Copyright
©2008 The WJG Press and Baishideng.
World J Gastroenterol. Feb 14, 2008; 14(6): 831-844
Published online Feb 14, 2008. doi: 10.3748/wjg.14.831
Published online Feb 14, 2008. doi: 10.3748/wjg.14.831
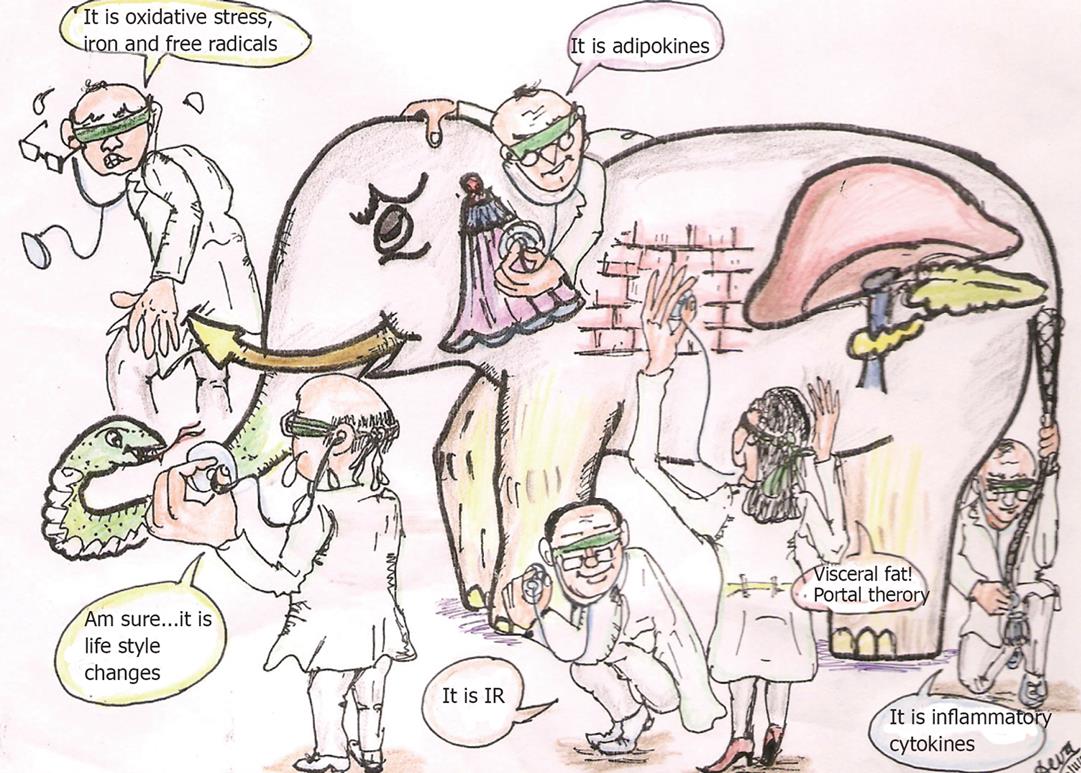
Figure 1 The pathogenesis of NAFLD has been disputed by many loud and long.
The term “nonalcoholic fatty liver disease” is imprecisely defined; there is uncertainty regarding its pathogenesis. The elephant (NAFLD) has been interpreted by many and several possible etiologies and pathogenesis models have been assigned to it like ‘insulin resistance’, mitochondrial defects, adipokine imbalance, visceral fat, inflammatory cytokines and oxidant stress.
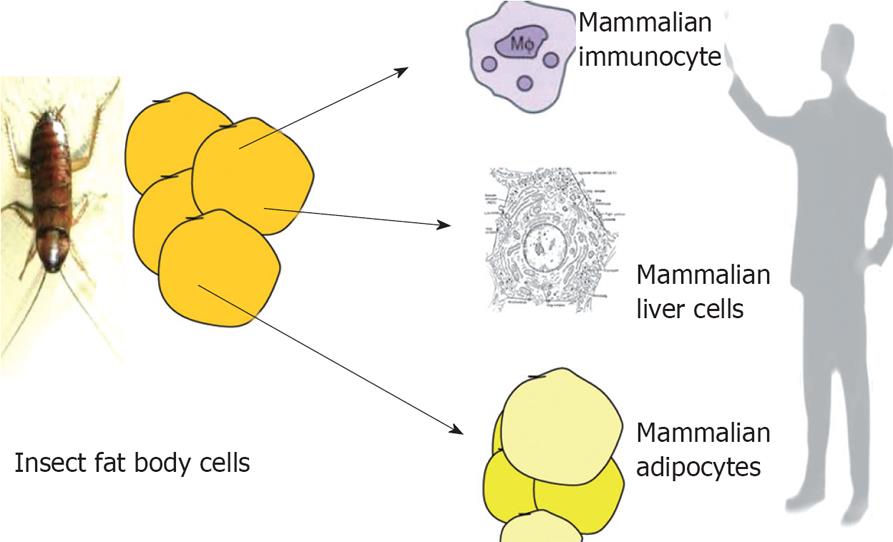
Figure 2 It is interesting to note that adipose tissue, liver and immunocytes has same ancestor-the “fat body” which is the mammalian homologue of all the three tissues in insects.
Over expressing PPAR-γ in hepatocytes can induce adipocyte like features in hepatocytes. Similarly gene expression profile of macrophage is very similar to adipocytes. All the three cell systems are affected in disorders like NAFLD and obesity.
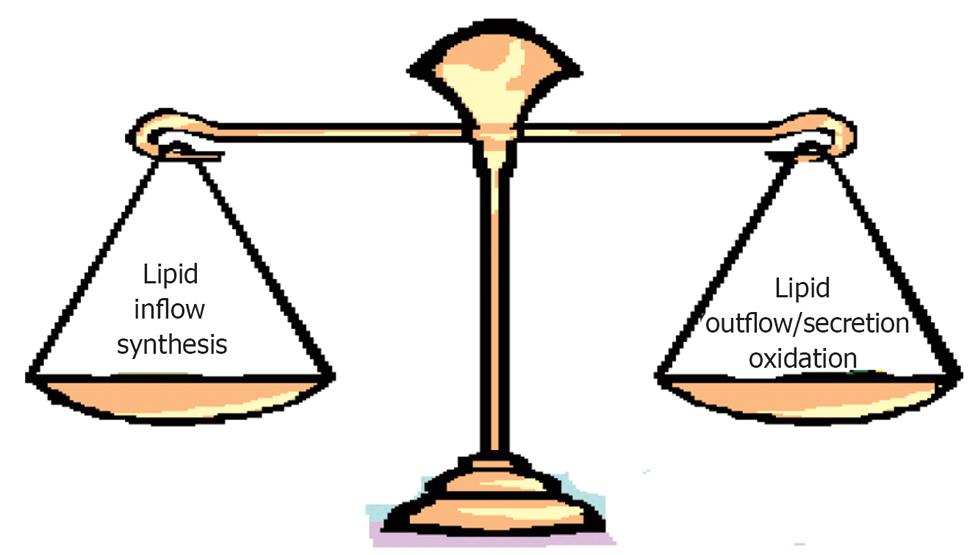
Figure 3 Fat gets accumulated in liver when there an imbalance between inflow (increased availability of lipids and lipid precursors) and outflow/secretion, synthesis and oxidation.
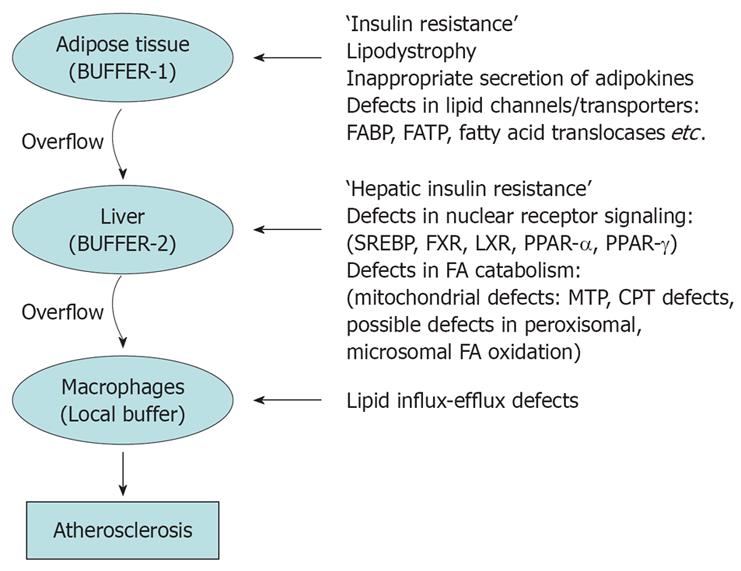
Figure 4 Adipose tissue functions as an energy reserve; where energy can be stored in the most concentrated form viz.
lipids safely. In other tissues lipid accumulation could cause ‘lipid toxicity’. Adipose tissue ‘buffers’ the lipids and energy rich compounds which are pumped into the blood stream soon after meals. However when excess calories overload adipose tissue the buffering action ceases, resulting in overflow of lipids to liver which is the next buffer. But as soon as liver also is overloaded, lipids overflow and gets accumulated in various other organs. In blood vessel walls macrophages takes up the excess lipids to form foam cells initiating a cascade which results in fibrosis of vessel walls.
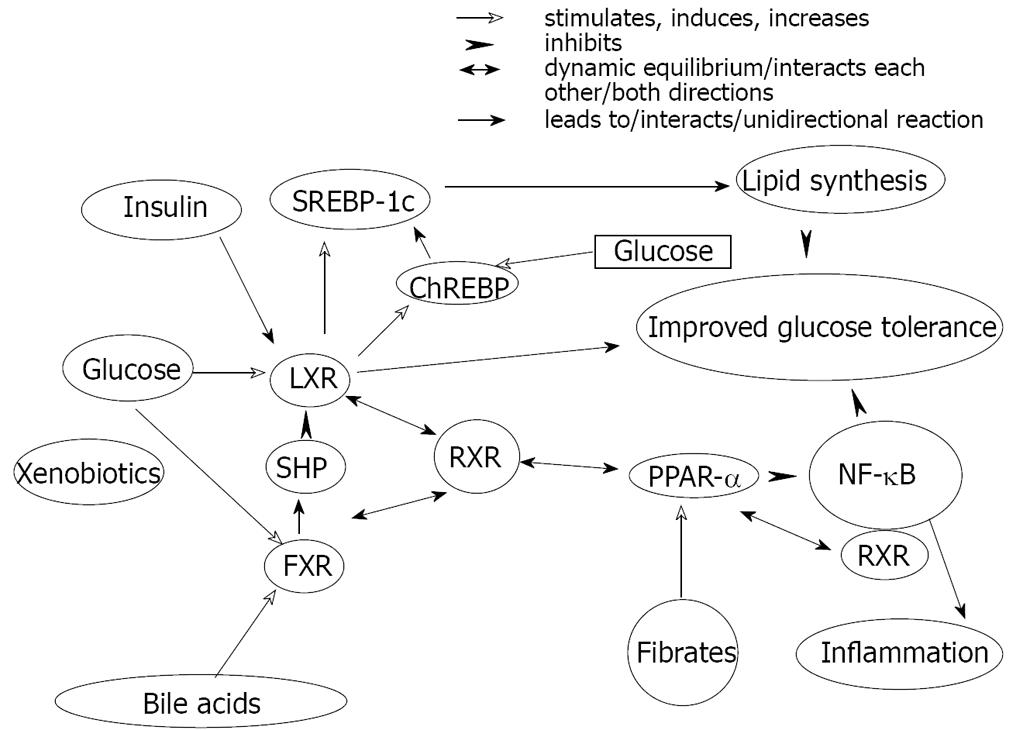
Figure 5 The complex interactions between various nuclear receptors involved in lipid-carbohydrate-bile acid homeostasis determines fat accumulation in liver.
SREBP-Sterol regulatory element-binding proteins LXR-liver xenobiotic receptor, FXR-farnesoid xenobiotic receptor, SHP-short heterodimer partner, RXR-retinoid xenobiotic receptor, PPAR-peroxisome proliferator-activated receptor, ChREBP-carbohydrate responsive element binding protein. NF-κB-nuclear factor κB.
Figure 6 With the progress of medicine at molecular level diseases like type 2 diabetes and ‘NAFLD’ which results from a spectrum of genetic defects would split into hundreds if not thousands of diseases with ‘individual’ identity providing patients’ accurate diagnosis of the disease and accurate treatment with minimum ‘side effects’ which is unavoidable in today’s ‘blind clinical practice’.
- Citation: Sanal MG. The blind men ‘see’ the elephant-the many faces of fatty liver disease. World J Gastroenterol 2008; 14(6): 831-844
- URL: https://www.wjgnet.com/1007-9327/full/v14/i6/831.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.831