Copyright
©2007 Baishideng Publishing Group Co.
World J Gastroenterol. Mar 7, 2007; 13(9): 1313-1332
Published online Mar 7, 2007. doi: 10.3748/wjg.v13.i9.1313
Published online Mar 7, 2007. doi: 10.3748/wjg.v13.i9.1313
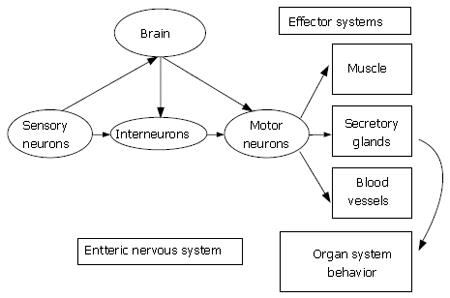
Figure 1 The heuristic model for the enteric nervous system is the same as for the central nervous system.
Sensory neurons, interneurons and motor neurons are synaptically interconnected to form the neural networks of the ENS. Like the central nervous system, sensory neurons, interneurons and motor neurons are connected by chemical synapses for directional flow of information from sensory neurons to interneuronal integrative networks to motor neurons to effector systems. The ENS organizes and coordinates the activity of each effector system into meaningful behavior of the whole organ. Bidirectional communication occurs between the ENS and the central nervous system.
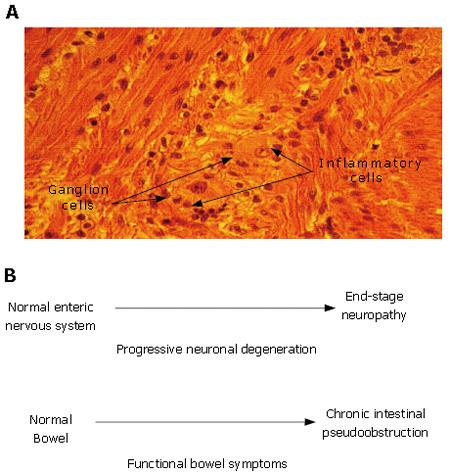
Figure 2 Enteric neuropathy underlies the pathophysiology in a subset of patients with functional gastrointestinal disorders.
A: Idiopathic myenteric ganglionitis. Histology of the myenteric plexus in a full-thickness small intestinal biopsy taken during exploratory laparotomy for intestinal obstruction. A diagnosis of neuropathic pseudoobstruction was made on the basis of neuronal degeneration associated with an inflammatory infiltrate localized to the myenteric plexus. The patient had a multiple year history of complaints suggestive of IBS. Histological section and patient history courtesy of Dr. Claudio Fiocchi, The Cleveland Clinic, Cleveland, Ohio; B: Enteric neuronal degeneration and associated symptoms progress in parallel (e.g. symptoms of IBS). Most individuals start postnatal life with a normal ENS and normally functioning bowel. Neuronal degeneration mediated by autoimmune attack directed to the ENS (e.g. paraneoplastic syndrome, Chagas disease, idiopathic inflammatory neuropathy) can progress to a stage where ENS function is lost. Functional bowel symptoms appear and worsen as neurons are progressively lost from the ENS microcircuits required for integrated function of the whole organ. Chronic intestinal pseudoobstruction of the neuropathic form occurs when the loss of neurons progresses to a stage where the neuronal circuits for propulsive motility are no longer functional.
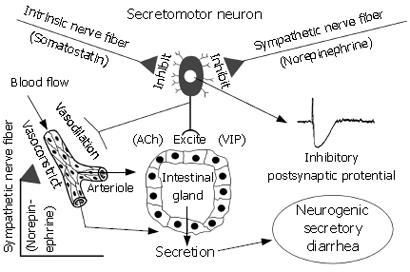
Figure 3 Intestinal secretory glands (i.
e. crypts of Lieberkühn and Brunner’s glands) are innervated by secretomotor neurons in the ENS. Neurotransmitters (e.g. ACh and VIP), which evoke secretion, are released at the neuro-epithelial junctions when secretomotor neurons fire. Axon collaterals to blood vessels simultaneously dilate submucosal vessels to increase blood flow in support of stimulated secretion. Noradrenergic input from the sympathetic nervous system and somatostatinergic input from intrinsic ENS neurons suppress firing of secretomotor neurons and thereby inhibit secretion. Factors that cause hyperactivity of secretomotor neurons enhance secretion and lead to neurogenic secretory diarrhea. In inflammatory states (e.g. ulcerative colitis and Crohn’s disease) the release of inflammatory mediators elevates activity of secretomotor neurons leading to diarrhea. Certain pathogens and enterotoxins (e.g. cholera toxin and Clostridium difficille toxin A) activate secretomotor neurons to produce a diarrheal state. In food allergies, presence of sensitizing food antigens (e.g. shellfish, tree nuts) triggers mast cell degranulation and releases mediators such as histamine, serotonin and prostaglandins, all of which activate secretomotor neurons to evoke diarrhea. When secretomotor neurons are hypoactive, secretion is reduced, the liquidity of the intestinal contents is reduced and a state of constipation can follow. Activating opioid or somatostatinergic receptors (e.g. by opioid analgesics or octreotide) on secretomotor neurons inhibits their excitability and suppresses secretion.
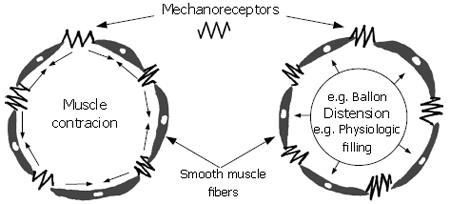
Figure 4 Mechanoreceptors are connected “in-series” with the long axes of the smooth muscle fibers that form the intestinal circular muscle layer.
Either contraction of the muscle or distension of the intestinal wall “stretches” the receptors and evokes firing of impulses in the sensory afferent fibers connected to the receptor. Firing frequency of the receptor increases in direct relation to the amount of “stretch” or, in some receptors, the rate at which the length changes. Mechanosensory information generated in this manner is transmitted by spinal afferents to the spinal cord or vagal nerve afferents to the dorsal vagal motor complex in the brain stem.
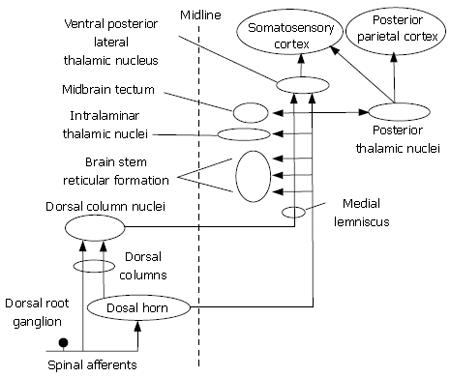
Figure 5 Visceral nociceptive information is transmitted along multiple ascending spinal pathways to multiple processing centers in the brain.
Projection pathways in the dorsal spinal columns transmit information from tactile receptors in the skin and visceral pain from nociceptors in the bowel.
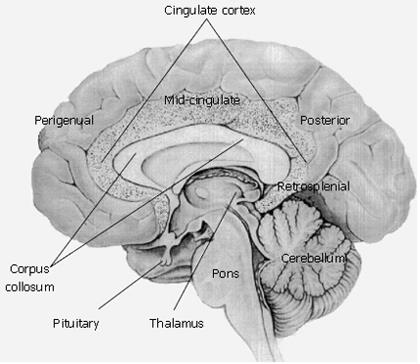
Figure 6 Conscious perception of visceral sensations emerge from the cingulate region of the cerebral cortex.
Medial view of the brain showing organization of the cingulate cortex. Perigenual, midcingulate, posterior and retrosplenial are important structural and functional regions of the cingulate cortex with the following properties: (1) Perigenual anterior cingulate cortex surrounds the rostral portion of the cingulate cortex and is primarily involved in affect with a subsector devoted to visceromotor control via projections to the cranial parasympathetic and spinal sympathetic divisions of the autonomic nervous system. (2) Midcingulate cortex is involved in response selection and motivation and has a skeletomotor control subsector with neurons that project directly to the spinal cord. (3) Retrosplenial cingulate cortex is associated with memory recall. (4) Posterior cingulate cortex overlays the entire surface of the cingulate gyrus and has a caudomedial subsector that overlaps with the retrosplenial cingulate cortex. The retrosplenial cingulate cortex and posterior cingulate cortex are both involved in memory and activation of the caudomedial sector of the posterior cingulate cortex during memory of emotional events (e.g. emotional events associated with affect and gut function) provide an important framework for assessment of visceral function and disorder in brain imaging studies.
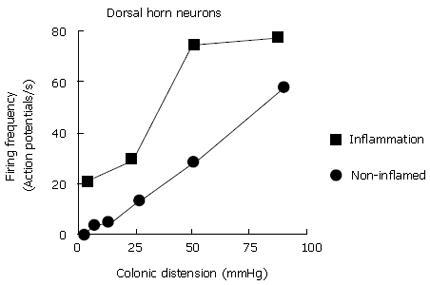
Figure 7 Firing frequency of neurons in the dorsal horns of the spinal cord in response to distension of the colon is increased during experimentally induced inflammation of the colon in rats.
Increased sensitivity is reflected by a decreased firing threshold and increased gain (increased slope) of the relation of firing frequency to degree of distension. Data extracted from Cervero and Laird[177].
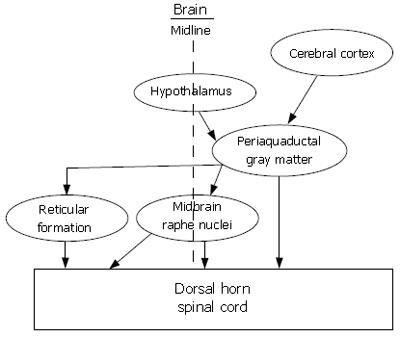
Figure 8 Multiple descending pathways from integrative centers in the brain project to the dorsal horn of the spinal cord where they release neurotransmitters that modulate the transmission of nociceptive signals after entry into the spinal cord.
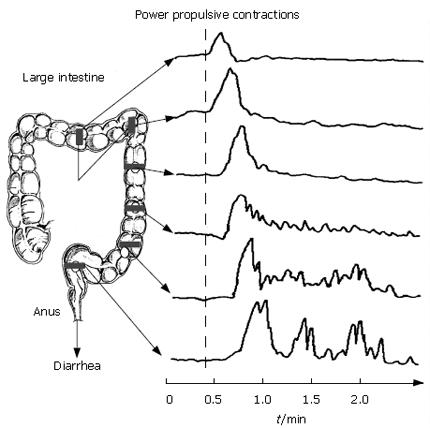
Figure 9 Power propulsion is a protective response to the presence of food allergens or other threatening agents in the intestinal lumen.
It is an ENS programmed motility pattern that can be recorded with sensors (e.g. force transducers) as strong, long lasting contractions of the circular muscle that propagate rapidly for extended distances along the intestine. Power propulsion quickly strips the lumen clean as it travels along extended lengths of intestine. Abdominal cramping, urgency, diarrhea and threat of incontinence are associated with this motor program. Application of irritants to the mucosa, the introduction of luminal parasites, enterotoxins from pathogenic bacteria, allergic reactions and exposure to ionizing radiation each can trigger the propulsive motor program.
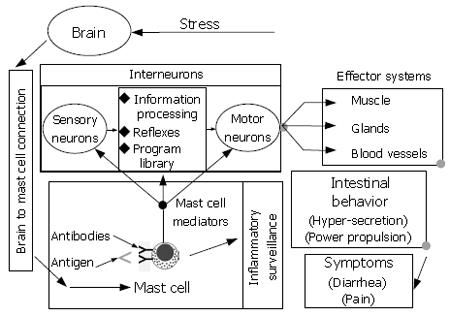
Figure 10 Heuristic model for brain-gut interaction in response to stress.
The ENS is a minibrain located in close apposition to the gastrointestinal effectors it controls. Enteric mast cells are positioned to detect foreign antigens and signal their presence to the ENS. When, stimulated mast cells release several paracrine mediators simultaneously. Some of the mediators signal the ENS while others act as attractant factors for polymorphonuclear leukocytes responsible for acute inflammatory responses. The ENS responds to the mast cell signal by initiating a defensive program of coordinated secretion and propulsive motility that functions to rapidly expel the source of antigenic stimulation from the bowel. Symptoms of abdominal pain, fecal urgency and watery diarrhea result from operation of the defense program. Neural inputs to enteric mast cells from the brain stimulate simultaneous release of chemoattractant factors for inflammatory cells and chemical signals to the ENS with effects that mimic the symptoms of antigenic detection by the mast cells. Stress activates this brain-to-mast cell connection.
- Citation: Wood JD. Neuropathophysiology of functional gastrointestinal disorders. World J Gastroenterol 2007; 13(9): 1313-1332
- URL: https://www.wjgnet.com/1007-9327/full/v13/i9/1313.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i9.1313