosis factor (TNF)-α IgG + I/R antibody (E, × 25000) or anti-TNF-α R1 antibody; (F, × 30000) before I/R injury.
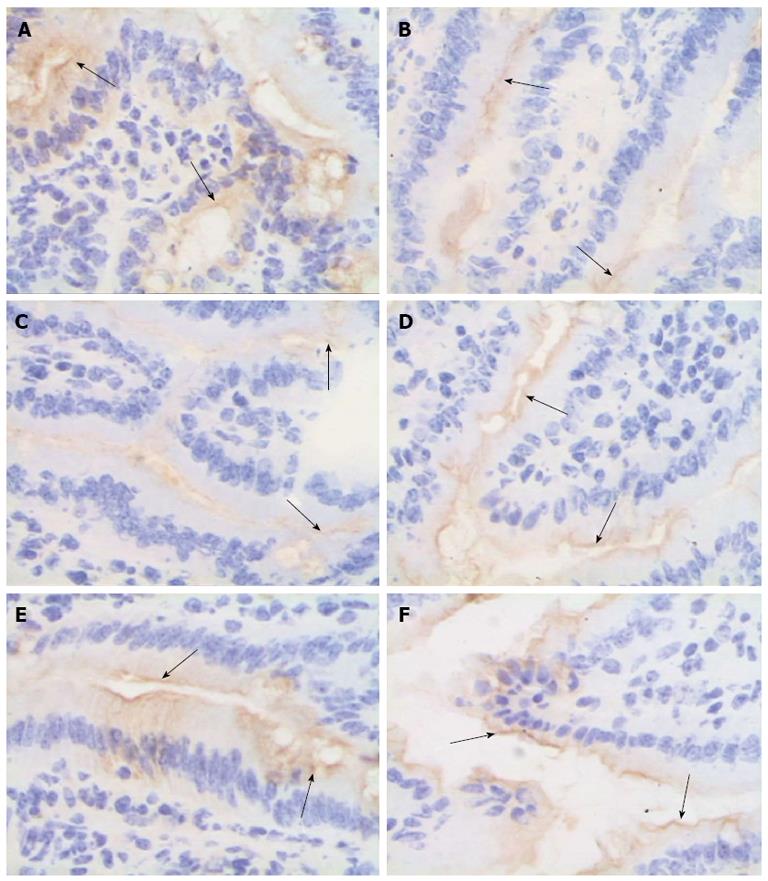
Figure 6 Bone-marrow mesenchymal stem cells and tumor necrosis factor-α blockade attenuate zona occludens 1 downregulation after intestinal ischemia/reperfusion injury.
The mucosal tissue sections after ischemia/reperfusion (I/R) injury were immunohistochemically labeled for zona occludens 1 (ZO-1) (brown) and counterstained with hematoxylin (blue). A: Sham group; B and C: In the normal saline + I/R injury group, decreased ZO-1 staining (arrows) was observed in the epithelial cells 2 h (B) and 6 h (C) after I/R; D-F: ZO-1 was not obviously affected in the bone-marrow mesenchymal stem cells + I/R injury group (D), anti-tumor necrosis factor (TNF)-α + I/R injury group (E), or anti-TNF-α R1 antibody + I/R injury group (F) at 6 h; original magnification, × 400.
Figure 7 Bone-marrow mesenchymal stem cells attenuate zona occludens 1 downregulation after intestinal ischemia/reperfusion injury.
Representative western blots and quantification of zona occludens 1 (ZO-1) protein expression in the intestinal mucosa. ZO-1 expression was significantly lower in the saline (NS) + ischemia/reperfusion (I/R) injury group than the bone-marrow mesenchymal stem cells (BM MSCs) + I/R injury group at 6 h (25.35 ± 4.58% vs 42.32 ± 1.26%; P < 0.01). Actin was used as a loading control. Values are shown as the mean ± SD (n = 3 rats per group); bP < 0.01 BM MSCs +I/R injury group vs the NS + I/R injury group [one-way ANOVA followed by the least significant difference (LSD) test].
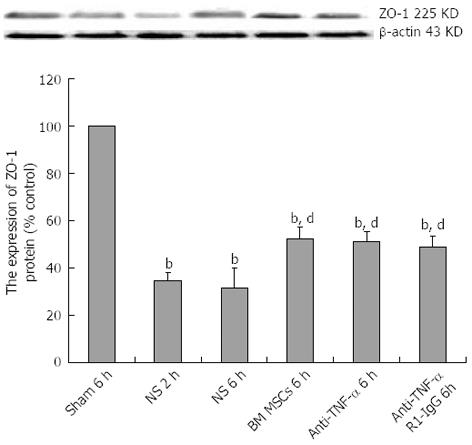
Figure 8 Bone-marrow mesenchymal stem cells and tumor necrosis factor-α blockade attenuate zona occludens 1 downregulation after intestinal ischemia/reperfusion injury.
Representative western blots and quantification of zona occludens 1 (ZO-1) protein expression in the intestinal mucosa. Actin was used as a loading control. Values are the mean ± SD (n = 3 rats per group); bP < 0.01 vs Sham group; dP < 0.01 vs the saline (NS) + ischemia/reperfusion (I/R) injury group (one-way analysis of variance followed by the LSD test). BM MSCs: Bone-marrow mesenchymal stem cells; Anti-TNF: Anti-tumor necrosis factor.
-
Citation: Shen ZY, Zhang J, Song HL, Zheng WP. Bone-marrow mesenchymal stem cells reduce rat intestinal ischemia-reperfusion injury, ZO-1 downregulation and tight junction disruption
via a TNF-α-regulated mechanism. World J Gastroenterol 2013; 19(23): 3583-3595 - URL: https://www.wjgnet.com/1007-9327/full/v19/i23/3583.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i23.3583