Copyright
©2006 Baishideng Publishing Group Co.
World J Gastroenterol. Mar 7, 2006; 12(9): 1452-1457
Published online Mar 7, 2006. doi: 10.3748/wjg.v12.i9.1452
Published online Mar 7, 2006. doi: 10.3748/wjg.v12.i9.1452
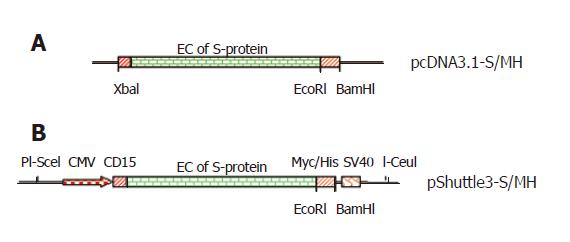
Figure 1 Construct of codon-optimized SARS S-protein genes.
The full-length of SARS codon-optimized S-protein gene was modified by inserting Myc/His gene immediately downstream extracellular domain of S-protein gene to generate pcDNA3.1-S/MH (A) and the S-protein fused with Myc/His gene was transferred into pShuttle3 vector to produce pShuttle3-S/MH vector (B).
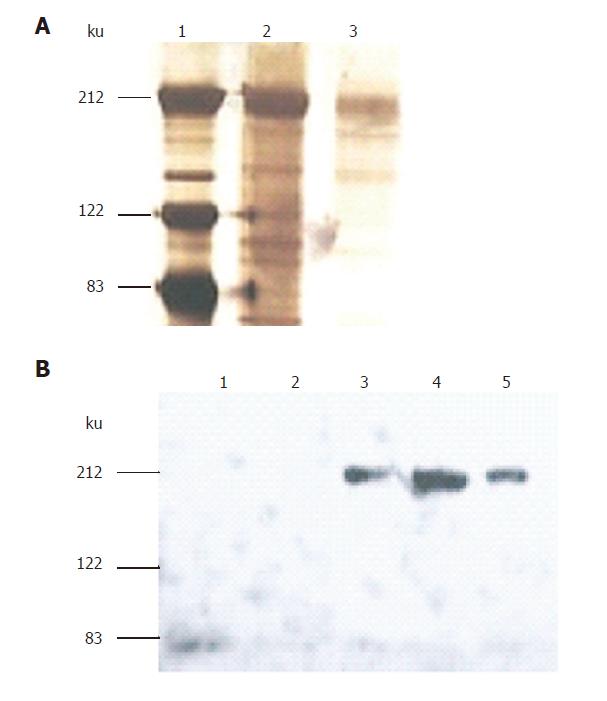
Figure 2 Expression and purification of fused S-protein soluble.
S-protein fused with Myc/His was expressed in HEK293 cells mediated by adenovirus and purified on Ni-NTA-agarose column and eluted with imidazole at 6 fractions. The eluted proteins were separated by 7% SDS-PAGE gels and identified by both silver staining (A) for elutions 4, 5 and Western-blotting using anti-Myc antibody (B) for elutions 1-5.
Figure 3 Effects of tunicamycin on expression of S-protein.
HEK293 cells were infected with Ad-S/MS virus for 48h in the presence or absence of tunicamycin (7μg/mL). The media were mixed with Ni-NTA-agarose beads and bound protein was eluted with imidazole elution buffer. The pelleted cells were lysed. After centrifugation, the supernatant with eluted protein from the media was separated on SDS-PAGE gels (7%, v/v). Separated proteins were detected by Western blotting using anti-Myc antibody.
Figure 4 Analysis of expression conditions.
A: HEK293 cells were infected with Ad-S/MH viruses at different MOI of 5-100. The culture media were harvested at 24, 48 and 72 h after infection. B: Cells were infected with same viruses at MOI of 50. The media were harvested at 10, 20, 30, 40, 50, 60 and 70h after infection. The expressed protein was purified with Ni-NTA-agarose and measured with protein assay kit. The dada of expression levels were the mean of three separate experiments.
Figure 5 Binding of S-protein to Vero and 293T cells.
Vero and 293T cells were detached from culture flask with 0.5 mmol/L EDTA/ PBS, washed and incubated with purified S-protein in 0.5% BSA/ PBS. Binding was measured by flow cytometry using anti-Myc antibody and goat anti-mouse IgG antibody conjugated with RPE (open profiles). As control (filled profiles), the cells were not incubated with S-protein but stained with the same antibodies.
- Citation: Zhong F, Zhong ZY, Liang S, Li XJ. High expression level of soluble SARS spike protein mediated by adenovirus in HEK293 cells. World J Gastroenterol 2006; 12(9): 1452-1457
- URL: https://www.wjgnet.com/1007-9327/full/v12/i9/1452.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i9.1452