Copyright
©2007 Baishideng Publishing Group Co.
World J Gastroenterol. Oct 7, 2007; 13(37): 4931-4937
Published online Oct 7, 2007. doi: 10.3748/wjg.v13.i37.4931
Published online Oct 7, 2007. doi: 10.3748/wjg.v13.i37.4931
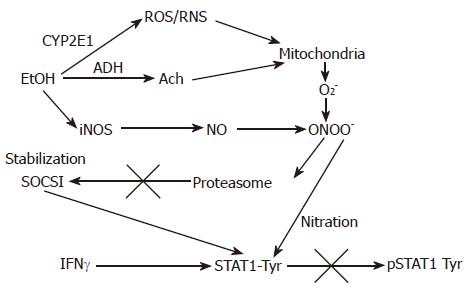
Figure 2 The proposed effects of EtOH metabolism on IFNγ-induced STAT1 phosphorylation in VL-17A cells.
EtOH is metabolized by ADH and CYP2E1 to Ach and ROS/RNS and increase a formation of PN (peroxynitrite, ONOO-), a reaction product of O2- and NO, which at high concentrations blocks proteasome function. This causes stabilization of SOCS1, a negative regulator of Jak-STAT1 signaling as well as prevents STAT1 phosphorylation on tyrosine residues.
- Citation: Osna NA, Jr TMD. Implication of altered proteasome function in alcoholic liver injury. World J Gastroenterol 2007; 13(37): 4931-4937
- URL: https://www.wjgnet.com/1007-9327/full/v13/i37/4931.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i37.4931