Copyright
©2010 Baishideng.
World J Gastroenterol. Jun 21, 2010; 16(23): 2873-2880
Published online Jun 21, 2010. doi: 10.3748/wjg.v16.i23.2873
Published online Jun 21, 2010. doi: 10.3748/wjg.v16.i23.2873
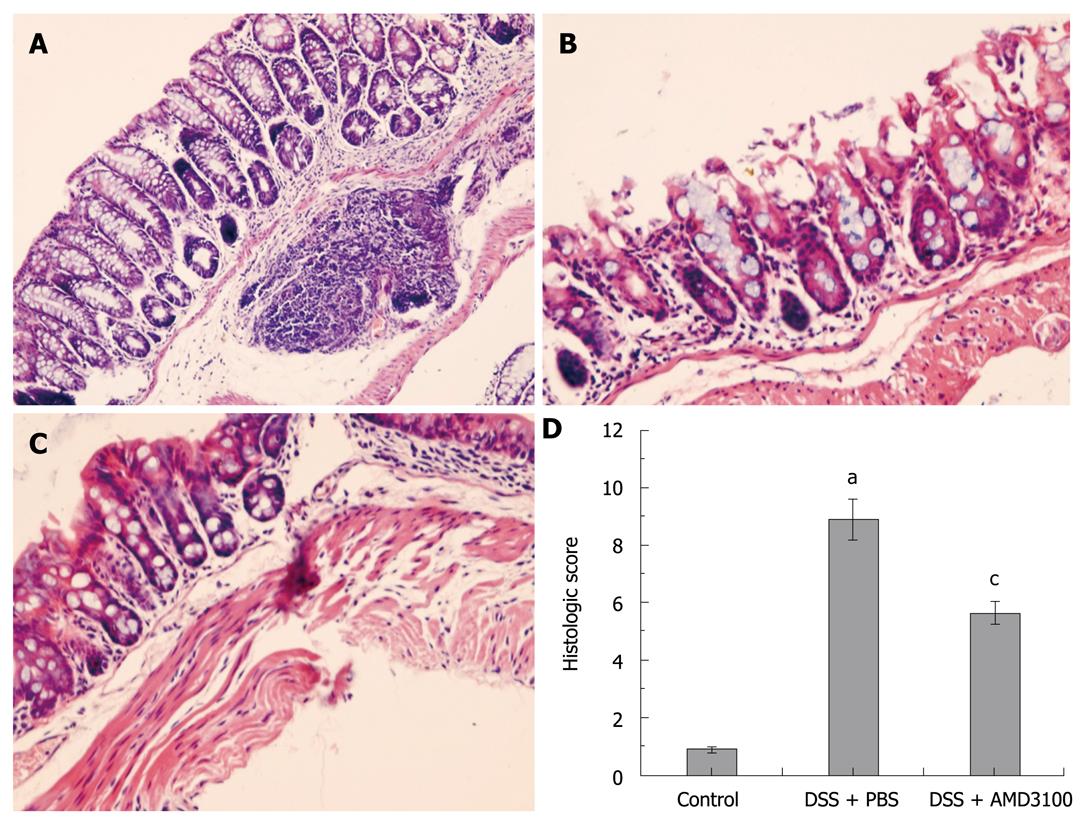
Figure 1 Effects of a chemokine stromal cell-derived factor-1 receptor (CXCR4) antagonist on mucosal damage in mice.
Colitis was induced by administration of 5% dextran sulfate sodium (DSS) for 7 d. A: Common colonic morphologic characteristics are observed in tissue sections from control groups; B: Marked colonic injury is observed in tissue sections from the colitis group; C: Treatment with CXCR4 antagonist, AMD3100, significantly attenuates colonic damage; D: The histological score in mice. Eight mice were studied in each experimental group. Results are mean ± SE. aP < 0.05 vs control group; cP < 0.05 vs DSS + PBS group. Original magnification, × 200.
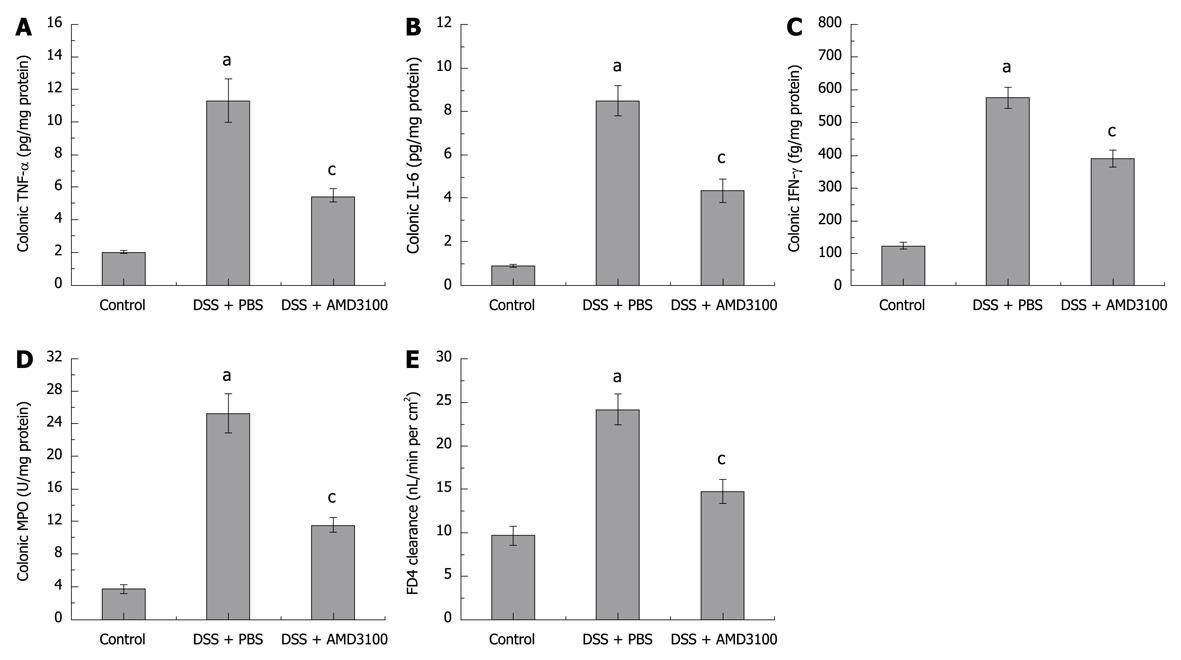
Figure 2 Effects of CXCR4 antagonist on colonic myeloperoxidase (MPO), proinflammatory cytokines, and gut permeability in mice.
Colitis was induced by administration of 5% DSS for 7 d. Colonic levels of tumor necrosis factor-α (TNF-α) (A), interleukin-6 (IL-6) (B), interferon-γ (IFN-γ) (C), MPO (D), and FD4 clearance (E) are shown. Eight mice were studied in each experimental group. Results are mean ± SE. aP < 0.05 vs control group; cP < 0.05 vs DSS + PBS group.
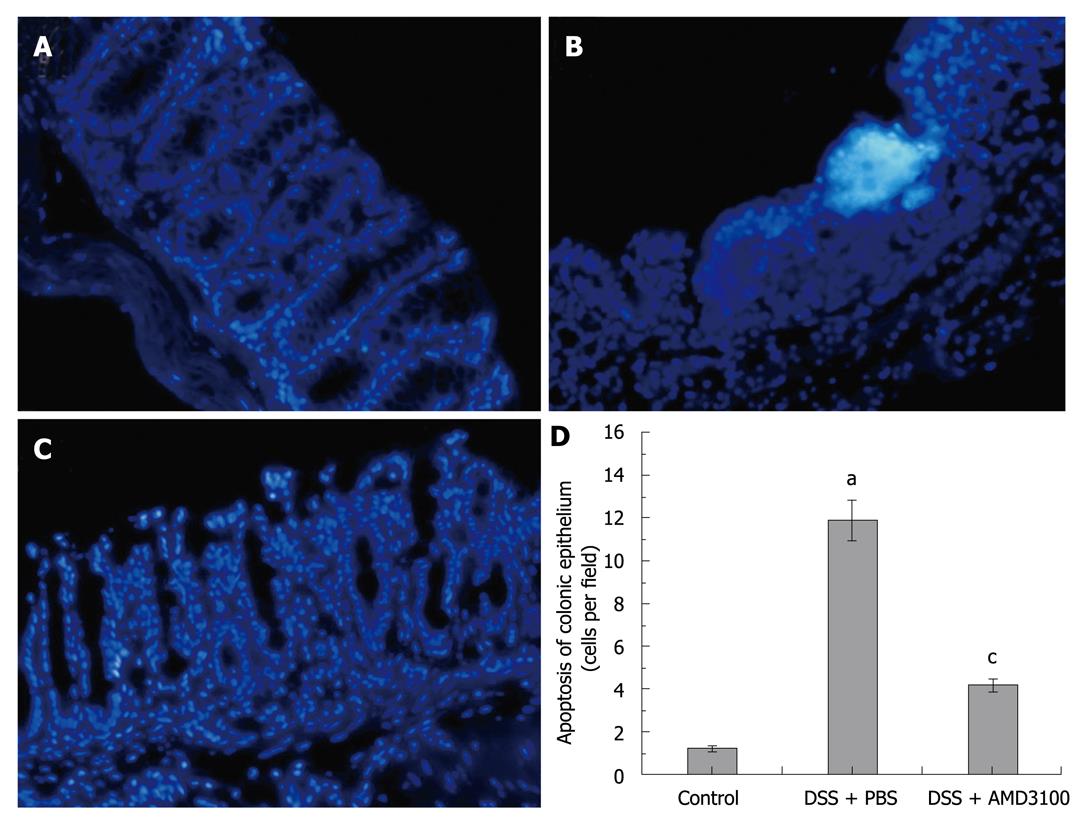
Figure 3 Effects of CXCR4 antagonist on colonic epithelial apoptosis in mice.
Colitis was induced by administration of 5% DSS for 7 d. Tissue sections were stained with Hoechst-33342. A: Sporadic apoptotic cells were observed in intestinal segments from the control group; B: Clustered apoptotic cells were observed in tissue sections from the colitis group; C: Treatment with CXCR4 antagonist AMD3100 obviously decreased epithelial apoptosis; D: The numbers of apoptotic cells in intestinal segments. Eight mice were studied in each experimental group. Results are mean ± SE. aP < 0.05 vs control group; cP < 0.05 vs DSS + PBS group. Original magnification, × 200.
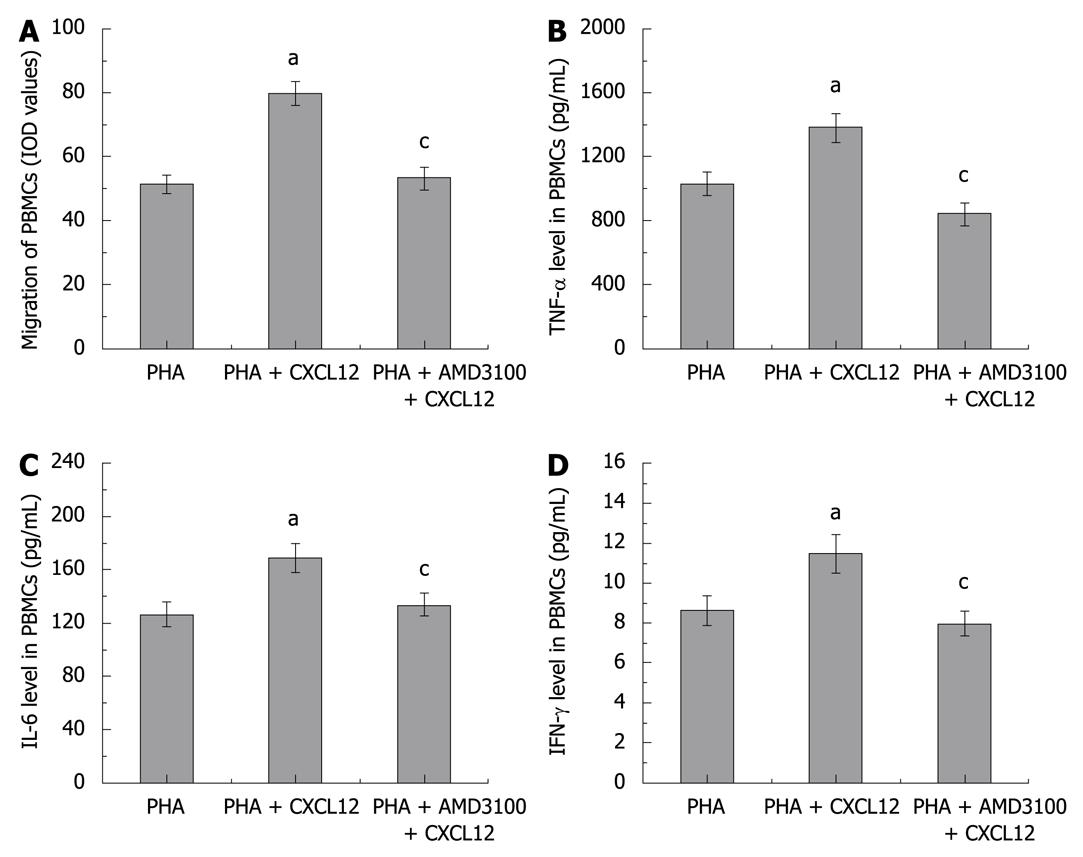
Figure 4 Effects of CXCR4 antagonist on migration and cytokine production of isolated PBMCs.
To mimic the in vivo inflammatory response, the PBMCs were pre-activated with PHA. Migration of PBMCs (A) and levels of TNF-α (B), IL-6 (C), and IFN-γ ( D) in PBMCs are shown. Six wells were studied in each experimental group. Results are mean ± SE. aP < 0.05, chemokine stromal cell-derived factor-1 (CXCL12) group vs control group; cP < 0.05, AMD3100 group vs CXCL12 group.
- Citation: Xia XM, Wang FY, Xu WA, Wang ZK, Liu J, Lu YK, Jin XX, Lu H, Shen YZ. CXCR4 antagonist AMD3100 attenuates colonic damage in mice with experimental colitis. World J Gastroenterol 2010; 16(23): 2873-2880
- URL: https://www.wjgnet.com/1007-9327/full/v16/i23/2873.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i23.2873