Published online Sep 16, 2015. doi: 10.12998/wjcc.v3.i9.793
Peer-review started: February 1, 2015
First decision: March 6, 2015
Revised: April 10, 2015
Accepted: June 15, 2015
Article in press: June 16, 2015
Published online: September 16, 2015
Processing time: 104 Days and 20 Hours
AIM: To determine the role of leukocyte function-associated antigen-1 (LFA-1) in polymicrobial sepsis model in mice.
METHODS: Cecal ligation and puncture model was used to study polymicrobial sepsis in wild type and LFA-1 knockout (KO) (= CD11a KO) mice. Their survivals were examined. Neutrophil recruitment to the abdominal cavity, bacterial tissue load and bacterial killing by neutrophils, tissue cytokine profiles, and serum cytokines were examined. Apoptosis of tissues was assessed using cleaved-caspase 3 and TUNNEL staining. The recruitment of neutrophils to various tissues was assessed using myeloperoxidase staining or measuring myeloperoxidase activity.
RESULTS: LFA-1 deficiency significantly decreased survival (P = 0.0024) with the reduction of neutrophil recruitment to the abdominal cavity and higher bacterial load in blood. It was also associated with increased apoptosis in spleen and more organ injuries probed by interleukin-6 mRNA level. However, the deficiency of LFA-1 did not prevent neutrophil recruitment to lung, liver, spleen or kidney, which suggested the existence of LFA-1 independent recruitment mechanism in these organs.
CONCLUSION: LFA-1 deficiency did not attenuate neutrophil recruitment to various organs to adequately mitigate secondary tissue injury in sepsis. It was associated with decreased neutrophil recruitment to the abdominal cavity, higher bacterial load, leading to increased mortality in an abdominal, polymicrobial sepsis.
Core tip: We report our result on the role of leukocyte function-associated antigen-1 (LFA-1) in polymicrobial abdominal sepsis model induced by cecal ligation and puncture. LFA-1 is a key player in neutrophil migration, but its role in neutrophil migration to tissues in polymicrobial sepsis is yet to be determined. This study demonstrated that LFA-1 deficiency blocked neutrophil migration to the abdominal cavity, but maintained migration to other organs, and reduced survival in sepsis.
- Citation: Liu JR, Han X, Soriano SG, Yuki K. Leukocyte function-associated antigen-1 deficiency impairs responses to polymicrobial sepsis. World J Clin Cases 2015; 3(9): 793-806
- URL: https://www.wjgnet.com/2307-8960/full/v3/i9/793.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v3.i9.793
Sepsis remains to be associated with high morbidity and mortality and accounts for a large expenditure in public health. The recent study demonstrated that the mortality was still over 30% in Europe and the United States despite the implementation of sepsis management protocols[1]. This suggests that our understanding of the pathophysiology of sepsis remains quite limited.
Accumulating evidence demonstrated that the innate immune system is activated and works as the first line of defense against sepsis. This inflammatory response is characterized by leukocyte infiltration into the extravascular space. Leukocytes are not only bactericidal but also mediate local tissue damage and alternations in microvascular perfusion. Adhesion molecules present on leukocytes and endothelial cells control leukocyte adhesion and extravasation.
Leukocyte-function associated antigen-1 (LFA-1, CD11aCD18) is an adhesion molecule expressed ubiquitously on the surface of all leukocytes and plays a central role in leukocyte recruitment. LFA-1 is composed of two subunits CD11a and CD18 and binds to their counter ligands, intercellular adhesion molecule-1 and -2 (ICAM-1 and ICAM-2). While LFA-1 does not bind its ligands in a resting state, in inflammation it becomes active and binds them. The interactions between them facilitate leukocyte rolling, arrest on the blood vessels and transmigration to facilitate leukocyte recruitment. This is a critical, immunological process to fight against and reduce infection.
The role of LFA-1 in infection has been examined in several in vivo models with mixed results. Deficiency of LFA-1 worsened the outcome of Streptococcus pneumoniae infection[2] and Mycobacterium tuberculosis[3], but was protective in listeriosis[4] and lipopolysaccharide shock[5]. Specifically neutrophils are innate immune cells for the first-line defense against infection. Their bactericidal properties emanates from phagocytosis and release of proteases and reactive oxygen species. However, these indiscriminant processes can also harm host tissues[6]. Sepsis often results in an exaggerated inflammatory response that leads to secondary tissue injury and organ dysfunction. We hypothesize that the LFA-1 deficiency will attenuate tissue injury and ameliorate bacterial sepsis. To test this hypothesis we used cecal ligation and puncture (CLP) model, an abdominal polymicrobial model which mimics human sepsis physiology[7] and measured the extent of histopathological damage and cytokine response in LFA-1 deficient and wild-type (WT) mice.
All the mice were purchased from Jackson laboratory (Bar Harbor, Maine, United States) and inbred in the Boston Children’s Hospital animal facility. CD11a knockout (KO) mice (LFA-1 KO mice) have been previously described[8]. Male mice on the C57BL/6J background aged 8-10 wk were used for the current experiments. They were housed under 12 h day and night cycles and specific pathogen free conditions.
All the experimental protocols were approved by the Review Board in Boston Children’s Hospital and followed the Animal Research: Reporting of in vivo experiments guidelines. All the appropriate measures were taken to minimize pain and discomfort as described below. Polymicrobial sepsis was induced by cecal ligation and puncture model as described in our previous report for the details[7]. The mice were anesthetized with ketamine 60 mg/kg and xylazine 5 mg/kg. The cecum was punctured through and through using a 18-G needle. Following the completion of CLP surgery, 0.1 mL/kg of warmed saline was given subcutaneously. Postoperative surgery pain was treated with buprenorphine. Activity levels were recorded as follows: 5 = full response and activity (baseline activity), 4 = little less than full response and activity, 3 = responsive, active, but decreased appetite, 2 = responds, but barely reacts, 1 = barely responsive, and 0 = no response. In some of the experiments, LFA-1 blocking antibody (M17/4; BioXcell, West Lebanon, NH, United States) 2 mg/kg was given intravenously prior to CLP surgery.
Peritoneal lavage samples were collected at 6 and 12 h after CLP as described in our previous report[7]. The number and differential of peritoneal leukocytes was assessed using TURK blood diluting fluid and Giemsa staining.
The residual bacterial loads in the organs and blood were determined by loading serially diluted tissue homogenates and blood on agar plates as described in our previous report[7].
As previously described[9], we isolated neutrophils from mouse bone marrow. We performed bacterial killing assays as described in our previous report[7]. Briefly, 50% diluted plasma from either WT or CD11a KO mice was mixed with 5 × 105 CFU of bacteria derived from tissues after CLP for opsonization. Then 5 × 104 of neutrophils were added and kept for one hour in a shaker at 37 °C, and then the number of residual bacteria was determined by plating on blood agar.
VetScan HM2 (Abaxis, Union City, CA, United States) was used for complete blood count counts. Levels of various serum cytokines except interleukin-6 (IL-6) were determined by using mouse Th1/Th2 kit (Meso Scale Discovery; Gaithersburg, MD, United States). Serum IL-6 was measured using mouse IL-6 enzyme-linked immunosorbent assay (ELISA) kit (Life Technologies, Grand Island, NY, United States). Serum mouse immunoglobulin levels were measured with a commercially available ELISA kit (BD, Sparks, MD, United States).
Following the harvest, tissues were snap frozen and stored at -80 °C for a short period. Total RNA was extracted using TRIzol reagent (Life Technologies) and subject to DNase digestion. 1 μg of RNA were subject to reverse transcription using Superscript III RNase reverse transcriptase (life technologies). Real time quantitative polymerase chain reaction (PCR) was performed using SYBR green master mix (Applied Biosystems, South Francisco, CA, United States). As examples of proinflammatory and anti-inflammatory cytokines, we quantitated mRNA level of tumor necrosis factor (TNF)-α, IL-1β, IL-6 and IL-10. glyceraldehyde-3-phosphate dehydrogenase was used as housekeeping gene. The sequences of primers used for this assay were listed in Table 1.
| Gene | Forward primer | Reverse primer |
| TNF-α | CTGTAGCCCACGTCGTAGC | TTGAGATCCATGCCGTTG |
| IL-1b | TGTAATGAAAGACGGCACACC | TCTTCTTTGGGTATTGCTTGG |
| IL-6 | GCTACCAAACTGGATATAATCAGGA | CCAGGTAGCTATGGTACTCCAGAA |
| IL-10 | CAGAGCCACATGCTCCTAGA | GTCCAGCTGGTCCTTTGTTT |
| GAPDH | GCACAGTCAAGGCCGAGAAT | GCCTTCTCCATGGTGGTGAA |
Mice tissues were fixed using 4% paraformaldehyde. Tissues for histology analysis were prepared in Boston Children’s Hospital pathology core.
The degree of splenic apoptosis was determined by Western blot analysis and immunohistochmistory probed by anti-cleaved caspase-3 antibody and TUNEL staining as we previously described for details[7,10]. For Western blot analysis, the total protein extracted from spleen was used. For immunohistochemistry analysis, anti-cleaved caspase 3 antibody was probed with biotin-labeled anti-rabbit IgG and peroxidase-conjugated streptavidin. 3,3’-diaminobenzidine (DAB) was used for a chromogenic reaction. TUNEL staining was performed according to the company protocol (Millipore, Serological Corporation, Norcross, GA, United States).
The degree of neutrophil recruitment to various organs was assessed by probing myeloperoxidase activity (MPO) in the tissues. Histology sections were deparaffinized as we previously described[7] and probed with anti-myeloperoxidase antibody, which was captured by anti-rabbit IgG-HRP and DAB.
Bronchoalveolar lavag fluid was obtained as described in our previous report[7]. The number and differential of leukocytes was determined with TURK blood diluting fluid and Giemsa staining.
MPO level was measure in lung samples obtained from mice at 12 h after CLP. MPO assay was well described in the past[11]. Briefly, MPO was extracted from tissues into the supernatant by homogenization in 50 mmol/L KPO4 buffer containing 0.05% hexadecyltrimethlammonium bromide, followed by repeated freeze and thaw, and centrifugation. O-dianisodine and H2O2 were added to the supernatant. The wave length of 450 nm was used for absorbance measurement.
The data were shown as mean ± SD. Differences were statistically analyzed using PRISM software (GraphPad software, Inc. La, CA, United States). The statistical methods used were detailed in corresponding figure legends. P < 0.05 was used for a statistical significance. The statistical methods of this study were reviewed by Amber Hall, MPH (Boston Childrens Hospital).
In the previous study using less severe CLP model (survival about 80%), administration of LFA-1 blocking antibody did not improve survival despite the attenuation of lung injury[12]. The use of blocking antibody carries the potential problem because (1) in the case of physical approximation of LFA-1 with an other surface receptor, anti-LFA-1 antibody may directly interfere with the receptor, and (2) blocking antibody may activate Fc receptors and induce functional changes in neutrophils[13]. Therefore, we studied using CD11a KO mice here.
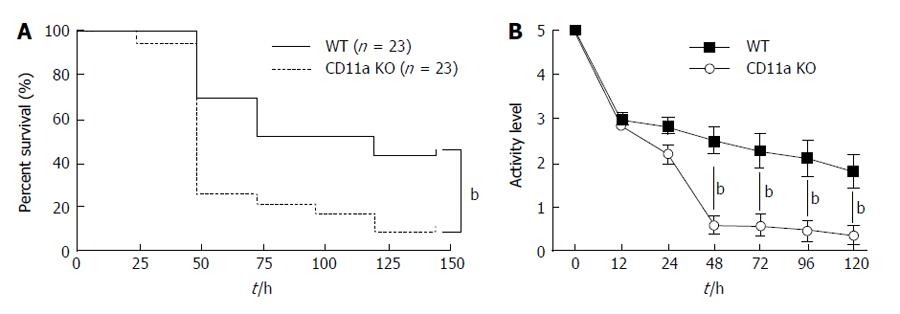
Contrary to our hypothesis that LFA-1 deficiency would attenuate sepsis, KO mice had significantly higher mortality (Figure 1A). Their activity level was significantly reduced 48 h after CLP compared to WT mice (Figure 1B), which was in line with the observed pattern of mortality.
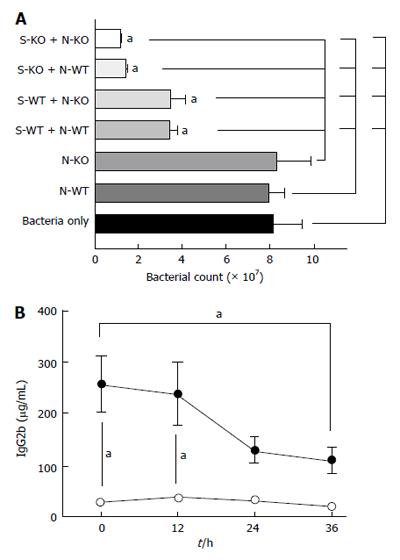
LFA-1 plays an important role in leukocyte recruitment. Now we evaluated the degree of leukocyte recruitment to the peritoneal space. The total number of peritoneal leukocytes was greater in WT mice (Figure 2A), which were primarily neutrophils (data not shown). Previously, the peritoneal emigration ratio was defined as (peritoneal leukocyte number)/(peripheral leukocyte number) by Prince et al[2]. When we compared this parameter, KO mice had much lower peritoneal emigration ratio than WT mice (Figure 2B), suggesting that leukocyte recruitment to the peritoneal cavity was significantly reduced in the former. Similarly, the administration of the blocking antibody was associated with lower peritoneal emigration ratio than isotype control (Figure 2C). This observation is compatible with thioglycollate-induced peritonitis model[14] and Streptococcus pneumoniae abdominal infection model[2].
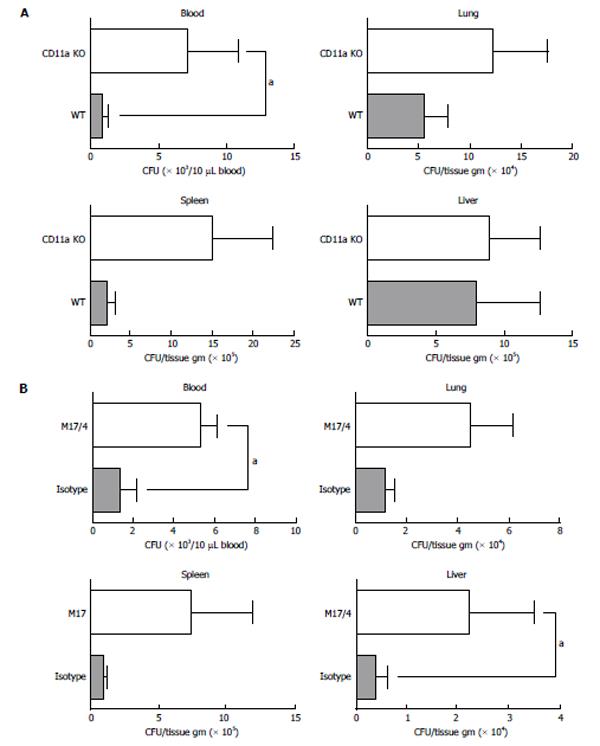
Since KO mice had significantly reduced leukocyte recruitment to the peritoneal cavity (site of infection), we reasoned that the higher mortality in KO mice might be due to the difference of bacterial clearance between WT and KO mice. Bacterial loads in blood and various tissues were measured. Even at 6 h after CLP surgery, the KO mice had a significantly higher bacterial load in blood than the WT (Figure 3A). Similarly the presence of CD11a blocking antibody M17/4 was associated with higher bacterial load in blood and liver than the presence of isotype-control antibody (Figure 3B).
Because KO mice had higher bacterial load, we assessed any alternation of bacterial killing in KO mice in addition to the reduced leukocyte recruitment to the abdominal cavity. We performed assays using bacteria derived from the cecal flora. Isolated neutrophils themselves were not bactericidal, but the addition of serum to the neutrophils effectively killed bacteria in both WT and KO mice (Figure 4A). The serum itself killed bacteria and KO serum was more efficient in bacterial killing. Furthermore neutrophils were more bactericidal with KO serum than with WT serum. Therefore, higher bacterial load in KO mice is attributed to the significantly reduced number of leukocytes at the site of infection. The observation that KO serum was more efficient in bacterial killing with and without neutrophils may suggest the possibility that KO serum have more complement activity, or it may be due to significant reduced serum IgG2b level in KO mice (Figure 4B). IgG2b can bind to inhibitory Fc receptor and impair optimal opsonization through binding to antigenic sites on the bacteria[15]. Therefore, the reduced IgG2b level may be in favor for opsonization in KO mice.
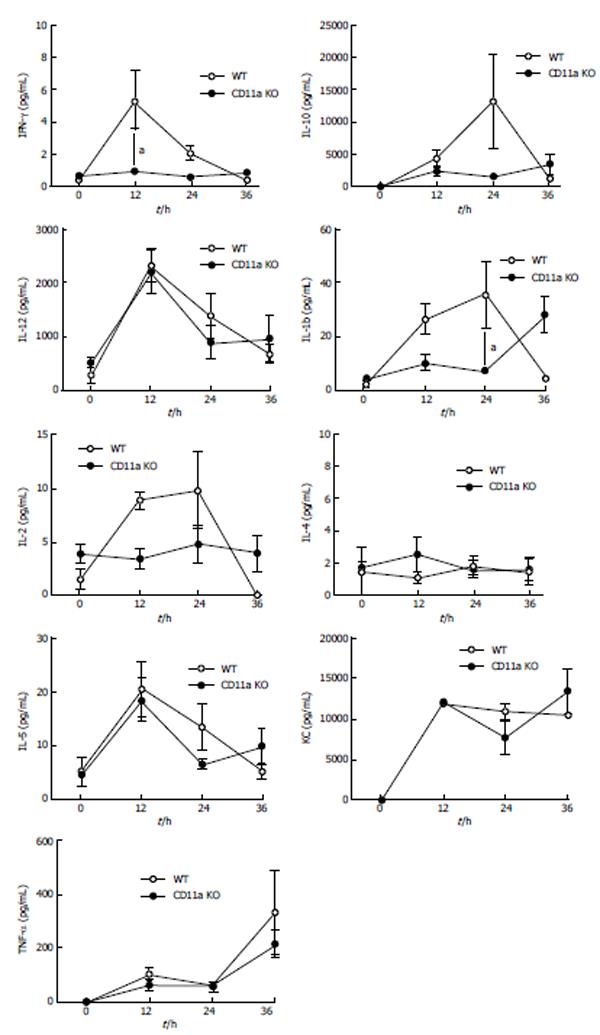
Systemic activation of the innate immune system plays an important role in the pathophysiology of sepsis. The initial immune response is proinflammatory, but this rapidly shifts to hypoinflammatory (anti-inflammatory) process[16]. The attenuation of proinflammatory cytokines was previously associated with the improved outcomes in this model[17]. Since KO mice had higher mortality, we compared proinflammatory [TNF-α, interferon (IFN)-γ, CXCL1, IL-1β, IL-2, and IL-12] and anti-inflammatory (IL-4, IL-5, and IL-10) cytokines (Figure 5). There was a slight increase of IFN-γ level in KO mice at 12 h after CLP procedure, but there was no difference at 24 and 36 h. IL-1β level of KO mice was significantly higher at 24 h after CLP procedure, but at 36 h no difference was observed. IL-6 is a cytokine with both proinflammatory and anti-inflammatory properties. Previously the serum level of IL-6 was elevated in septic patients[18] and shown to correlate with their outcomes[19,20]. Serum IL-6 level was higher at 12 h in KO mice. At 24 and 36 h, serum IL-6 level in KO mice was not statistically different (Figure 5).
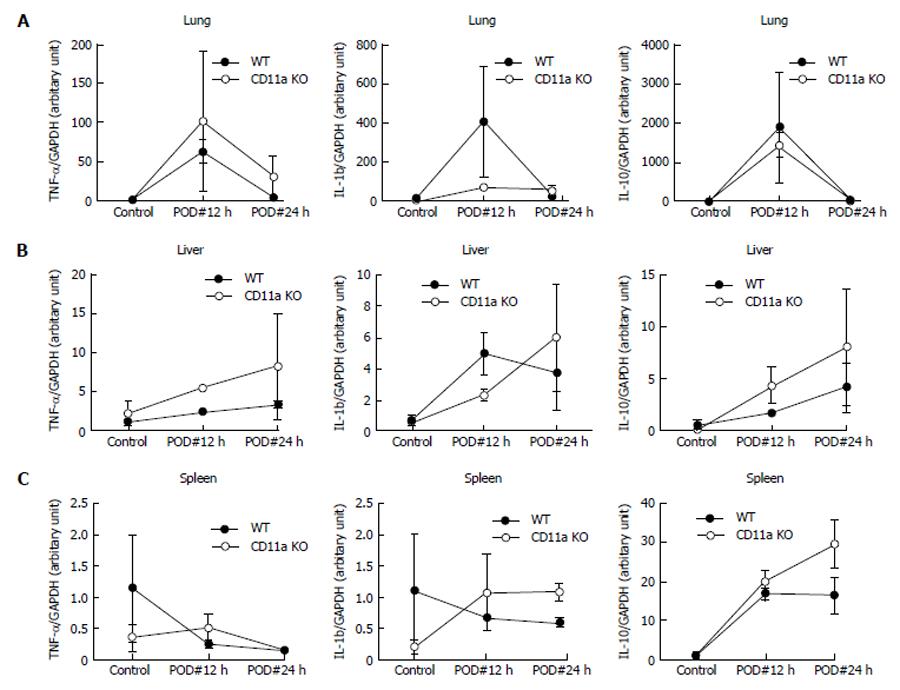
Next we evaluated local injury to see if there was any difference between WT and KO mice. IL-6 serves as sensitive marker of tissue inflammation (injury)[21]. In both lung and spleen, the level of IL-6 mRNA was significantly higher in KO mice than WT mice (Figure 6), suggesting that there was more tissue injury in KO mice. mRNA levels of TNF-α, IL-1β and IL-10 were not statistically significant (Figure 7).
Apoptosis in the spleen is linked to the severity of sepsis[22]. Therefore, we evaluated the degree of apoptosis in our model. We first evaluated the cleaved-caspase-3 expression in spleen using western blot. The expression of cleaved caspase-3 was peaked at 12 h following CLP procedure in both WT and CD11a KO mice (Figure 8). Expression was higher in KO mice. Next we evaluated apoptosis using TUNEL stain and cleaved caspase-3 staining on the spleen histology. As predicted, apoptotic cells of spleen were present more in KO mice (Figure 9). Taken together, LFA-1 deficiency contributed to severity in tissue injuries in CLP model.
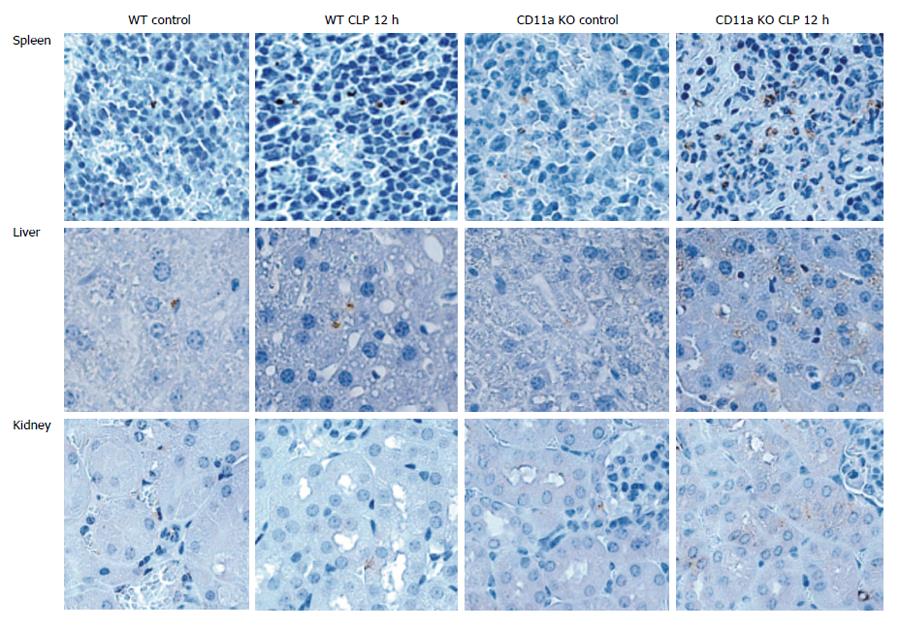
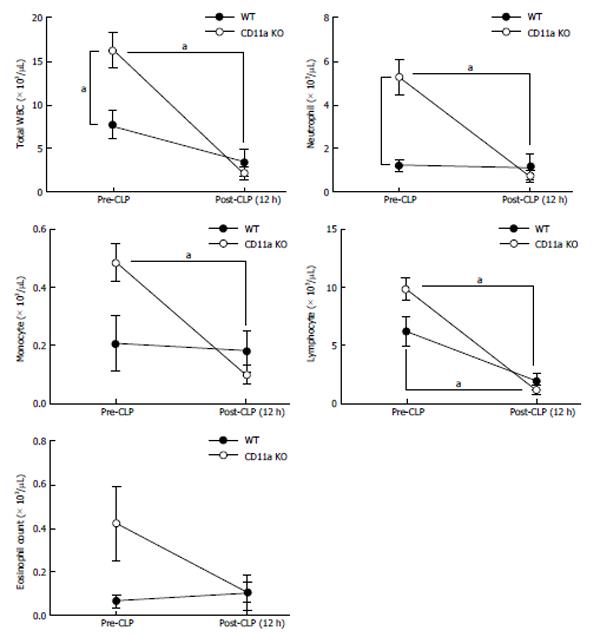
Since KO mice had increased tissue injury, we examined neutrophil recruitment to spleen, liver, kidney and lung. MPO is a peroxidase enzyme that is most abundantly expressed in the granules of neutrophils. We examined the expression of MPO in tissues as a surrogate of neutrophil number. There were more MPO positive cells on tissues of KO mice in organs tested after CLP (Figure 10). Activated leukocytes are often trapped in lung in sepsis, causing lung injury. The number of leukocytes in bronchial lavage fluid was not statistically different between WT and KO mice (Figure 11A). The majority of the population was neutrophils (data not shown). However, MPO value was significantly higher in lung tissue in KO mice than in WT mice (Figure 11B), suggesting that neutrophils in KO mice were trapped in lung tissue with less migration out into the alveolar space. Although the baseline neutrophil count was higher in KO mice as previously reported due to the elevated level in G-CSF level[23], there was no difference at 12 h after CLP (Figure 12). This larger reduction in peripheral neutrophil counts could be well explained by more neutrophil recruitment to the tissues in KO mice. In addition, we tested neutrophil recruitment to lung using LFA-1 blocking antibody M17/4. The result demonstrated more neutrophil recruitment to lung in the group that received LFA-1 blocking antibody, consistent with our KO mice data (Figure 11C). These results suggested that neutrophils migrated in a LFA-1 independent fashion to the tissues tested.
Here we demonstrated that both LFA-1 blockade and deficiency were detrimental in polymicrobial sepsis. CD11a KO mice demonstrated higher mortality with more bacterial load in blood than WT mice in CLP model. The reduction of available leukocytes at the site of infection (the abdominal cavity) was significant. Contrary to our hypothesis, LFA-1 deficiency did not attenuate neutrophil recruitment to various organs, suggesting that tissue damage by neutrophils was not attenuated. Therefore, we conclude that the loss of LFA-1 did not alleviate organ injury in this experimental model of abdominal sepsis.
Neutrophil phagocytosis of a certain bacteria strain (such as Streptococcus pyogenes) was previously reported to be potentiated by LFA-1/ICAM-1 interaction[24]. The capability of bacteria killing was not different between WT and KO mice, suggesting that neutrophils devoid of LFA-1 could phagocytize bacteria effectively. Reduction of leukocyte recruitment to the abdominal cavity can certainly reduce the efficiency of bacteria killing. Therefore, higher bacteria load in blood may be simply due to the impaired recruitment of neutrophils to the abdominal cavity.
The process of leukocyte (neutrophil) recruitment to organs was previously assumed to uniformly follow an orchestrated progression consisting of initial tethering and rolling by selectins, firm adhesion by integrins such as LFA-1 and macrophage-1 antigen (Mac-1, CD11bCD18) and transmigration. Molecules involved in the recruitment of leukocytes to the abdominal cavity were examined in thioglycollate-induced inflammation[25]. The study demonstrated that two integrins LFA-1 and α4β1 contributed to neutrophil recruitment. In our study migration of leukocytes to the peritoneal cavity was much less in CD11a KO mice than WT mice, suggesting that LFA-1 played a major role. Microcirculation of peritoneum seems to fit this traditional scheme. Microcirculation in tissues such as mesentery, skeletal muscle, and skin also follow the original scheme[26]. In contrast, LFA-1 deficiency did not have an effect on neutrophil recruitment to liver, kidney, spleen and lung in our model, suggesting that other processes are involved.
Recruited leukocytes are predominantly neutrophils early in sepsis. Neutrophils efficiently kill bacteria via various methods, but the secondary damage by neutrophils could be detrimental. The importance of early, enhanced neutrophil recruitment by the chemokines CXCL1 (= KC) and CXCL2 (macrophage inflammatory protein 2; MIP-2) administration in peritonitis-induced sepsis was previously shown to improve bacterial clearance and survival[27]. However, blockade of CXCL2 was associated with the reduced percentage of neutrophils in the peritoneal fluid but with better survival[28]. Furthermore, leukotriene B4 receptor (BLT1) deficiency was associated with reduction of neutrophils migration to the abdominal cavity with a better survival[29]. The impact of these molecules on other effects such as neutrophils recruitment to various tissues and cytokine productions were not fully explored in these studies. However the completely opposite outcomes despite similarly reduced neutrophil recruitment to the abdominal cavity suggested that there are important factors other than local infection control. The function of CXCL2 includes the activation of neutrophils. Blockade of CXCL2 or BLT1 deficiency may possibly reduce neutrophil migration to various organs outside of the abdominal cavity, limiting organ injury. It is important to understand how we modulate the behavior of neutrophils (leukocytes) in the whole body rather than simple control of neutrophils to the site of infection.
In the present report we demonstrated that in CD11a KO mice, neutrophil recruitment seems organ specific. Neutrophil recruitment to liver in lipopolysaccharide shock was previously shown to be independent on LFA-1 and dependent on CD44[5,30]. Interestingly, neutrophil utilizes Mac-1 when fMLP was injected locally in liver, suggesting that main players can change depending on circumstances[30]. Lung microvasculature is a network of thin capillaries. Neutrophils have to deform to pass through these small capillaries, which may make selectins and integrins unnecessary[31]. However, the other report suggests that integrins play a significant role in leukocyte recruitment to lung[12,32]. In the study by Asaduzzaman et al[12], the administration of LFA-1 blocking antibody did not change the outcome of mice in mild CLP model (survival around 80% in both vehicle and blocking antibody injected groups) despite the less neutrophil recruitment to lung. In our more severe model, LFA-1 blocking antibody did not attenuate neutrophil recruitment to lung. Whether or not the pattern of neutrophil recruitment to lung varies in different disease processes as well as severity of diseases remains to be clarified. In kidney, Mac-1 is shown to be involved in neutrophil recruitment[33]. Various insights into neutrophil recruitment at various organs start to emerge. However, it is required to have further investigations on clear mechanism of neutrophil recruitment to each organ in the future. Our study highlighted the importance of this approach.
Since LFA-1 works by interacting with its ligands, it is important to study the role of ICAM-1 and ICAM-2, both of which are ligands for LFA-1. They are expressed on leukocytes, epithelial cells, endothelial cells, and fibroblasts. ICAM-1 is uniformly expressed on the surface of resting endothelium at low level and its expression is upregulated by inflammatory cytokines. On the other hand, ICAM-2 shows high expression constitutively, particularly at endothelial cell junctions. The predominant role of ICAM-1 is to establish stable leukocyte adhesion while ICAM-2 mediates transmigration[34]. The role of ICAM-2 in polymicrobial sepsis has not been studied. ICAM-1 KO mice subjected to CLP surgery had reduction of serum TNF-α, IL-1β, IL-6 and IL-10 levels as well as leukocyte migration to liver and lung, and had significantly higher survival over WT mice in CLP model[35]. The degree of leukocyte migration to the abdominal cavity and bacterial tissue load were not assessed in this study, but it is highly likely that neutrophils recruitment to the abdominal cavity was reduced by disrupted LFA-1/ICAM-1 interaction. The significantly lower cytokine levels and attenuation of leukocyte migration to organs such as liver in ICAM-1 KO mice might reduce their mortality. In our study, we did not see a significant difference of cytokine levels between WT and CD11a KO mice. Because ICAM-1 also binds to Mac-1 and p150,90, the elimination of interaction of ICAM-1 with these targets other than LFA-1 might contribute to this paradoxical result in ICAM-1 KO mice. The recruitment of neutrophils to liver is dependent on Mac-1[31], and the reduction of leukocyte recruitment to liver might be due to the abolishment of Mac-1 interaction. It is interesting to investigate in the future if ICAM-1 KO mice have less neutrophil recruitment to the abdominal cavity as well as other organs.
We tried to address the degree of tissue injury by measuring IL-6 levels. IL-6 is induced by various signals including IL-1β, TNF-α, IL-2 and endotoxin, and it was reported as a marker of severity in sepsis and correlated with the outcome of patients. Remick et al[21] stated it as a sensitive marker for tissue injury. We demonstrated that IL-6 mRNA levels in various tissues were more elevated in CD11a KO mice. This was in line with our result of more neutrophil recruitment to various organs in KO mice. It remains to be studied whether or not other markers suit better for this purpose.
In conclusion, we demonstrated that LFA-1 blockade selectively reduced neutrophil recruitment to the site of infection without attenuating various organ injuries. Blockade of LFA-1 may not have a therapeutic implication in the setting of abdominal polymicrobial sepsis.
Neutrophils are the double-edge swords in sepsis because they eradicate bacteria as the first-line innate cells, but cause tissue injury. Leukocyte function-associated antigen-1 (LFA-1) is an important adhesion molecule in leukocyte recruitment. The effect of LFA-1 deficiency on leukocyte recruitment and tissue injury has not been well characterized in polymicrobial sepsis model.
The author aimed to study the role of LFA-1 in neutrophil migration to various organs in polymicrobial sepsis model.
To illustrate the contribution of LFA-1 in neutrophil migration to various tissues so as to understand the impact of LFA-1 deficiency (or blockade) in polymicrobial sepsis model.
The author hypothesized that LFA-1 deficiency would reduce neutrophil recruitment to various organs, thereby reducing tissue injury and improving the outcome in polymicrobial sepsis model. However, the authors results showed that LFA-1 reduced neutrophil migration to the abdominal cavity, but did not reduce to other organs. The blockade of LFA-1 does not likely improve sepsis outcomes.
LFA-1 is an adhesion molecule expressed on neutrophils and binds to its ligands such as intercellular adhesion molecule-1 on the endothelium, allowing neutrophils to stop on the blood vessel. Traditionally, this step is considered to be a critical step in neutrophil migration.
This is a clear cut study using LFA-1 knockout mice. The authors revealed that the absence of LFA-1 results in survival disadvantage in polymicrobial sepsis. The experiments were performed properly, and the paper is well-written with reasonable discussions.
P- Reviewer: Nosaka T, Spasojevic SD, Teimourian S S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
| 1. | Levy MM, Artigas A, Phillips GS, Rhodes A, Beale R, Osborn T, Vincent JL, Townsend S, Lemeshow S, Dellinger RP. Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet Infect Dis. 2012;12:919-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 381] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 2. | Prince JE, Brayton CF, Fossett MC, Durand JA, Kaplan SL, Smith CW, Ballantyne CM. The differential roles of LFA-1 and Mac-1 in host defense against systemic infection with Streptococcus pneumoniae. J Immunol. 2001;166:7362-7369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Ghosh S, Chackerian AA, Parker CM, Ballantyne CM, Behar SM. The LFA-1 adhesion molecule is required for protective immunity during pulmonary Mycobacterium tuberculosis infection. J Immunol. 2006;176:4914-4922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Miyamoto M, Emoto M, Emoto Y, Brinkmann V, Yoshizawa I, Seiler P, Aichele P, Kita E, Kaufmann SH. Neutrophilia in LFA-1-deficient mice confers resistance to listeriosis: possible contribution of granulocyte-colony-stimulating factor and IL-17. J Immunol. 2003;170:5228-5234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Emoto M, Emoto Y, Brinkmann V, Miyamoto M, Yoshizawa I, Stäber M, van Rooijen N, Hamann A, Kaufmann SH. Increased resistance of LFA-1-deficient mice to lipopolysaccharide-induced shock/liver injury in the presence of TNF-alpha and IL-12 is mediated by IL-10: a novel role for LFA-1 in the regulation of the proinflammatory and anti-inflammatory cytokine balance. J Immunol. 2003;171:584-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Brown KA, Brain SD, Pearson JD, Edgeworth JD, Lewis SM, Treacher DF. Neutrophils in development of multiple organ failure in sepsis. Lancet. 2006;368:157-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 502] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 7. | Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4:31-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1609] [Cited by in RCA: 1634] [Article Influence: 102.1] [Reference Citation Analysis (0)] |
| 8. | Ding ZM, Babensee JE, Simon SI, Lu H, Perrard JL, Bullard DC, Dai XY, Bromley SK, Dustin ML, Entman ML. Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J Immunol. 1999;163:5029-5038. [PubMed] |
| 9. | Carbo C, Yuki K, Demers M, Wagner DD, Shimaoka M. Isoflurane inhibits neutrophil recruitment in the cutaneous Arthus reaction model. J Anesth. 2013;27:261-268. [PubMed] |
| 10. | Liu JR, Liu Q, Li J, Baek C, Han XH, Athiraman U, Soriano SG. Noxious stimulation attenuates ketamine-induced neuroapoptosis in the developing rat brain. Anesthesiology. 2012;117:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Hoesel LM, Neff TA, Neff SB, Younger JG, Olle EW, Gao H, Pianko MJ, Bernacki KD, Sarma JV, Ward PA. Harmful and protective roles of neutrophils in sepsis. Shock. 2005;24:40-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 114] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Asaduzzaman M, Zhang S, Lavasani S, Wang Y, Thorlacius H. LFA-1 and MAC-1 mediate pulmonary recruitment of neutrophils and tissue damage in abdominal sepsis. Shock. 2008;30:254-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Zhou M, Todd RF, van de Winkel JG, Petty HR. Cocapping of the leukoadhesin molecules complement receptor type 3 and lymphocyte function-associated antigen-1 with Fc gamma receptor III on human neutrophils. Possible role of lectin-like interactions. J Immunol. 1993;150:3030-3041. [PubMed] |
| 14. | Berlin-Rufenach C, Otto F, Mathies M, Westermann J, Owen MJ, Hamann A, Hogg N. Lymphocyte migration in lymphocyte function-associated antigen (LFA)-1-deficient mice. J Exp Med. 1999;189:1467-1478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 274] [Cited by in RCA: 273] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 15. | Moitra R, Beal DR, Belikoff BG, Remick DG. Presence of preexisting antibodies mediates survival in sepsis. Shock. 2012;37:56-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2810] [Cited by in RCA: 2775] [Article Influence: 126.1] [Reference Citation Analysis (0)] |
| 17. | Dejager L, Pinheiro I, Dejonckheere E, Libert C. Cecal ligation and puncture: the gold standard model for polymicrobial sepsis? Trends Microbiol. 2011;19:198-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 536] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 18. | Waage A, Brandtzaeg P, Halstensen A, Kierulf P, Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J Exp Med. 1989;169:333-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 834] [Cited by in RCA: 839] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 19. | Casey LC, Balk RA, Bone RC. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Ann Intern Med. 1993;119:771-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 718] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 20. | Damas P, Ledoux D, Nys M, Vrindts Y, De Groote D, Franchimont P, Lamy M. Cytokine serum level during severe sepsis in human IL-6 as a marker of severity. Ann Surg. 1992;215:356-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 556] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 21. | Remick DG, Bolgos G, Copeland S, Siddiqui J. Role of interleukin-6 in mortality from and physiologic response to sepsis. Infect Immun. 2005;73:2751-2757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 179] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 22. | Dear JW, Yasuda H, Hu X, Hieny S, Yuen PS, Hewitt SM, Sher A, Star RA. Sepsis-induced organ failure is mediated by different pathways in the kidney and liver: acute renal failure is dependent on MyD88 but not renal cell apoptosis. Kidney Int. 2006;69:832-836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Anderson DC, Schmalsteig FC, Finegold MJ, Hughes BJ, Rothlein R, Miller LJ, Kohl S, Tosi MF, Jacobs RL, Waldrop TC. The severe and moderate phenotypes of heritable Mac-1, LFA-1 deficiency: their quantitative definition and relation to leukocyte dysfunction and clinical features. J Infect Dis. 1985;152:668-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 416] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 24. | Schnitzler N, Haase G, Podbielski A, Lütticken R, Schweizer KG. A co-stimulatory signal through ICAM-beta2 integrin-binding potentiates neutrophil phagocytosis. Nat Med. 1999;5:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Henderson RB, Lim LH, Tessier PA, Gavins FN, Mathies M, Perretti M, Hogg N. The use of lymphocyte function-associated antigen (LFA)-1-deficient mice to determine the role of LFA-1, Mac-1, and alpha4 integrin in the inflammatory response of neutrophils. J Exp Med. 2001;194:219-226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 147] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 26. | Lee WY, Kubes P. Leukocyte adhesion in the liver: distinct adhesion paradigm from other organs. J Hepatol. 2008;48:504-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 27. | Craciun FL, Schuller ER, Remick DG. Early enhanced local neutrophil recruitment in peritonitis-induced sepsis improves bacterial clearance and survival. J Immunol. 2010;185:6930-6938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 28. | Walley KR, Lukacs NW, Standiford TJ, Strieter RM, Kunkel SL. Elevated levels of macrophage inflammatory protein 2 in severe murine peritonitis increase neutrophil recruitment and mortality. Infect Immun. 1997;65:3847-3851. [PubMed] |
| 29. | Scott MJ, Cheadle WG, Hoth JJ, Peyton JC, Subbarao K, Shao WH, Haribabu B. Leukotriene B4 receptor (BLT-1) modulates neutrophil influx into the peritoneum but not the lung and liver during surgically induced bacterial peritonitis in mice. Clin Diagn Lab Immunol. 2004;11:936-941. [PubMed] |
| 30. | Menezes GB, Lee WY, Zhou H, Waterhouse CC, Cara DC, Kubes P. Selective down-regulation of neutrophil Mac-1 in endotoxemic hepatic microcirculation via IL-10. J Immunol. 2009;183:7557-7568. [PubMed] |
| 31. | Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159-175. [PubMed] |
| 32. | Kadioglu A, De Filippo K, Bangert M, Fernandes VE, Richards L, Jones K, Andrew PW, Hogg N. The integrins Mac-1 and alpha4beta1 perform crucial roles in neutrophil and T cell recruitment to lungs during Streptococcus pneumoniae infection. J Immunol. 2011;186:5907-5915. [PubMed] |
| 33. | Devi S, Li A, Westhorpe CL, Lo CY, Abeynaike LD, Snelgrove SL, Hall P, Ooi JD, Sobey CG, Kitching AR. Multiphoton imaging reveals a new leukocyte recruitment paradigm in the glomerulus. Nat Med. 2013;19:107-112. [PubMed] |