Published online Aug 12, 2016. doi: 10.5318/wjo.v6.i3.20
Peer-review started: May 26, 2016
First decision: July 5, 2016
Revised: July 29, 2016
Accepted: August 7, 2016
Article in press: August 8, 2016
Published online: August 12, 2016
To study activation of extracellular signal-regulated kinase-1/2 (ERK1/2) and pro-matrix metalloproteinases (pro-MMPs) secretion from isolated primary human ciliary muscle (h-CM) cells in response to bradykinin (BK) and other agonists.
Serum-starved h-CM cells were challenged with vehicle, BK agonists or antagonists. Cell lysates were evaluated for phosphorylated ERK1/2 using homogeneous time-resolved fluorescence technology based on a sandwich immunoassay. Rabbit polyclonal anti-pro-MMP antibodies were used to measure pro-MMPs using immunoblot analysis.
A 10 min incubation time using 5 × 104 h-CM cells/well was optimum condition for studying stimulation of ERK1/2 phosphorylation. BK (100 nmol/L) caused a 1.86 ± 0.26 fold (n = 3) increase in ERK1/2 phosphorylation above baseline. BK analogs, Met-Lys-BK and RMP-7 (100 nmol/L), also stimulated ERK1/2 phosphorylation by 1.57 ± 0.04 and 1.55 ± 0.09 fold, respectively. However, Des-Arg9-Bradykinin, a B1 receptor-selective agonist (0.1-1 μmol/L), was essentially inactive. HOE-140 or WIN-64338 (B2-antagonists) appreciably blocked phosphorylation of ERK1/2 induced by various BK agonists. Pre-treatment of cells with a prostaglandin (PG) synthase inhibitor (bromfenac; 1 μmol/L) failed to alter kinin-induced ERK1/2 activation. BK and a non-peptide BK agonist (FR-190997) (10 nmol/L-1 μmol/L) also enhanced pro-MMPs secretion (pro-MMP-1 > pro-MMP-3 > pro-MMP-2; 1.45-1.75-fold over baseline) from h-CM cells.
These collective data suggest that B2 kinin receptors initiate signaling in h-CM cells by a relatively rapid mechanism (within minutes) involving ERK1/2 activation which in turn regulates MMPs production (within hours). The latter process does not involve PGs.
Core tip: Bradykinin (BK), its peptide and non-peptide analogs and mimetic were potently and efficaciously able to stimulate extracellular signal-regulated kinase 1/2 phosphorylation in human ciliary muscle (h-CM) cells in vitro. Additionally, these agonists also induced the production and secretion of pro-matrix metalloproteinases in a prostaglandin-independent manner. Selective antagonists helped link these responses to be mediated via the B2-receptor sub-type in h-CM cells. These mechanistic studies show how BK can lower intraocular pressure in animals when delivered to the eye.
- Citation: Sharif NA, Patil R, Li L, Husain S. Human ciliary muscle cell responses to kinins: Activation of ERK1/2 and pro-matrix metalloproteinases secretion. World J Ophthalmol 2016; 6(3): 20-27
- URL: https://www.wjgnet.com/2218-6239/full/v6/i3/20.htm
- DOI: https://dx.doi.org/10.5318/wjo.v6.i3.20
Bradykinin (BK) and Lys-BK are generated from precursor polypeptides (kininogens) by the proteolytic actions of the enzymes kallikreins[1,2]. BK, its synthetic components and constituents, and its signal-transducing receptor proteins (B1- and B2-receptors) are all present in the mammalian eye, including in the ciliary muscle, ciliary epithelium, trabecular meshwork, and the retina[3-7]. Whilst there has been confusion about the physiological and pathological roles of BK in ocular function, in particular related to elevation or reduction of intraocular pressure (IOP)[8-15], recent studies have clearly demonstrated that BK has some beneficial effects in the anterior chamber. Thus, BK and two non-peptide mimics of BK (FR-190997 and BKA278), cause IOP lowering in rabbits, mice and Cynomolgus monkeys when delivered intravitreally and/or topical ocularly[16-18]. Additionally, BK and FR-190997 cause the IOP reduction by increasing uveoscleral outflow in living monkey eyes[17] and by conventional outflow in isolated perfused bovine eye anterior chambers[16-19].
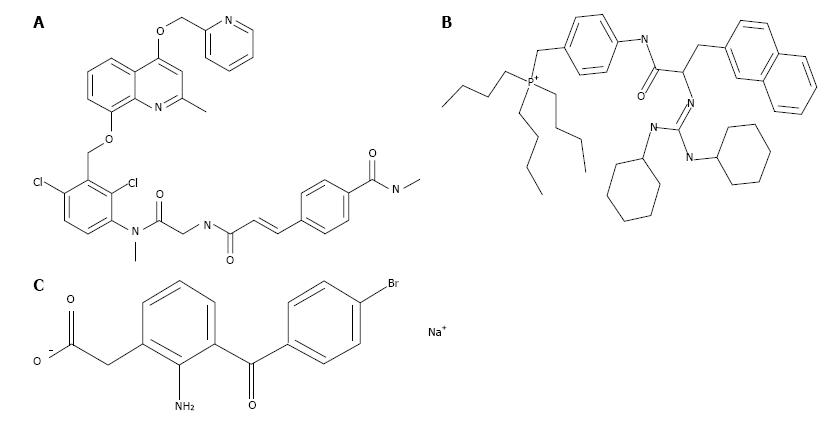
The cellular and molecular pathways activated by BK B2-receptors involve phosphoinositide hydrolysis, intracellular Ca2+-mobilization, and prostaglandin (PG) release[16-19]. However, the elements linking these signal transduction pathways in the Human ciliary smooth muscle (h-CM) cells to IOP reduction have not been reported to our knowledge. Therefore, we embarked on the current studies in order to investigate the possible stimulation of extracellular signal-regulated kinase 1/2 (ERK1/2) and pro-MMPs secretion from isolated h-CM cells in response to BK and FR-190997. We utilized a number of BK-related peptides, a non-peptide BK agonist, two receptor-specific antagonists (Figure 1) to delineate the receptor-subtype mediating some of the actions of BK in h-CM cells in these mechanistic studies. In addition, we used a PG synthase inhibitor [bromfenac (BF); cyclooxygenase inhibitor; Figure 1] in order to determine whether PGs were involved in mediating the effects of BK agonists on ERK1/2 activation. A preliminary account of these studies was presented at a meeting of Association for Research in Vision and Ophthalmology (ARVO)[20].
h-CM cells were prepared from normal human cadaveric eyes using the procedure previously described earlier[21-23]. In brief, human eyes were obtained from NDRI (Philadelphia, PA). Ciliary muscles were dissected with the aid of a dissecting microscope under sterile conditions, cleaned, and cut into 1-2 mm pieces. The explants were placed in DMEM containing 2 mg/mL collagenase type IA, 10% fetal bovine serum (FBS), and 50 μg/mL gentamicin and then incubated for 1-2 h at 37 °C with occasional shaking. When a major part of the explant was dispersed into single cells or groups of cells, the cell suspension was centrifuged at 200 g for 10 min and re-suspended in DMEM supplemented with 10% FBS, 100 U/mL penicillin G, 100 μg/mL streptomycin, and 0.25 μg/mL amphotericin B and maintained in a 5% CO2 humidified atmosphere. The confluent cells were sub-cultured at a split ratio of 1:4 using 0.05% trypsin and 0.02% EDTA. Cells of passage number 2-8 were used in the current studies obtained from numerous human donor eyes.
h-CM cells were initially seeded at different densities ranging from 1 × 104 to 1 × 105 and grown over 2 d in 96-well plates to 80%-90% confluence. Using cells at this confluence level prevented any potential contact inhibition, and thus the responses to pharmacological agents were not confounded by the latter factor. Once the optimum number of cells/well was determined, all subsequent experiments utilized 5 × 104 cells/well. Cells were then starved off serum overnight before being challenged with BK agonists or antagonists (Figure 1) for various times and with different ligands at various concentrations. Cells were lysed using lysis buffer provided in the Cellul’erk kit from CisBio (Bedford, MA) for direct detection of phosphorylated ERK1/2 at room temperature with shaking for 30 min after the ligand treatment. The cell lysates then received homogeneous time-resolved fluorescence (HTRF) conjugates containing phospho-ERK1/2 antibodies and the incubation continued for 2 additional hours at 23 °C. Phosphorylated ERK1/2 was then detected using an anti-phospho-ERK1/2 antibody labeled with d2 and an anti-ERK1/2 antibody labeled with Eu3+-Cryptate using HTRF technology based on a sandwich immunoassay. Recording of the fluorescence signals was performed at 620 nm for the donor and 665 nm for the acceptor[20]. A 10-min incubation with agonist ligands using 5 × 104 cells was found to be optimum for such studies. B2-receptor antagonists (HOE-140; WIN-64338) or an enzyme inhibitor (BF; PG synthase inhibitor) (Figure 1), when used, were added to the cells 15 min prior to the agonist compound.
For these experiments, 12 h-serum-starved (almost confluent) h-CM cells were incubated with the test compound or buffer vehicle for 6 h. This time-point was chosen based on the peak IOP-reduction observed following intravitreal injection of BK[16]. Centricon concentrators (10-kDa cutoff; Centricon-10; Amicon Beverly, MA) were them used to concentrate the incubation medium (10-fold) from each well and adjusted to a 10:1 concentration. The concentrated incubation media (40 μL) were then loaded on SDS-polyacrylamide (10%) gels. This was followed thereafter by transfer to nitrocellulose membranes. The latter were first blocked with non-fat dry milk (5%) (Biorad, Hercules, CA), and then incubated with specific antibodies for pro-MMP-1 (Dilution at 1:1000; Cat# AV42039; Sigma-Aldrich, St. Louis, MO), pro-MMP-2 (Dilution at 1:1000; Cat# 1M-33, Calbiochem, Billerica, MA), or pro-MMP-3 (Dilution at 1:1000; Cat# Ab2963; Millipore, Billerica, MA) (12 h with gentle shaking at 4 °C). Synthetic peptides directed towards N-termini of human pro-MMP-1, pro-MMP-2, or pro-MMP-3 were used by the manufacturers to generate all the antibodies for pro-MMPs. Appropriate secondary antibodies (HRP-conjugated; dilution 1:3000) were incubated with the membranes for 1 h at 20 °C. Standard molecular-weight markers and pre-stained proteins were used in the same experiments for comparisons. At the appropriate time, these membranes were treated with enhanced chemiluminescent reagent (Amersham Pharmacia, Piscataway, NJ) and a Biorad Versadoc imaging system (Biorad, Hercules, CA) utilized to monitor the chemiluminescent signal[21,22]. After this, quantitative densitometry was used to quantify the band intensities followed by normalization with cellular protein for each respective band. This process was followed to account for potential differences in cell densities/well. Student’s t-test was used to determine potential statistical differences between all treatments, with P < 0.05 being the minimally acceptable level of statistical significance.
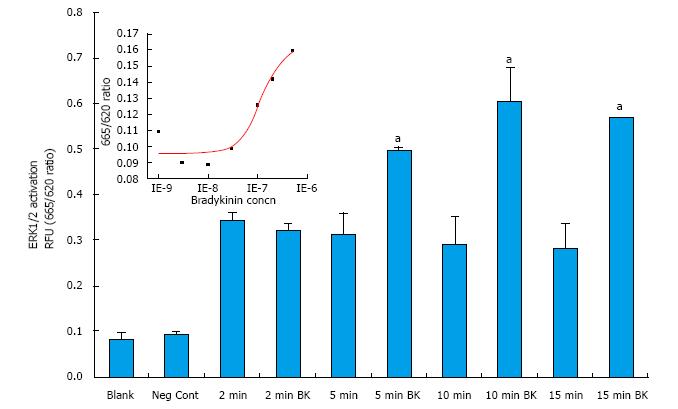
Initial studies focused on determining the optimal conditions of ERK1/2 phosphorylation in primary h-CM cells. In response to BK (100 nmol/L), h-CM ERK1/2 phosphorylation was induced in a time (2-15 min)-dependent manner, being highest at 10-15 min relative to the basal levels (Figure 2). All subsequent experiments utilized 5 × 104 cells/well and a 10 min incubation with the agonist compound. When antagonists or an enzyme inhibitor were tested, they were added to the cells 15 min prior to agonist exposure.
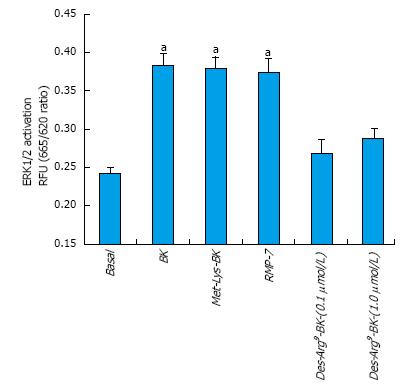
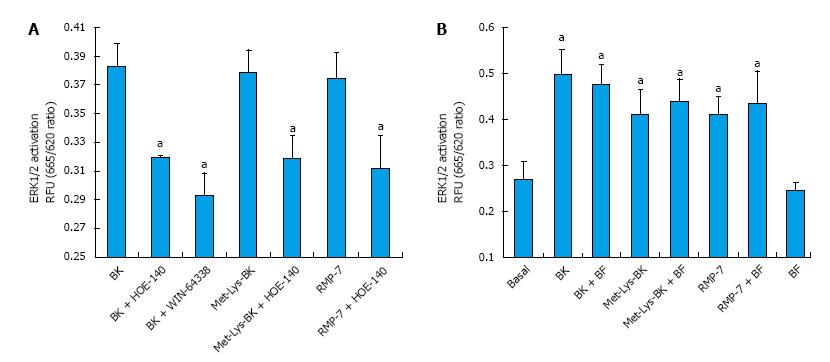
BK concentration-dependently stimulated ERK1/2 phosphorylation in h-CM cells with a minimum at 3 nmol/L and a maximum at 1 µmol/L (Figure 2 inset). Such concentration-response studied yielded half-maximal responses (EC50s; efficacy) at 20 nmol/L and 80 nmol/L, from two independent experiments. While 100 nmol/L of the B2-receptor agonists, BK, Met-Lys-BK, and RMP-7 (a metabolically stabile analog of BK) stimulated ERK1/2 phosphorylation to approximately the same degree (1.6-fold above basal), the B1-agonist, Des-Arg9-BK (0.1-1 μmol/L) was much weaker at stimulating ERK1/2 phosphorylation in the same experiments (Figure 3). In the presence of the B2-receptor antagonists, WIN-64338 and HOE-140 (both at 100 nmol/L), ERK1/2 phosphorylation was significantly reduced for the afore-mentioned B2-agonists (Figure 4A). However, pretreatment of h-CM cells with the PG synthase inhibitor BF (1 μmol/L) failed to effect the ERK1/2 phosphorylation induced by BK, Met-Lys-BK or RMP-7 (Figure 4B). BF by itself was also inactive (Figure 4B).
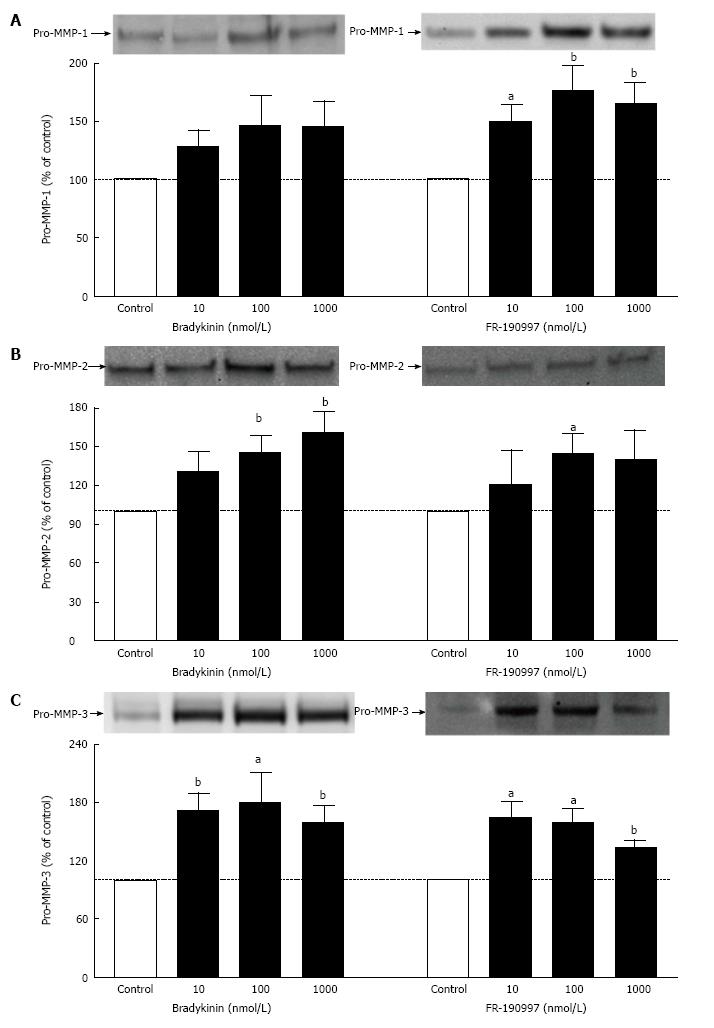
BK and FR-190997 (10 nmol/L-1 μmol/L) stimulated the production/secretion of pro-MMPs from h-CM cells but in a differential manner (pro-MMP-1 > pro-MMP-3 > pro-MMP-2) (Figure 5). However, while the FR-190997 and BK effects were similar on pro-MMP-3 and pro-MMP-2 secretion (Figure 5B and C), BK was a little less efficacious than FR-190997 at stimulating pro-MMP-1 (Figure 5A). However, these differences were statistically insignificant. In additional experiments, the latter agonists also enhanced pro-MMP-2 and -3 secretion (1.4-1.7-fold increase above baseline) from h-CM cells after 24 h incubation with the cells (data not shown). While a minor limitation of the current studies is that active forms of MMP-1-3 were not studied, previous work from our research has demonstrated a closely linked process of pro-MMP secretion and their conversion to the active species in human ocular cells[19,21].
Under normal physiological conditions BK primarily activates B2-receptors to mediate its biological effects in the mammalian body[1,2]. Various tissues and cells of the mammalian eye also respond this way as shown using a variety of cell- and tissue-based assays and models[4-7,16-20]. With respect to the actions of BK in h-CM cells, we have recently reported the existence of B2-receptor protein and its activation leading to generation of various intracellular second messengers such as inositol phosphates (IPs) and intracellular Ca2+ whose elevation then causes the release of various PGs (mainly PGE2 and PGF2α)[16-18]. In the current studies in h-CM cells we have extended those observations by demonstrating the relatively fast phosphorylation of ERK1/2 in response to BK and its close analogs, Met-Lys-BK and the stabilized BK-mimetic RMP-7. Consistent with only the stimulation of the B2-receptor, it was evident that the B1-receptor agonist Des-Arg9-BK lacked activity in this regard, and indeed two B2-receptor-selective antagonists, HOE-140 and WIN-64338, blocked the effects of BK on ERK1/2 phosphorylation. Since a PG synthase inhibitor, BF, failed to reduce BK, Met-Lys-BK and RMP-7-induced ERK1/2 activation in h-CM cells, it appears that ERK1/2 phosphorylation occurs directly and is not mediated indirectly by PGs, at least within the first 10 min of receptor activation in vitro. Further down-stream from B2-receptor activation by BK or a non-peptide BK mimetic agonist, FR-190997, is for instance the generation of pro-MMPs from h-CM cells. This is a time-dependent process that apparently requires several hours. Accordingly, both BK and FR-190997 up-regulated the production of pro-MMP-1-3 in h-CM cells to a similar extent after the agonists were incubated with h-CM cells. BK has also been shown to stimulate ERK1/2 phosphorylation in isolated human trabecular meshwork (h-TM) cells followed by release of MMP-9[24]. Thus, similar series of events ensue following B2-receptor activation by BK in h-CM and h-TM cells. A unifying signal transduction pathway applicable to the h-CM and h-TM cell responses to kinins appears to be as follows: BK, or its stabilized-mimetic (RMP-7) or a synthetic non-peptide analog (FR-190997), bind to the B2-receptor with high affinity that induces the G-protein (Gq) that couples to the receptor and activates phospholipase C which then hydrolyzes membrane-bound phospholipids to liberate IPs[25] and diacyl glycerol (DAG) into the cellular cytoplasm. IPs then liberate intracellular Ca2+ from the endoplasmic reticulum[15-18] while DAG activates protein kinase C which then activates ERK1/2 via phosphorylation by a mitogen-activated protein kinase[21]. These events are followed by specific induction and release of pro-MMPs into the extracellular space where they are activated. Digestion of extracellular matrix by MMPs[26] leads to the creation of several gaps between bundles of ciliary muscle and scleral tissues, and perhaps within the TM, to promote outflow of aqueous humor that then causes reduction in the IOP[17-19]. Accordingly, FR-190997 perfused into bovine anterior segments increased outflow of fluid, starting within an hour and reaching an apparent plateau around 4 h of perfusion[17]. It is interesting to note that similar findings were also reported for BK when it was perfused in the same model[19]. In a correlative manner, intravitreally delivered BK in Dutch-belt rabbits has been demonstrated to lower IOP starting around 4-6 h after injection and peaking at 8 h post-injection[16]. Both these events require several hours to be fully operational thereby correlating with the time-course of pro-MMPs production/secretion induced by BK and FR-190997 from h-CM cells (our current study; and from h-TM cells[24]), and stimulation of outflow of fluid in the perfused anterior eye segment model mentioned above[17-19].
These collective data provide further evidence for the involvement of B2-receptors in h-CM cells mediating ERK1/2 activation and pro-MMP-1-3 secretion in response to BK and other B2-receptor agonists. This information correlates well with the time-course of activity of intravitreally injected BK causing IOP-reduction in rabbits[16], without the involvement of B1-receptors[17-19,24].
Prior studies in human ciliary muscle (h-CM) cells revealed that activation of bradykinin (BK) B2-receptors results in generation of two second messengers (inositol phosphates, Ca2+) that then cause prostaglandin E2 and other prostaglandins (PGs) secretion. The net result of such events in vivo, for instance when BK is injected into the posterior eye segment of rabbits, is the stimulation of aqueous humor outflow from the front of the eye to cause a reduction in intraocular pressure (IOP). However, The authors envisaged that there may be other key biochemical steps involved after B2-receptors activation in h-CM cells, and thus embarked on unravelling these potential pathways.
The authors discovered that indeed, BK and its close peptide and non-peptide analogs/mimetic, stimulate extracellular signal-regulated kinase 1/2 (ERK1/2) and promote secretion of pro-matrix metalloproteinases (pro-MMPs) from isolated h-CM cells. The B2-receptor involvement in these responses was confirmed utilizing two selective receptor antagonists. The authors also showed that PGs were not involved in such processes since a PG-synthase inhibitor did not influence those responses to BK.
The innovative portion of the studies involved utilizing classical pharmacological tools coupled with modern cellular and molecular biochemistry to delineate the early-stage and late-phase actions of BK in h-CM cells in vitro. These studies may help explain the time-course of activity of this endogenous peptide in causing and controlling IOP reduction in vivo when it is delivered to the inside of the eye.
It is hoped that other researchers would be encouraged and excited by the use of such multidisciplinary techniques, tools and technologies, and the overall results, to undertake additional mechanistic studies. Additional exploration of similar mechanisms in other ocular cells involved in aqueous humor dynamics, including non-pigmented ciliary epithelial cells, scleral fibroblasts and other scleral tissue-derived cells, and Schlemm’s canal cells would be very informative.
Since some readers may not be so familiar with various terms and abbreviations used in the current paper, a list of the most commonly used terms is provided below. IOP is controlled by the rate of aqueous humor (AQH) formation by the ciliary processes and its drainage from the anterior chamber of the eye. Since IOP is a major risk factor for glaucoma, it is important to learn what and how it can be modulated to help preserve vision. AQH: Aqueous humor; BK: Bradykinin; EC50: Concentration of agonist that produces half-maximal response; ERK1/2: Extracellular signal-regulated kinase-1/2; h-CM: Human ciliary muscle; IOP: Intraocular pressure; PG: Prostaglandin; pro-MMPs: Pro-matrix metalloproteinases.
The manuscript is a mechanistic study describing the molecular sequences by which activated bradykinin receptors initiate a rapid signalling cascade through ERK1/2 which in turn activate the MMP-1, -2 and -3 production leading to subsequent events (previously published by the authors). The topic of study is good.
Manuscript source: Unsolicited manuscript
Specialty type: Ophthalmology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Felix K, Fang Y, Soriano-Ursua MA S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Bhoola KD, Figueroa CD, Worthy K. Bioregulation of kinins: kallikreins, kininogens, and kininases. Pharmacol Rev. 1992;44:1-80. [PubMed] [Cited in This Article: ] |
| 2. | Leeb-Lundberg LM, Marceau F, Müller-Esterl W, Pettibone DJ, Zuraw BL. International union of pharmacology. XLV. Classification of the kinin receptor family: from molecular mechanisms to pathophysiological consequences. Pharmacol Rev. 2005;57:27-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 732] [Cited by in F6Publishing: 713] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 3. | Igić R. Kallikrein and kininases in ocular tissues. Exp Eye Res. 1985;41:117-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Ma JX, Song Q, Hatcher HC, Crouch RK, Chao L, Chao J. Expression and cellular localization of the kallikrein-kinin system in human ocular tissues. Exp Eye Res. 1996;63:19-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 71] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Crook RB, Polansky JR. Neurotransmitters and neuropeptides stimulate inositol phosphates and intracellular calcium in cultured human nonpigmented ciliary epithelium. Invest Ophthalmol Vis Sci. 1992;33:1706-1716. [PubMed] [Cited in This Article: ] |
| 6. | Wiernas TK, Davis TL, Griffin BW, Sharif NA. Effects of bradykinin on signal transduction, cell proliferation, and cytokine, prostaglandin E2 and collagenase-1 release from human corneal epithelial cells. Br J Pharmacol. 1998;123:1127-1137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Webb JG, Yang X, Crosson CE. Expression of the kallikrein/kinin system in human anterior segment. Exp Eye Res. 2009;89:126-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Zeller EA, Shoch D, Czerner TB, Hsu MY, Knepper PA. Enzymology of the refractory media of the eye. X. Effects of topically administered bradykinin, amine releasers, and pargyline on aqueous humor dynamics. Invest Ophthalmol. 1971;10:274-281. [PubMed] [Cited in This Article: ] |
| 9. | Chiang TS. Effects of intravenous infusions of histamine 5-hydroxytryptamine, bradykinin and prostaglandins on intraocular pressure. Arch Int Pharmacodyn Ther. 1974;207:131-138. [PubMed] [Cited in This Article: ] |
| 10. | Cole DF, Unger WG. Action of bradykinin on intraocular pressure and papillary diameter. Ophthalmol Res. 1974;6:308-314. [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Green K, Elijah D. Drug effects on aqueous humor formation and pseudofacility in normal rabbit eyes. Exp Eye Res. 1981;33:239-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Kaufman PL, Bárány EH, Erickson KA. Effect of serotonin, histamine and bradykinin on outflow facility following ciliary muscle retrodisplacement in the cynomolgus monkey. Exp Eye Res. 1982;35:191-199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 13. | Bynke G, Håkanson R, Hörig J, Leander S. Bradykinin contracts the pupillary sphincter and evokes ocular inflammation through release of neuronal substance P. Eur J Pharmacol. 1983;91:469-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Yokoyama K, Awaya S, Mizumura K, Kumazawa T. Implication of polymodal receptor activities in intraocular pressure elevation by neurogenic inflammation. Jpn J Ophthalmol. 1990;34:245-255. [PubMed] [Cited in This Article: ] |
| 15. | Llobet A, Gual A, Palés J, Barraquer R, Tobías E, Nicolás JM. Bradykinin decreases outflow facility in perfused anterior segments and induces shape changes in passaged BTM cells in vitro. Invest Ophthalmol Vis Sci. 1999;40:113-125. [PubMed] [Cited in This Article: ] |
| 16. | Sharif NA, Xu S, Li L, Katoli P, Kelly CR, Wang Y, Cao S, Patil R, Husain S, Klekar L. Protein expression, biochemical pharmacology of signal transduction, and relation to intraocular pressure modulation by bradykinin B2 receptors in ciliary muscle. Mol Vis. 2013;19:1356-1370. [PubMed] [Cited in This Article: ] |
| 17. | Sharif NA, Katoli P, Scott D, Li L, Kelly C, Xu S, Husain S, Toris C, Crosson C. FR-190997, a nonpeptide bradykinin B2-receptor partial agonist, is a potent and efficacious intraocular pressure lowering agent in ocular hypertensive cynomolgus monkeys. Drug Dev Res. 2014;75:211-223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Sharif NA, Li L, Katoli P, Xu S, Veltman J, Li B, Scott D, Wax M, Gallar J, Acosta C. Preclinical pharmacology, ocular tolerability and ocular hypotensive efficacy of a novel non-peptide bradykinin mimetic small molecule. Exp Eye Res. 2014;128:170-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Webb JG, Husain S, Yates PW, Crosson CE. Kinin modulation of conventional outflow facility in the bovine eye. J Ocul Pharmacol Ther. 2006;22:310-316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 20. | Patil R, Li L, Sharif NA. Activation of ERK signaling pathway by bradykinin B2-receptors in isolated human primary ciliary muscle cells. Invest Ophthalmol Vis Sci. 2012;ARVO Abst. # 1971. [Cited in This Article: ] |
| 21. | Husain S, Jafri F, Crosson CE. Acute effects of PGF2alpha on MMP-2 secretion from human ciliary muscle cells: a PKC- and ERK-dependent process. Invest Ophthalmol Vis Sci. 2005;46:1706-1713. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Sharif NA, McLaughlin MA, Kelly CR, Katoli P, Drace C, Husain S, Crosson C, Toris C, Zhan GL, Camras C. Cabergoline: Pharmacology, ocular hypotensive studies in multiple species, and aqueous humor dynamic modulation in the Cynomolgus monkey eyes. Exp Eye Res. 2009;88:386-397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Sharif NA, Kelly CR, Crider JY, Davis TL. Serotonin-2 (5-HT2) receptor-mediated signal transduction in human ciliary muscle cells: role in ocular hypotension. J Ocul Pharmacol Ther. 2006;22:389-401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Webb JG, Yang X, Crosson CE. Bradykinin activation of extracellular signal-regulated kinases in human trabecular meshwork cells. Exp Eye Res. 2011;92:495-501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Sharif NA, Xu SX. Pharmacological characterization of bradykinin receptors coupled to phosphoinositide turnover in SV40-immortalized human trabecular meshwork cells. Exp Eye Res. 1996;63:631-637. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Weinreb RN, Kashiwagi K, Kashiwagi F, Tsukahara S, Lindsey JD. Prostaglandins increase matrix metalloproteinase release from human ciliary smooth muscle cells. Invest Ophthalmol Vis Sci. 1997;38:2772-2780. [PubMed] [Cited in This Article: ] |