Published online Jun 16, 2022. doi: 10.12998/wjcc.v10.i17.5861
Peer-review started: December 29, 2021
First decision: February 8, 2022
Revised: February 21, 2022
Accepted: April 22, 2022
Article in press: April 22, 2022
Published online: June 16, 2022
Although minimal change disease (MCD) and focal segmental glomerulosclerosis (FSGS) have been described as two separate forms of nephrotic syndrome (NS), they are not completely independent. We report a case of a patient transitioning from MCD to FSGS, review the literature, and explore the relationship between the two diseases.
A 42-year-old male welder, presenting with lower extremity edema and elevated serum creatinine, was diagnosed with NS and end-stage kidney disease (ESKD) based on laboratory test results. The patient had undergone a kidney biopsy for NS 20 years previously, which indicated MCD, and a second recent kidney biopsy suggested FSGS. The patient was an electric welder with excessive levels of cadmium and lead in his blood. Consequently, we suspect that his aggravated pathology and occurrence of ESKD were related to metal nephrotoxicity. The patient eventually received kidney replacement therapy and quit his job which involved long-term exposure to metals. During the 1-year follow-up period, the patient was negative for metal elements in the blood and urine and recovered partial kidney function.
MCD and FSGS may be different stages of the same disease. The transition from MCD to FSGS in this case indicates disease progression, which may be related to excessive metal contaminants caused by the patient’s occupation.
Core Tip: Minimal change disease (MCD) and focal segmental glomerulosclerosis (FSGS) are the two major forms of nephrotic syndrome. Although MCD and FSGS are defined as different types of primary glomerular disease, there is some overlap between them in clinical features and pathological changes. We present a rare case that highlights that MCD and FSGS are actually different histological manifestations of the same disease progression. FSGS may be MCD of advanced stage, and metal overexposure may be an important cause of disease progression.
- Citation: Tang L, Cai Z, Wang SX, Zhao WJ. Transition from minimal change disease to focal segmental glomerulosclerosis related to occupational exposure: A case report. World J Clin Cases 2022; 10(17): 5861-5868
- URL: https://www.wjgnet.com/2307-8960/full/v10/i17/5861.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i17.5861
Nephrotic syndrome (NS) is a common glomerulopathy characterized by nephrotic proteinuria, hypoproteinemia, edema, and hyperlipidemia. Most patients with NS have similar clinical manifestations, but their pathology can be different. Minimal change disease (MCD) and focal segmental glomerulosclerosis (FSGS) are the two major forms of NS. MCD is the most frequent glomerular disease leading to NS in childhood, accounting for 70%-90% of cases, and is an important cause of primary NS in adolescents, with the reported rates varying between 10% and 15% of NS patients[1]. Most MCD patients are responsive to corticosteroid therapy. Active treatment can achieve complete remission, and rarely result in progression to end-stage kidney disease (ESKD). Unlike MCD, more FSGS patients are resistant to corticosteroids, and NS due to FSGS can appear at any age. A failure to respond to corticosteroids in MCD patients may predict the presence of FSGS[2].
In most textbooks, MCD and FSGS are often described as two separate diseases based on their respective characteristics, histology and outcomes, but there is still considerable evidence that they are different manifestations of the same progressive disease[3]. Steroid-sensitive NS, which is associated with MCD, might progress to steroid-resistant NS, which is associated with FSGS. Some studies have proposed that they are actually different histological manifestations of the same disease progression, and some patients experience a transition from the initial state of a collapsed cytoskeleton (MCD) to the decompensated state of permanent podocyte loss and replacement with scar (FSGS)[4].
We report a patient with MCD who underwent a second kidney biopsy due to deterioration of renal function, and the result showed a change in pathological type from MCD to FSGS, which may be related to excessive metal exposure caused by the patient’s occupation. To the best of our knowledge, this is the first case report to demonstrate the transition from MCD to FSGS associated with lead and cadmium exposure.
A 42-year-old Asian male welder, was admitted to our hospital with bilateral lower extremity edema and elevated serum creatinine for 1 wk.
The patient’s symptoms started 1 wk ago with recurrent episodes of bilateral lower extremity edema. At the local hospital, he was found to have an elevated serum creatinine level of 1002 μmol/L, which had reached uremic levels.
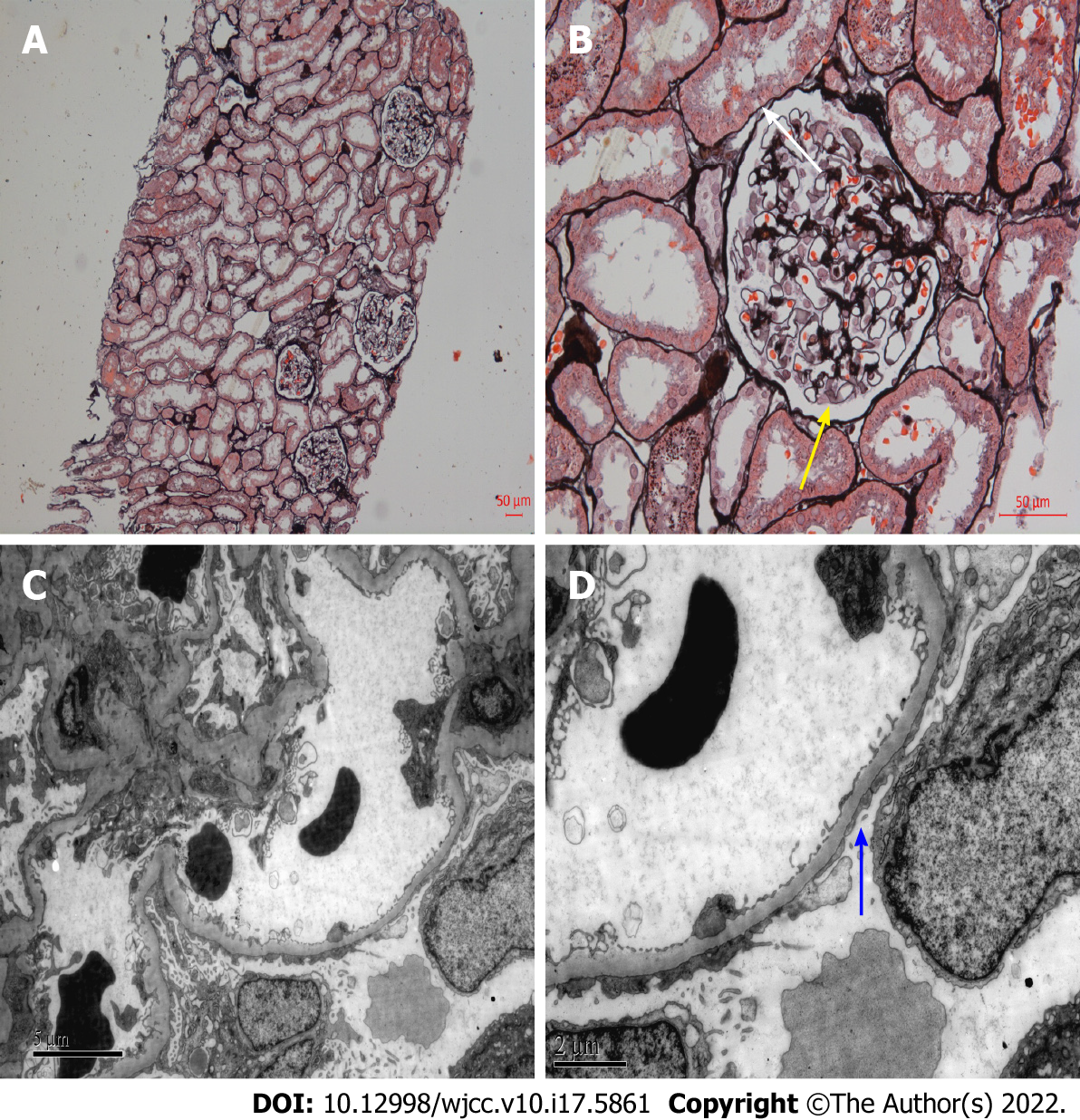
The patient had a history of MCD dating back 20 years, but no other relevant medical history. The patient was diagnosed with NS 20 years ago, and MCD was confirmed by kidney biopsy (Figure 1). At that time, the patient was regularly treated with corticosteroids. Prednisolone 40 mg/d was initially prescribed, which was gradually reduced and then stopped within 12 mo. The clinical symptoms of NS were completely relieved after treatment, and his proteinuria turned negative. The patient’s NS did not relapse within 1 year after glucocorticoid discontinuation. Subsequently, the patient did not regularly return visit, and routine urinalysis or kidney function was not monitored.
The patient had worked as a welder for about 10 years and had been frequently exposed to welding work containing cadmium and lead in the past 1 year. The patient denied having family history.
The patient’s vital signs upon admission were as follows: temperature, 36.5 °C; heart rate, 78 bpm; respiratory rate, 17 breaths/min; blood pressure, 150/90 mmHg, and oxygen saturation in room air, 99%. No evident abnormalities were found during a physical examination except for mild edema in both his lower extremities.
The results of laboratory tests showed urinalysis: Protein 3+, occult blood 3+, red blood cells 18-20/HP, glucose 3+, ketones -; 24-h urinary protein quantitation 10 920 mg/24 h; serum albumin 22.3 g/L; serum creatinine 1096 μmol/L; blood urea nitrogen 34.26 mmol/L; estimated glomerular filtration rate 4.4 mL/min/1.73 m2; blood cadmium 2.8 μg/L and blood lead 32.8 μg/L. Urine metal elements, plasma complement C3, C4, serum and urine free light chain, immunofixation electrophoresis, anti-GBM antibody, ANCA, anti-nuclear antibody series, serology for HBV, HCV, HIV, and PLA2R were all negative.
Nephrosonography revealed enhanced echogenic parenchyma without any significant kidney shrinkage, and his echocardiography, kidney artery ultrasound, and chest computed tomography were normal.
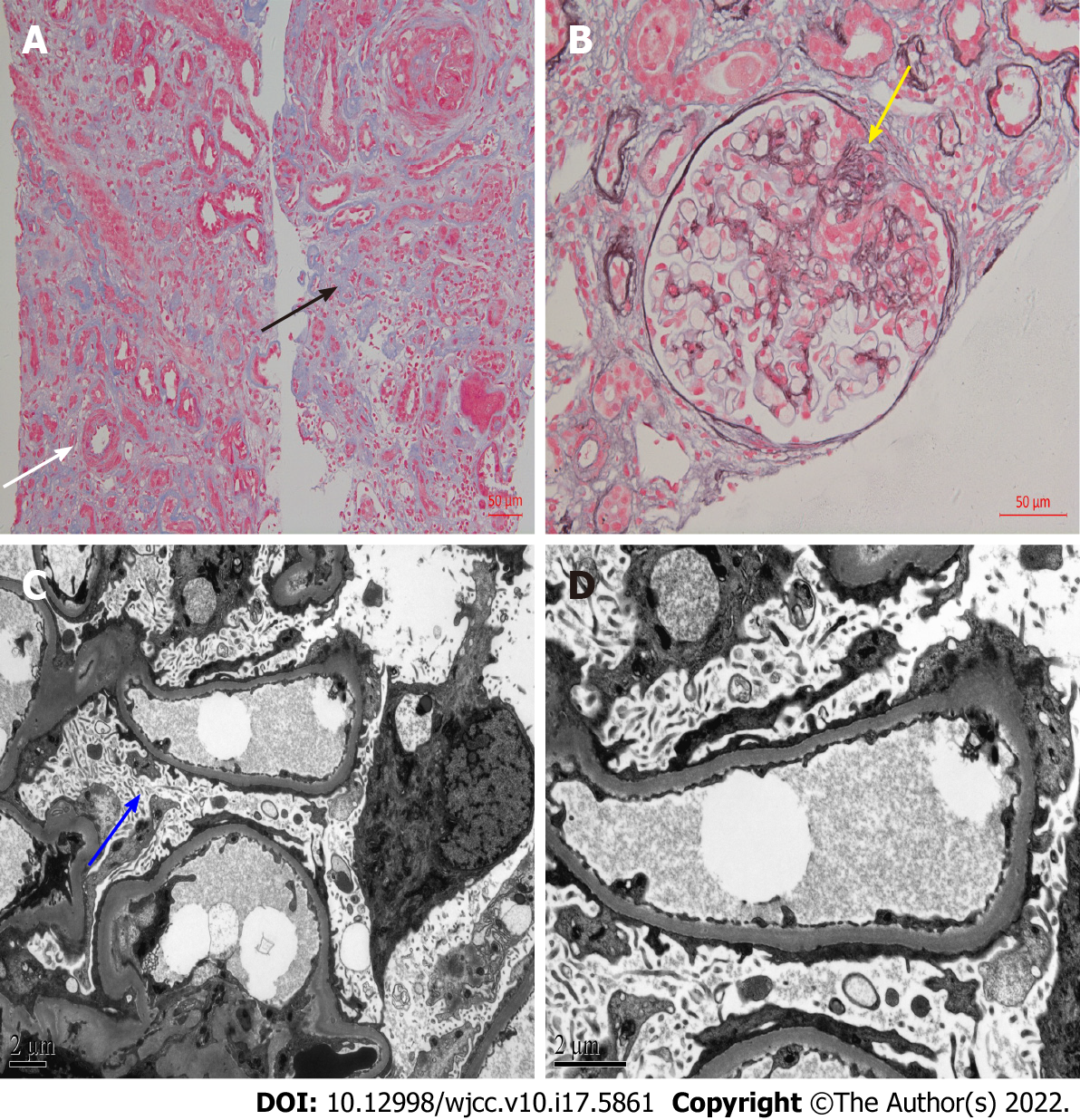
After obtaining consent from the patient, we performed another kidney biopsy and the results indicated FSGS (Figure 2).
The final diagnosis of the presented case was ESKD, FSGS-induced NS, and the pathological type transitioned from MCD to FSGS.
Our primary diagnosis was recurrent MCD-induced acute kidney injury (AKI). Considering that the patient had experienced an episode of worsening acute kidney failure, and the re-examination showed no recovery trend of serum creatinine, urgent hemodialysis was performed. After ward rounds and case discussions, the patient was recommended to try prednisolone acetate tablets orally at a dose of 30 mg/d. Other adjuvant therapies during dialysis included diuretics and traditional Chinese medicine. Although the use of oral prednisolone during hemodialysis is controversial, our aim was to help this patient alleviate MCD or AKI through aggressive treatment, and avoid ESKD or long-term dialysis. However, edema was relieved after 1 mo of treatment, but his kidney function did not significantly improve. The previous kidney biopsy of the patient showed MCD with a favorable prognosis, which was insufficient to explain the current ESKD. Therefore, we did another kidney biopsy and the results showed FSGS. Coupled with the kidney biopsy results, the patient eventually received continuous kidney replacement therapy with rapid corticosteroid reduction and withdrawal.
The patient resigned from his job after being discharged from the hospital, which discontinued his exposure to metal. During the regular 1-year follow-up period, the patient was negative for metal elements in the blood and urine and recovered partial kidney function (Table 1).
| Parameter | Time | |||||||
| 1 wk1 | 2 wk | 3 wk2 | 4 wk3 | 1 yr4 | ||||
| Urinalysis | ||||||||
| 24hUP (mg/24 h) | 10920 | 10320 | 11482.8 | - | 3320 | |||
| mALB/Cr (mg/mmol) | 866.95 | - | - | - | 339.06 | |||
| RBCs (cells/HP) | 18-20 | 8-12 | 7-10 | - | 1-2 | |||
| DysEry (%) | > 80 | > 75 | > 70 | - | - | |||
| Biochemical test | ||||||||
| CR (μmol/L) | Pre-HD | 1096 | 848.3 | 902.8 | 760 | 703 | 901.5 | 504.4 |
| Post-HD | 587.7 | 528.6 | 596 | |||||
| BUN (mmol/L) | Pre-HD | 34.26 | 22.27 | 22.8 | 14.64 | 13.48 | 23.11 | 15.38 |
| Post-HD | 18.1 | 13.82 | 10.88 | |||||
| ALB (g/L) | 22.3 | 21.2 | 22.4 | 22.7 | 36 | |||
| CHO (mmol/L) | 7.4 | - | 4.27 | - | 3.89 | |||
| TG (mmol/L) | 1.53 | - | 1.31 | - | 1.33 | |||
| Blood toxicity elements test | ||||||||
| Pb (μg/L) | 32.8 | - | - | - | 24.3 | |||
| Cd (μg/L) | 2.8 | - | - | - | 1.5 | |||
| As (μg/L) | 6.4 | - | - | - | 5.1 | |||
| Hg (μg/L) | 0.2 | - | - | - | < 0.1 | |||
| Tl (μg/L) | < 0.1 | - | - | - | 0 | |||
| Al (μg/L) | 48.1 | - | - | - | 30 | |||
| Mn (μg/L) | < 0.1 | - | - | - | < 0.1 | |||
Our patient underwent a kidney biopsy at the time of initial diagnosis of NS, which pathologically revealed MCD. MCD is considered to be a benign disease with a favorable long-term prognosis, which rarely progresses to ESKD. There was no glomerular injury detected under light microscopy, and only the foot process of podocytes disappeared under electron microscopy, but no podocytes loss[5]. Although the proportion of adult-onset MCD patients in NS is low, unlike children, adults are less responsive to corticosteroids and more prone to AKI. Moreover, steroid-resistant MCD patients may progress to ESKD, and these patients may be the missed FSGS patients. In contrast to MCD, patients with FSGS have a higher risk of corticosteroid resistance and kidney failure. Glomerular injury caused by FSGS leads to irreversible scar formation, which has a poor prognosis and eventually develops into ESKD. Irreversible glomerular damage caused in the context of FSGS can be explained by podocyte depletion. Compensatory hypertrophy of the remaining podocytes, cell-to-cell propagation of podocyte injury, and segmental solidification of the glomerular tuft can lead to progressive focal and segmental sclerosis[6].
As far as we know, cases of transition between MCD and FSGS are not rare in clinical practice, but few cases have been reported. Some cases have appeared in observational studies without proper in-depth analysis, and most of these studies involved children or adolescents[4,7,8]. In the above literature, most scholars placed FSGS at the onset of the disease. The focal and segmental nature of FSGS leads to sample error or diagnosis error, which may result in MCD misdiagnosis, or FSGS being missed, we agree, especially in patients with early lesions or limited glomeruli in the biopsy specimens. Early FSGS can only show the diffuse effacement of the foot process, which is consistent with MCD. There was no difference in the decrease of podocyte density labeled by WT1 between MCD and FSGS[9]. These factors sometimes result in confusion between the two diseases for clinicians. In fact, FSGS lesions may be absent in the early stages of NS, and the presence of FSGS lesions in the repeated biopsy tissue reflected the progression of MCD. The dose dependence of animal models supports the hypothesis that MCD and FSGS are two successive pathological processes of podocyte disease. Both models are based on the induction of podocyte injury and subsequent podocyte loss, and the difference depends on the degree of podocyte injury and the severity of podocyte loss. Only foot process of podocyte exfoliation similar to that in MCD is observed in the initial phase, while persistent podocyte loss results in the development of FSGS[10]. In the initial stages, this disease is steroid-sensitive. With relapses and delays, continuous proteinuria and podocyte loss lead to a decrease or loss of steroid sensitivity. When podocyte loss is more than 30%-40%, an ESKD outcome seems inevitable[3]. In this case, after MCD diagnosis, the patient was treated regularly with corticosteroid and achieved complete remission. Although there was no regular long-term follow-up, no serious relapses occurred, according to his records. A typical NS recurrence materialized 20 years later, at which time his clinical course and repeated kidney biopsy results both supported the FSGS diagnosis. In order to avoid misdiagnosis, we retrieved his kidney tissue sample (24 glomeruli) from 20 years ago for a pathological re-examination, and the results still supported the diagnosis of MCD. Therefore, we suggest that the patient progressed from MCD to FSGS, rather than FSGS being missed at the first diagnosis.
It is no coincidence that pathological changes occurred before and after the repeated kidney biopsy. FSGS is not a diagnosis of a specific disease, but a progressive glomerular pathological change caused by podocyte depletion. Most glomerular diseases eventually result in loss of renal function due to FSGS, and FSGS lesions can be described as an outcome of persistent damage in certain glomerular diseases or as a common endpoint event in some glomerular diseases[11]. MCD and FSGS are both podocyte diseases, and when FSGS is excluded from being missed or misdiagnosed as MCD, their sequential occurrence often suggests that FSGS is the progression of MCD[6]. The patient did not have regular follow-up visits when his NS was in remission, which was also a limitation of this case. Since recurrence of MCD is based on proteinuria, this patient most likely had an undetected recurrence of MCD. We hypothesized that the neglected recurrent MCD lead to persistent podocyte damage that ultimately caused FSGS, and heavy metal exposure is involved in exacerbating this process. Primary FSGS is usually caused by circulating factors, and the etiology of secondary FSGS includes infection, drugs, maladaptive responses, familial/genetic form, and variation of the APOL1 gene[12]. The patient did not have the above background, but a possible related factor was his occupation. The patient was an electric welder with excessive levels of cadmium and lead in his blood. Consequently, we suspect that his aggravated pathology and ESKD occurrence were related to metal nephrotoxicity. Nephrotoxicity induced by excess exposure to certain metals is well known, and lithium has been shown to cause MCD and FSGS[13]. Although there is not enough literature to confirm that lead and cadmium can cause FSGS, it has been proven that both of them induce podocytotoxicity and can even induce podocyte apoptosis[14,15]. Kidney exposure to cadmium and lead mainly causes proximal renal tubule dysfunction, acute exposure can lead to Fanconi syndrome, and long-term exposure leads to a persistent decline in kidney function[16,17]. This may explain the positive urine sugar content of the patient, and it may also be the reason for the occurrence of NS and ESKD in this patient. The levels of cadmium and lead in the blood of this patient were not that high compared to the normal range, but chronic, long-term exposure to low doses of the metal can cause kidney damage. Both lead and cadmium can increase the risk of chronic kidney disease, even at low levels (Blood lead < 5 mg/dL; Blood cadmium < 0.6 mg/L)[18,19]. The kidney damage caused by lead and cadmium is long-term and chronic, and combination of the two has a more profound nephrotoxicity. We hope to provide convincing evidence for the contention that lead and cadmium cause the transition from MCD to FSGS through future in-depth research.
Although, unfortunately, the patient ultimately required kidney replacement therapy, this case is still thought-provoking. MCD and FSGS are defined as different types of primary glomerular disease, but there is some overlap between them in clinical features and pathological changes. MCD and FSGS are both podocyte diseases, clinically presenting with sudden-onset NS, characterized by the absence of immune deposits under immunofluorescence[20]. However, their treatment response and prognosis are different. The differential diagnosis of MCD and FSGS is difficult, but it is important to distinguish between the two. A positive attitude towards second, or multiple kidney biopsies is needed for uncertain or recurrent MCD patients and early stage FSGS patients. In addition to invasive kidney puncture, urinary myo-inositol, parietal epithelial cell marker staining, and IgG/albumin staining ratio in tubular protein reabsorption droplets are also potential diagnostic markers to differentiate between MCD and FSGS[21-23].
We recognize through this rare case and a literature review that FSGS could be the later stage of MCD. FSGS lesions found in a repeated kidney biopsy can often reflect disease progression, and metal overexposure may be one of the possible reasons. Patients with MCD who have a slower and less effective response to steroid hormones should be alerted by clinicians to the risk of conversion to FSGS, or the possibility of coexistence with FSGS lesions. Due to its focal and segmental nature, we propose a higher requirement for nephrologists, at least to ensure the number of glomeruli.
We gratefully thank the patient for allowing us to publish this case report. We acknowledge technical assistance from members of the Pathological Centre, Peking University First Hospital.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Urology and nephrology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Trevino S, Mexico; Yorioka N, Japan S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Lionaki S, Mantios E, Tsoumbou I, Marinaki S, Makris G, Liapis G, Vergandis C, Boletis I. Clinical Characteristics and Outcomes of Adults with Nephrotic Syndrome Due to Minimal Change Disease. J Clin Med. 2021;10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Wang CS, Greenbaum LA. Nephrotic Syndrome. Pediatr Clin North Am. 2019;66:73-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 3. | Maas RJ, Deegens JK, Smeets B, Moeller MJ, Wetzels JF. Minimal change disease and idiopathic FSGS: manifestations of the same disease. Nat Rev Nephrol. 2016;12:768-776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 4. | Wieder N, Greka A. Calcium, TRPC channels, and regulation of the actin cytoskeleton in podocytes: towards a future of targeted therapies. Pediatr Nephrol. 2016;31:1047-1054. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Vivarelli M, Massella L, Ruggiero B, Emma F. Minimal Change Disease. Clin J Am Soc Nephrol. 2017;12:332-345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 280] [Cited by in F6Publishing: 276] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 6. | Rosenberg AZ, Kopp JB. Focal Segmental Glomerulosclerosis. Clin J Am Soc Nephrol. 2017;12:502-517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 342] [Cited by in F6Publishing: 299] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 7. | Ahmad H, Tejani A. Predictive value of repeat renal biopsies in children with nephrotic syndrome. Nephron. 2000;84:342-346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Maas RJ, Deegens JK, Beukhof JR, Reichert LJ, Ten Dam MA, Beutler JJ, van den Wall Bake AWL, Rensma PL, Konings CJ, Geerse DA, Feith GW, Van Kuijk WH, Wetzels JF. The Clinical Course of Minimal Change Nephrotic Syndrome With Onset in Adulthood or Late Adolescence: A Case Series. Am J Kidney Dis. 2017;69:637-646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | da Silva CA, Monteiro MLGDR, Araújo LS, Urzedo MG, Rocha LB, Dos Reis MA, Machado JR. In situ evaluation of podocytes in patients with focal segmental glomerulosclerosis and minimal change disease. PLoS One. 2020;15:e0241745. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Pippin JW, Brinkkoetter PT, Cormack-Aboud FC, Durvasula RV, Hauser PV, Kowalewska J, Krofft RD, Logar CM, Marshall CB, Ohse T, Shankland SJ. Inducible rodent models of acquired podocyte diseases. Am J Physiol Renal Physiol. 2009;296:F213-F229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 198] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 11. | Müller-Deile J, Schenk H, Schiffer M. [Minimal change disease and focal segmental glomerulosclerosis]. Internist (Berl). 2019;60:450-457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Shabaka A, Tato Ribera A, Fernández-Juárez G. Focal Segmental Glomerulosclerosis: State-of-the-Art and Clinical Perspective. Nephron. 2020;144:413-427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 13. | Łukawska E, Frankiewicz D, Izak M, Woźniak A, Dworacki G, Niemir ZI. Lithium toxicity and the kidney with special focus on nephrotic syndrome associated with the acute kidney injury: A case-based systematic analysis. J Appl Toxicol. 2021;41:1896-1909. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Eichler TE, Ransom RF, Smoyer WE. Differential induction of podocyte heat shock proteins by prolonged single and combination toxic metal exposure. Toxicol Sci. 2005;84:120-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Eichler T, Ma Q, Kelly C, Mishra J, Parikh S, Ransom RF, Devarajan P, Smoyer WE. Single and combination toxic metal exposures induce apoptosis in cultured murine podocytes exclusively via the extrinsic caspase 8 pathway. Toxicol Sci. 2006;90:392-399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Satarug S, C Gobe G, A Vesey D, Phelps KR. Cadmium and Lead Exposure, Nephrotoxicity, and Mortality. Toxics. 2020;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 17. | Jalili C, Kazemi M, Cheng H, Mohammadi H, Babaei A, Taheri E, Moradi S. Associations between exposure to heavy metals and the risk of chronic kidney disease: a systematic review and meta-analysis. Crit Rev Toxicol. 2021;51:165-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 18. | Nakhaee S, Amirabadizadeh A, Brent J, Mehrpour O. Impact of chronic lead exposure on liver and kidney function and haematologic parameters. Basic Clin Pharmacol Toxicol. 2019;124:621-628. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Madrigal JM, Ricardo AC, Persky V, Turyk M. Associations between blood cadmium concentration and kidney function in the U.S. population: Impact of sex, diabetes and hypertension. Environ Res. 2019;169:180-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 20. | Ahn W, Bomback AS. Approach to Diagnosis and Management of Primary Glomerular Diseases Due to Podocytopathies in Adults: Core Curriculum 2020. Am J Kidney Dis. 2020;75:955-964. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 21. | An JN, Hyeon JS, Jung Y, Choi YW, Kim JH, Yang SH, Oh S, Kwon S, Lee SH, Cho JH, Park SH, Ha H, Kim DK, Lee JP, Hwang GS. Urinary myo-inositol is associated with the clinical outcome in focal segmental glomerulosclerosis. Sci Rep. 2019;9:14707. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Suzuki T, Kohatsu K, Han W, Watanabe S, Yahagi K, Nakata M, Ueno T, Ichikawa D, Imai N, Shirai S, Koike J, Shibagaki Y. Morphological Features of Minimal Change Disease and Focal Segmental Glomerulosclerosis Using Repeat Biopsy and Parietal Epithelial Cell Marker. Kidney Dis (Basel). 2020;6:119-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Bu L, Mirocha J, Haas M. Immunoglobulin G/albumin staining in tubular protein reabsorption droplets in minimal change disease and focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2021;36:1016-1022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |