Copyright
©2007 Baishideng Publishing Group Co.
World J Gastroenterol. Oct 7, 2007; 13(37): 4931-4937
Published online Oct 7, 2007. doi: 10.3748/wjg.v13.i37.4931
Published online Oct 7, 2007. doi: 10.3748/wjg.v13.i37.4931
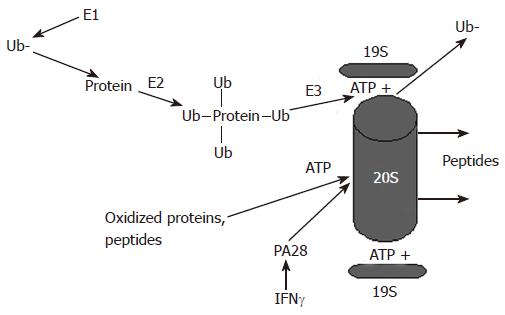
Figure 1 Proteasome-dependent degradation of proteins by the 26S and the 20S proteasome.
Proteins undergo ubiquitylation and are degraded in an ATP-dependent manner by the 26S proteasome, which activity is regulated by the 19S particle. Alternatively, oxidized proteins can be degraded by the 20S proteasome. This degradation is ATP-independent and is regulated by the 20S proteasome activator, PA28.
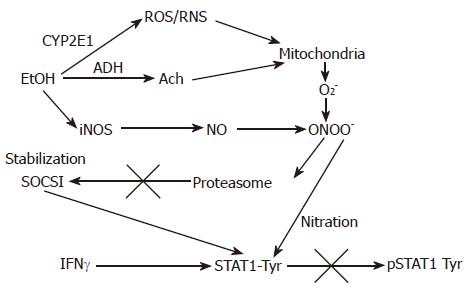
Figure 2 The proposed effects of EtOH metabolism on IFNγ-induced STAT1 phosphorylation in VL-17A cells.
EtOH is metabolized by ADH and CYP2E1 to Ach and ROS/RNS and increase a formation of PN (peroxynitrite, ONOO-), a reaction product of O2- and NO, which at high concentrations blocks proteasome function. This causes stabilization of SOCS1, a negative regulator of Jak-STAT1 signaling as well as prevents STAT1 phosphorylation on tyrosine residues.
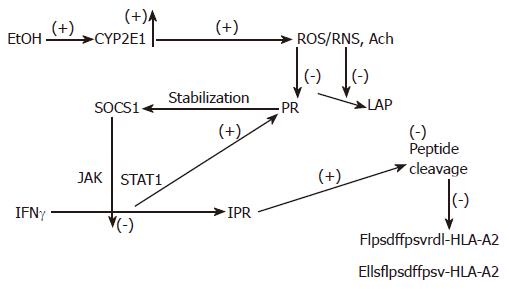
Figure 3 The proposed mechanism of regulation of peptide cleavage by ethanol metabolism.
Ethanol (EtOH) treatment induces CYP2E1 activity, which catalyzes production of oxidants. These products block proteasome (Pr) and leucine aminopeptidase (LAP) activities, stabilizing SOCS1 and preventing IFNγ-dependent activation of PA28 and immunoproteasome (IPr). Finally, generation of C-and N-extended peptides for MHC class I-restricted antigen presentation is blocked.
- Citation: Osna NA, Jr TMD. Implication of altered proteasome function in alcoholic liver injury. World J Gastroenterol 2007; 13(37): 4931-4937
- URL: https://www.wjgnet.com/1007-9327/full/v13/i37/4931.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i37.4931