Published online May 15, 2017. doi: 10.4291/wjgp.v8.i2.67
Peer-review started: September 1, 2016
First decision: October 31, 2016
Revised: December 1, 2016
Accepted: February 28, 2017
Article in press: March 2, 2017
Published online: May 15, 2017
Processing time: 260 Days and 16.5 Hours
To investigate the synergistic hepato-protective properties of curcumin and vitamin E in an Hfe-/- high calorie diet model of steatohepatitis.
Hfe-/- C57BL/6J mice were fed either a high calorie diet or a high calorie diet with 1 mg/g curcumin; 1.5 mg/g vitamin E; or combination of 1 mg/g curcumin + 1.5 mg/g vitamin E for 20 wk. Serum and liver tissue were collected at the completion of the experiment. Liver histology was graded by a pathologist for steatosis, inflammation and fibrosis. RNA and protein was extracted from liver tissue to examine gene and protein expression associated with fatty acid oxidation, mitochondrial biogenesis and oxidative stress pathways.
Hfe-/- mice fed the high calorie diet developed steatohepatitis and pericentral fibrosis. Combination treatment with curcumin and vitamin E resulted in a greater reduction of percent steatosis than either vitamin E or curcumin therapy alone. Serum alanine aminotransferase and non-alcoholic fatty liver disease (NAFLD) activity score were decreased following combination therapy with curcumin and vitamin E compared with high calorie diet alone. No changes were observed in inflammatory or fibrosis markers following treatment. Epididymal fat pad weights were significantly reduced following combination therapy, however total body weight and liver weight were unchanged. Combination therapy increased the mRNA expression of AdipoR2, Ppar-α, Cpt1a, Nrf-1 and Tfb2m suggesting enhanced fatty acid oxidation and mitochondrial biogenesis. In addition, combination treatment resulted in increased catalase activity in Hfe-/- mice.
Combination curcumin and vitamin E treatment decreases liver injury in this steatohepatitis model, indicating that combination therapy may be of value in NAFLD.
Core tip: The high prevalence of obesity and the metabolic syndrome suggests that many patients with liver disease of varying etiologies will have co-existent non-alcoholic fatty liver disease. Our model of co-toxic liver disease incorporates increased hepatic iron in combination with steatosis and was associated with necroinflammation and early hepatic fibrosis. Because of the beneficial effect of combination therapy, we believe vitamin E and curcumin should be investigated in other animal models of non-alcoholic steatohepatitis. Should beneficial effects be demonstrated, combination treatment with the development of appropriate dosing strategies could be rapidly moved to human studies allowing for an effective treatment strategy.
- Citation: Heritage M, Jaskowski L, Bridle K, Campbell C, Briskey D, Britton L, Fletcher L, Vitetta L, Subramaniam VN, Crawford D. Combination curcumin and vitamin E treatment attenuates diet-induced steatosis in Hfe-/- mice. World J Gastrointest Pathophysiol 2017; 8(2): 67-76
- URL: https://www.wjgnet.com/2150-5330/full/v8/i2/67.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v8.i2.67
Non-alcoholic fatty liver disease (NAFLD) is the most common liver disease in Western countries[1] and encompasses a range of hepatic pathologies from simple steatosis to the more aggressive non-alcoholic steatohepatitis (NASH)[2]. Homozygosity for the C282Y mutation in the HFE gene is the most common cause of hereditary hemochromatosis (HH)[3]. Steatosis is common in patients with HH[4] and is associated with increased hepatic fibrosis; conversely, heterozygosity for the C282Y mutation in HFE is common in patients with NAFLD[5]. Even mild increases in hepatic iron concentration (HIC) may play an important role in the transition from simple steatosis to NASH as Hfe-/- mice fed a high calorie diet develop NASH, impaired anti-oxidant activity and accelerated liver injury[6]. This relationship between HFE, altered iron metabolism and the development of NAFLD could be the target for therapeutic strategies that may attenuate disease progression in NASH. Curcumin, the yellow pigment of the plant Curcuma longa (turmeric), has potent antioxidant and chemo preventative effects[7]. Curcumin treatment produces beneficial effects in many animal models of liver diseases[8,9]. Curcumin ameliorates the early stages of experimental steatohepatitis and limits the development and progression of fibrosis in mice fed a methionine choline deficient diet[10,11]. In addition, a recent randomised controlled trial of curcumin in fatty liver patients demonstrated a reduction in NASH features[12]. The anti-oxidant, vitamin E has been shown to be beneficial in animal models of liver disease and in subjects with NASH[13-15].
Curcumin and vitamin E have been used with therapeutic benefit in experimental models of NASH[10,11,14]. To date, these agents have mostly been used at the initiation of injury which does not validate their utility in NASH in the usual clinical situation of already established injury and ongoing exposure to excess calorie intake. We hypothesized that these two agents may have synergistic activity in attenuating disease progression in NASH. Thus, in the present study, we investigated combination use of curcumin and vitamin E in the treatment of Hfe-/- mice with established liver injury induced by a high-fat, high-carbohydrate diet, and with ongoing exposure to the diet. Our results show that combination treatment of curcumin and vitamin E decreased liver injury and hepatic steatosis suggesting that this treatment may be of therapeutic value in NAFLD.
All animals received humane care under the guidelines and approval of the QIMR Berghofer Medical Research Institute and The University of Queensland Animal Ethics Committees. Eight-week-old male Hfe-/- mice (on a C57BL/6J background)[16], were fed a high calorie diet (HCD) (SF03-020, Speciality Feeds, Glen Forrest, WA, Australia) (n = 34) for a period of 10 wk. The mice were then randomly assigned (n = 7-9 per group) to receive either HCD alone, or HCD and 1 mg/g curcumin (Curcumin C3 complex, Sabinsa Corporation, Sydney, NSW, Australia), HCD and 1.5 mg/g vitamin E or HCD and a combination of 1 mg/g curcumin and 1.5 mg/g vitamin E for a further 10 wk (Specialty Feeds). The fat composition of the HCD by weight was 150 mg/g saturated fat, 20 mg/g polyunsaturated and 60 mg/g monounsaturated fat, derived from a predominant mixture of cocoa butter (50 mg/g), partially hydrogenated vegetable oil (131 mg/g) and canola oil (50 mg/g) with added cholesterol (1.9 mg/g). Animals were allowed ad libitum access to diets and drinking water. After 20 wk of dietary treatment, animals were sacrificed under anaesthesia following cardiac puncture for blood collection. The livers were removed and portions were snap-frozen in liquid nitrogen and stored at -80 °C prior to analysis. Separate portions were collected for histology or dried for the determination of hepatic iron concentration (HIC). Epididymal fat pads were weighed and stored at -80 °C.
Liver samples were fixed in 40 g/L neutral-buffered formalin, embedded in paraffin, sectioned and stained with haematoxylin-eosin (H and E). Histological parameters were staged and graded according to accepted criteria by a specialist liver pathologist in a blinded fashion[17]. Steatosis was graded according to the percentage of steatotic hepatocytes (grade 0, < 5% affected; grade 1, 5%-33% affected; grade 2, 34%-66% affected; and grade 3, > 66% affected). Lobular inflammatory activity was scored based on the number of inflammatory foci per 200x field (0, none; 1, < 2 seen; 2, 2-4 seen; and 3, > 4 seen) and ballooning was scored based on the degree of hepatocyte ballooning (0, none; 1, few; and 2, many). Activity was also scored using NAFLD activity score (NAS) established by the NASH Clinical Research Network (CRN), which is an unweighted sum of scores for steatosis (0-3), lobular inflammation (0-3) and ballooning (0-2)[17].
Fibrosis stage was assessed following Picro Sirius Red staining for collagen according to the criteria established by Brunt et al[18].
Serum concentrations of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured on a Cobas Integra 400 Chemistry Automated Analyser as per manufacturer’s instructions (Roche Diagnostics, Castle Hill, NSW, Australia). HIC was measured as previously described[6,19].
Total cellular glutathione peroxidase (GPx), reduced (GSH) and oxidized glutathione (GSSG), catalase and mitochondrial manganese superoxide dismutase (MnSOD) activities were measured on homogenized liver tissue using commercial assay kits as per manufacturer’s instructions (Cayman Chemical, Ann Arbor, MI, United States).
Total RNA was extracted using TRIzol reagent (Invitrogen, Mount Waverley, Victoria, Australia), subjected to deoxyribonuclease I digestion and transcribed into cDNA using Superscript III according to the manufacturer’s instructions (Invitrogen). Quantitative gene expression was performed by real-time polymerase chain reaction (RT-PCR) (ViiATM 7, Applied Biosystems, Life Technologies Corporation, Carlsbad, CA, United States) using Quantifast SYBR green as per manufacturer’s conditions (Qiagen, Chadstone Centre, VIC, Australia). The expression of individual genes were normalised to the geometric mean of three house-keeper genes: Basic transcription factor 3 (Btf-3), Glyceraldehyde-3-phosphate dehydrogenase (Gapdh) and β2-microglobulin (β2-m). Mouse primer sequences for genes investigated are shown in Supplementary Table 1.
| HCD (n = 9) | HCD + Cu (n = 9) | HCD + VE (n = 7) | HCD + Cu + VE (n = 9) | |
| Histology diagnosis | NASH (9) | Steatosis (2)/NASH (7) | Steatosis (2)/NASH (5) | Steatosis (4)/NASH (5) |
| Steatosis grade | 3 (3-3) | 3 (2-3) | 3 (2-3) | 3 (2-3) |
| % steatosis | 95 (80-100) | 80 (40-100) | 95 (70-100) | 75 (40-95)a |
| Lobular score | 2 (1-2) | 2 (1-3) | 1 (0-2) | 1 (0-3) |
| Ballooning | 2 (1-2) | 2 (1-2) | 1 (1-2) | 1 (0-2) |
| NAS score | 6 (5-7) | 6 (4-8) | 6 (4-6) | 5 (3-8) |
| Fibrosis stage | 1a (0-1a) | 0 (0-1a) | 0 (0-1a) | 0 (0-1a) |
Protein extracts (30 µg) from liver were electrophoresed in sodium dodecyl sulphate-10% polyacrylamide gel for 30 min at 100 V and blotted onto polyvinylidene fluoride membranes (Bio-Rad, Hercules, CA, United States) as previously described[6]. Membranes were immunostained with primary antibodies for ACOX (Abcam, Cambridge, MA, United States) and mitoNEET (Proteintech, Chicago, IL, United States). Signals were detected using standard chemiluminescence (Supersignal West Femto, ThermoFisher Scientific, Scoresby, Victoria, Australia) on an Image Station 4000MM Pro and quantified using Carestream Molecular Imaging software (Carestream Australia, East Melbourne, Victoria, Australia).
Statistical analysis was performed using IBM SPSS Statistics 22 (IBM, Armonk, NY, United States). Non-parametric tests were used to evaluate differences between groups, and data are depicted using box and whisker plots showing median, minimum and maximum values. A Kruskal-Wallis test with Dunn’s correction for multiple comparisons was performed to compare intervention groups. A cut-off P value of 0.05 was used to determine significance. Statistical review of the manuscript was performed by a biomedical statistician.
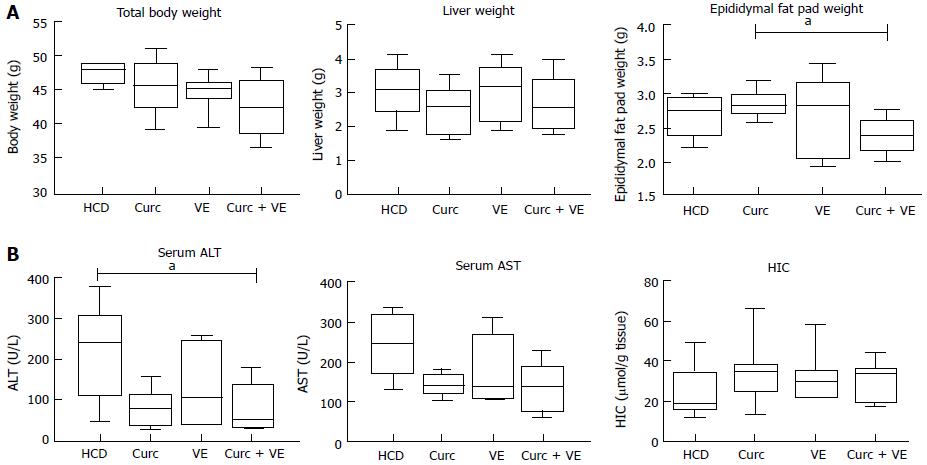
Total body weight and liver weight were not altered by dietary treatment in Hfe-/- mice (Figure 1A). Epididymal fat pad weight was significantly reduced in the combination treatment group compared to curcumin alone (P < 0.02, Figure 1A).
Serum ALT concentrations were significantly reduced by combination treatment compared to HCD alone (P = 0.02, Figure 1B). AST concentrations were not significantly different across groups (P = 0.12). There was no significant effect of dietary treatment on HIC (P < 0.30, Figure 1B).
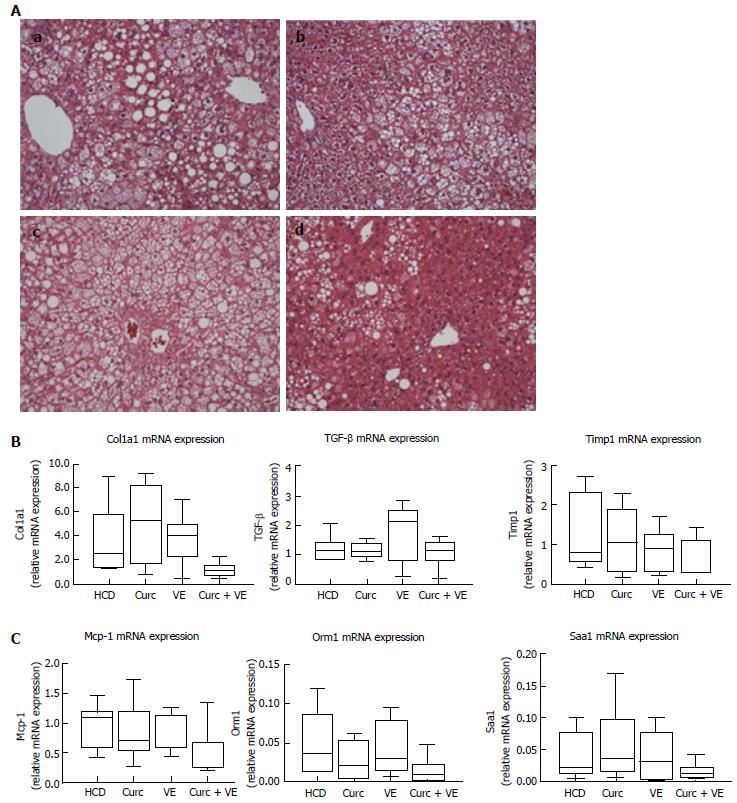
Hfe-/- HCD-fed mice developed moderate to severe steatosis with hepatocyte ballooning and inflammatory cell infiltration present in 100% of mice meeting the criteria for steatohepatitis (Table 1 and Figure 2A). Combination treatment with curcumin and vitamin E reduced the presence of NASH to only 55% of mice. Combination treatment also significantly reduced percent steatosis compared with HCD alone (P = 0.03). Lobular inflammatory score and NAS score were also reduced in combination treated Hfe-/- mice (Table 1 and Figure 2A). Treatment with either curcumin or vitamin E alone had minimal effects on hepatic histology (Figure 2A).
Centrilobular (stage 1) fibrosis (as detected by Sirius red staining) was observed in 7/9 Hfe-/- mice fed the HCD and only in 3/9 combination treated mice, as evidenced by reduced staining (data not shown). However, hepatic α1(I)-procollagen (Col1a1) mRNA, transforming growth factor β1 (Tgfβ1) and tissue inhibitor of metalloproteinase 1 (Timp1) gene expression were not significantly altered by dietary treatment (P = 0.05, P = 0.40 and P = 0.26 respectively, Figure 2B). Gene expression of monocyte chemoattractant protein-1 (Mcp-1) and two acute phase reactants, orosomucoid 1 (Orm-1) and serum amyloid A1 (Saa1) were not significantly altered by combination treatment when compared to HCD or monotherapy (P = 0.08, P = 0.07 and P = 0.17 respectively, Figure 2C).
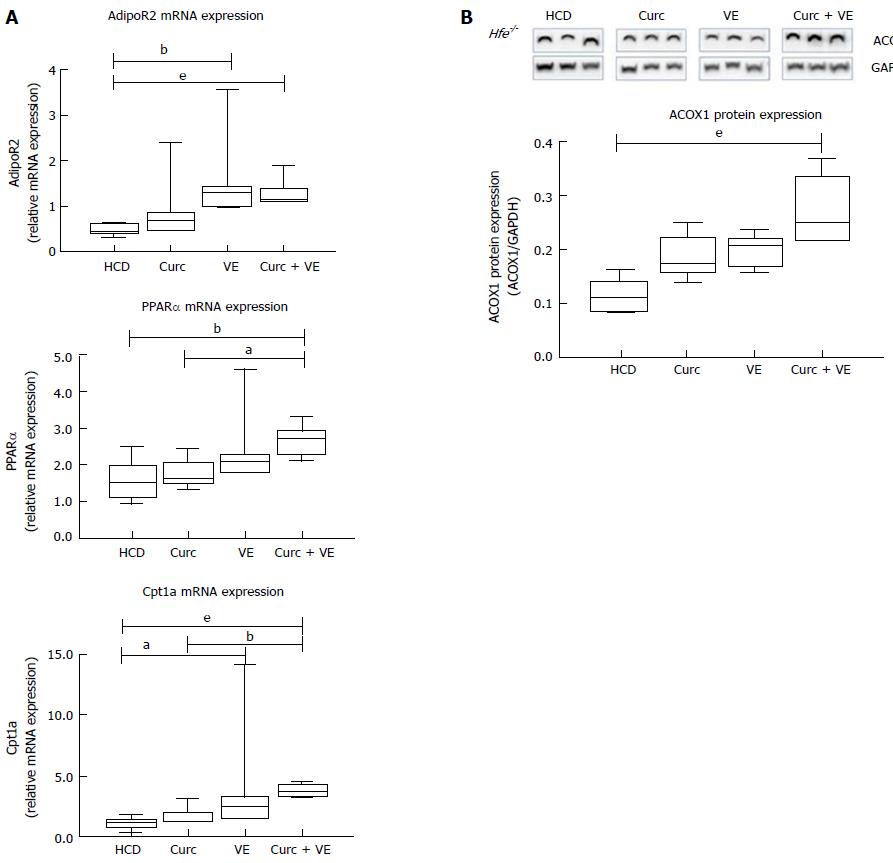
Hepatic adiponectin receptor 2 (AdipoR2) mRNA expression was significantly increased in vitamin E and combination treatment groups compared to HCD alone (P = 0.02 and P < 0.01, respectively).
Downstream of AdipoR2, peroxisome proliferator-activated receptor α (Pparα) mRNA expression was significantly increased in combination treated Hfe-/- mice compared to HCD alone and curcumin alone (P < 0.012 and P = 0.01, respectively, Figure 3A). Carnitine palmitoyl transferase 1A (Cpt1a) expression was also increased due to dietary treatments where vitamin E and combination treatment significantly increased Cpt1a expression in Hfe-/- mice compared to HCD alone (P = 0.05 and P < 0.01, respectively, Figure 3A). In addition, combination therapy increased Cpt1a expression above curcumin treatment alone (P < 0.01, Figure 3A). The expression of acyl-coenzymeA oxidase 1 (ACOX1) protein, the first enzyme in the β-oxidation pathway, was significantly different following dietary intervention. Combination treatment up-regulated ACOX1 expression compared with HCD alone (P < 0.01, Figure 3B). These results suggest an increase in lipid metabolism by β-oxidation which has been altered by long-term HCD feeding.
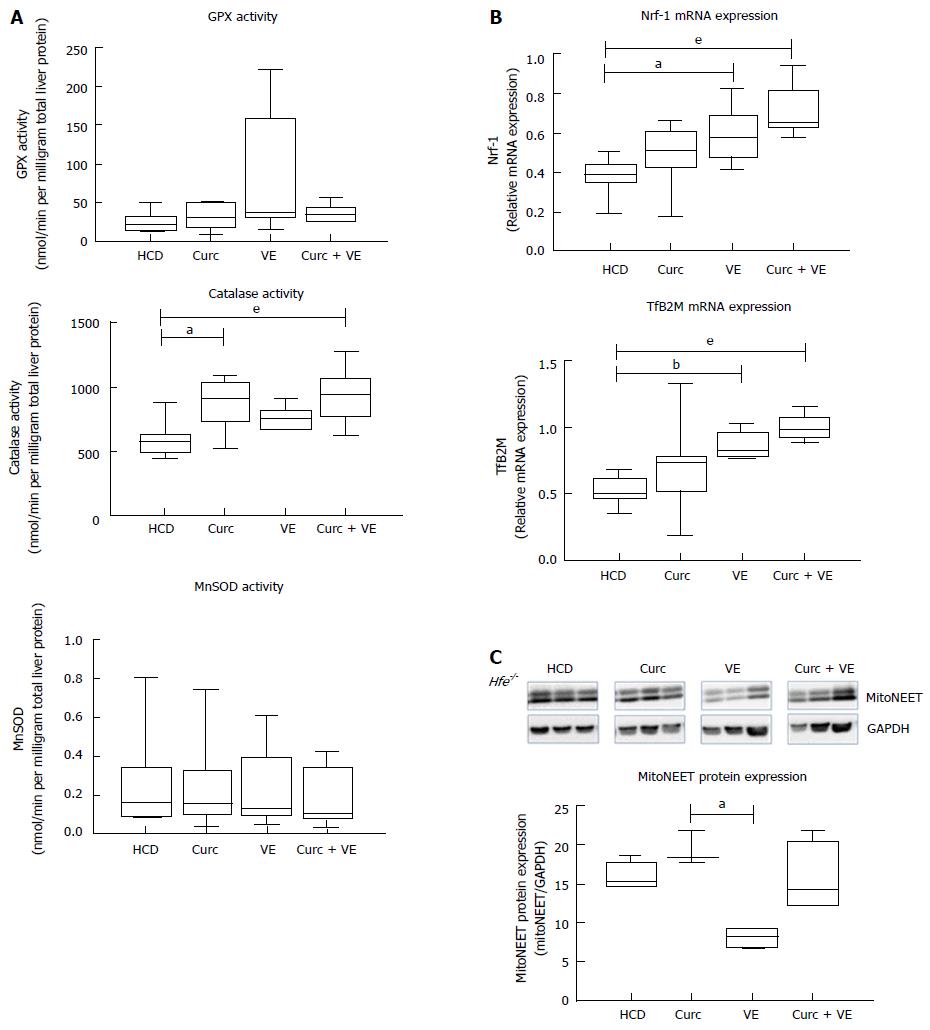
Neither glutathione peroxidase activity (GPx) nor hepatic mitochondrial manganese superoxide dismutase (MnSOD) activity were significantly altered by dietary treatments (P = 0.4 and P = 0.80, respectively, Figure 4A). However both curcumin and combination treatment increased catalase when compared to HCD alone (P = 0.01 and P < 0.01, respectively, Figure 4A).
We have previously shown mitochondrial dysfunction in Hfe-/- mice fed a HCD[6], therefore we then investigated if combination treatment could correct this defect. Indeed, nuclear transcription factor 1 (Nrf-1) mRNA expression was significantly altered by dietary treatments in Hfe-/- mice (Figure 4B), where vitamin E and combination treatment significantly increased expression compared to HCD alone (P = 0.01 and P < 0.01, respectively). Likewise, transcription factor B2 mitochondrial (TfB2M) mRNA was significantly increased in Hfe-/- mice treated with vitamin E and combination treatment compared to HCD (P < 0.01 and P < 0.01, respectively, Figure 4B). These results suggest an up-regulation in mitochondrial biogenesis due to vitamin E and combination treatment. Interestingly, mitoNEET, a protein that is a driving force behind mitochondrial biogenesis[20], was not significantly different in combination treated animals compared with HCD alone (Figure 4C).
In this study we demonstrated that the combination of curcumin and vitamin E therapy attenuated steatosis and lobular inflammation in Hfe-/- mice even with ongoing feeding of a HCD. Hfe-/- mice fed a HCD for 20 wk developed steatosis and 100% had histological features consistent with steatohepatitis and the majority of animals showed centrilobular fibrosis. Monotherapy with either vitamin E or curcumin had no effect on percent steatosis, but combination therapy reduced percent hepatic steatosis as well as lobular inflammation, ballooning degeneration and fibrosis. This implies that vitamin E and curcumin exert a synergistic effect over that provided by each individual therapy.
Our previous studies have shown that Hfe-/- mice fed a HCD develop steatosis and the pathophysiology of this injury involves altered β-oxidation, impaired fatty acid uptake and mitochondrial dysfunction[6]. To assess the mechanisms through which curcumin and vitamin E may exert their beneficial effect we examined fatty acid uptake, β-oxidation and mitochondrial function in this study.
Curcumin has been reported to increase the metabolism of lipids by β-oxidation[21,22] and this appears to be a mechanism by which combination therapy results in reduced hepatic steatosis in this study. This was evidenced by increased AdipoR2, Ppar-α and Cpt1a mRNA expression in the combination fed Hfe-/- mice; facilitating increased mitochondrial uptake of free fatty acids. AdipoR2 has been shown to activate Ppar-α and fatty acid oxidation genes[23], and these results imply that combination therapy induced AdipoR2 expression, resulting in the up-regulation of fatty acid oxidation pathways[24]. However, it is worth noting that the change in AdipoR2 and Cpt1 expression with vitamin E monotherapy alone was similar to that achieved by combination therapy. Increased ACOX1 expression, facilitating increased β-oxidation, was also observed in combination treated animals, providing further evidence of increased fatty acid β-oxidation.
We examined defence mechanisms against oxidative stress as another potential reason for the benefits of combination therapy since both curcumin and vitamin E are potent anti-oxidants. Curcumin and combination treatment in Hfe-/- mice raised catalase activity illustrating that an up-regulation in β-oxidation was counteracted by an up-regulation of an enzyme that removes excess reactive oxygen species. These observations are consistent with other models of hepatic injury where the anti-oxidant effects of curcumin and vitamin E are mediated by altering catalase and MnSOD activity[25,26].
Vitamin E and combination treatment increased gene expression of Nrf-1 and Tfb2m which are consistent with up-regulated mitochondrial biogenesis. It has been suggested that curcumin may be similar to resveratrol in this respect through activating PPARγ coactivator 1-alpha (PGC-1α)[21,22], a regulator of mitochondrial biogenesis[27]. Indeed, curcumin increases PGC-1α and Nrf-1 gene expression in vitro, protecting against mitochondrial impairment induced by high free fatty acids[28]. A recent study in a rat model of NASH demonstrated attenuation of liver injury by curcumin which the authors suggested was Nrf-1 mediated[29]. Combination treatment increased Nrf-1 and TfB2m expression in Hfe-/- mice but failed to increase the expression of mitoNEET. These results are similar to MnSOD activity where combination treatment failed to restore its activity above a HCD-induced suppression. Down-regulation of mitoNEET causes an increase in iron content into the mitochondria and decreases the mitochondria’s capacity to carry out electron transport and oxidative phosphorylation[20], potentially increasing oxidative stress and further damaging mitochondria. Indeed, mitochondrial dysfunction is associated with insulin resistance and the development of type 2 diabetes where diminished oxidative capacity is thought to be involved in disease pathogenesis[30]. Taken together, these findings of our study suggest that combination treatment manages to enhance the capacity for fatty acid disposal by β-oxidation without increasing oxidative stress.
This study is limited by the use of an animal model which cannot fully replicate the pathophysiology of human NASH. The dietary model incorporated high levels of curcumin which may not be easily replicated in humans. Further studies examining dosing of curcumin and vitamin E in humans are still required. While these studies have suggested potential mechanistic pathways involved in efficacy of combination treatment, we have not fully elucidated mechanisms responsible for the synergistic effects of combination therapy over monotherapy.
The high prevalence of obesity and the metabolic syndrome suggests that many patients with liver disease of varying etiologies will have co-existent non-alcoholic fatty liver disease. Indeed contemporary clinical practice in hepatology is often characterised by the need to address multiple co-toxins in one patient. The results of the present study illustrate of the concept of co-toxic liver disease since our model incorporates increased HIC in combination with steatosis and was associated with necroinflammation and early hepatic fibrosis. Curcumin and vitamin E therapy resulted in attenuation of steatosis through increased fatty acid β-oxidation, increased catalase activity and upregulated mitochondrial biogenesis. Because of the beneficial effect of combination therapy, we believe vitamin E and curcumin should be investigated in other animal models of NASH, and could be moved rapidly into human studies if a beneficial effect is demonstrated, and if appropriate dosing strategies can be developed.
Associate Professor Graeme Macdonald from the Gastroenterology and Hepatology unit of the Princess Alexandra Hospital for his knowledge and continual input into the project. Dr. Sarah McLeay for her review of the statistical methods in the manuscript.
Non-alcoholic fatty liver disease (NAFLD) is the most common liver disease in Western countries and encompasses a range of hepatic pathologies from simple steatosis to the more aggressive non-alcoholic steatohepatitis (NASH). Homozygosity for the C282Y mutation in the HFE gene is the most common cause of hereditary hemochromatosis (HH). Steatosis is common in patients with HH and is associated with increased hepatic fibrosis; conversely, heterozygosity for the C282Y mutation in HFE is common in patients with NAFLD. Even mild increases in hepatic iron concentration (HIC) may play an important role in the transition from simple steatosis to NASH. Curcumin and vitamin E have been used with therapeutic benefit in experimental models of NASH, therefore this study investigated the role of curcumin and vitamin E in ameliorating injury in a model of iron overload and fatty liver.
Therapies for NASH and fatty liver disease are lacking and due to the growing prevalence of fatty liver disease are increasing important for treatment of this growing number of patients.
This study utilised combination treatment with curcumin and vitamin E which differed from previous studies which only examined monotherapy. In addition, this study examined the efficacy of treatment in established fatty liver disease, not solely at the initiation of injury.
As both curcumin and vitamin E have been used clinically the authors suggest that combination treatment could be easily moved into human clinical trials.
Non-alcoholic fatty liver disease: Encompasses a range of hepatic pathologies from simple steatosis to the more aggressive non-alcoholic steatohepatitis; Haemochromatosis: An iron-overload disease which results in hepatic iron accumulation.
Manuscript’s content is interesting, overall well written and timely.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Trovato GM S- Editor: Gong ZM L- Editor: A E- Editor: Wu HL
| 1. | Ong JP, Younossi ZM. Epidemiology and natural history of NAFLD and NASH. Clin Liver Dis. 2007;11:1-16, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 323] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 2. | Smith BW, Adams LA. Non-alcoholic fatty liver disease. Crit Rev Clin Lab Sci. 2011;48:97-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 232] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 3. | Valenti L, Fracanzani AL, Bugianesi E, Dongiovanni P, Galmozzi E, Vanni E, Canavesi E, Lattuada E, Roviaro G, Marchesini G. HFE genotype, parenchymal iron accumulation, and liver fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2010;138:905-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 214] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 4. | Powell EE, Ali A, Clouston AD, Dixon JL, Lincoln DJ, Purdie DM, Fletcher LM, Powell LW, Jonsson JR. Steatosis is a cofactor in liver injury in hemochromatosis. Gastroenterology. 2005;129:1937-1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 5. | Nelson JE, Bhattacharya R, Lindor KD, Chalasani N, Raaka S, Heathcote EJ, Miskovsky E, Shaffer E, Rulyak SJ, Kowdley KV. HFE C282Y mutations are associated with advanced hepatic fibrosis in Caucasians with nonalcoholic steatohepatitis. Hepatology. 2007;46:723-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Tan TC, Crawford DH, Jaskowski LA, Murphy TM, Heritage ML, Subramaniam VN, Clouston AD, Anderson GJ, Fletcher LM. Altered lipid metabolism in Hfe-knockout mice promotes severe NAFLD and early fibrosis. Am J Physiol Gastrointest Liver Physiol. 2011;301:G865-G876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Sharma RA, Steward WP, Gescher AJ. Pharmacokinetics and pharmacodynamics of curcumin. Adv Exp Med Biol. 2007;595:453-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 297] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 8. | Baghdasaryan A, Claudel T, Kosters A, Gumhold J, Silbert D, Thüringer A, Leski K, Fickert P, Karpen SJ, Trauner M. Curcumin improves sclerosing cholangitis in Mdr2-/- mice by inhibition of cholangiocyte inflammatory response and portal myofibroblast proliferation. Gut. 2010;59:521-530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Rong S, Zhao Y, Bao W, Xiao X, Wang D, Nussler AK, Yan H, Yao P, Liu L. Curcumin prevents chronic alcohol-induced liver disease involving decreasing ROS generation and enhancing antioxidative capacity. Phytomedicine. 2012;19:545-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 10. | Leclercq IA, Farrell GC, Sempoux C, dela Peña A, Horsmans Y. Curcumin inhibits NF-kappaB activation and reduces the severity of experimental steatohepatitis in mice. J Hepatol. 2004;41:926-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 174] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 11. | Vizzutti F, Provenzano A, Galastri S, Milani S, Delogu W, Novo E, Caligiuri A, Zamara E, Arena U, Laffi G. Curcumin limits the fibrogenic evolution of experimental steatohepatitis. Lab Invest. 2010;90:104-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Rahmani S, Asgary S, Askari G, Keshvari M, Hatamipour M, Feizi A, Sahebkar A. Treatment of Non-alcoholic Fatty Liver Disease with Curcumin: A Randomized Placebo-controlled Trial. Phytother Res. 2016;30:1540-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 315] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 13. | Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003;98:2485-2490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 484] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 14. | Chung MY, Yeung SF, Park HJ, Volek JS, Bruno RS. Dietary α- and γ-tocopherol supplementation attenuates lipopolysaccharide-induced oxidative stress and inflammatory-related responses in an obese mouse model of nonalcoholic steatohepatitis. J Nutr Biochem. 2010;21:1200-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675-1685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2642] [Cited by in RCA: 2469] [Article Influence: 164.6] [Reference Citation Analysis (2)] |
| 16. | Zhou XY, Tomatsu S, Fleming RE, Parkkila S, Waheed A, Jiang J, Fei Y, Brunt EM, Ruddy DA, Prass CE. HFE gene knockout produces mouse model of hereditary hemochromatosis. Proc Natl Acad Sci USA. 1998;95:2492-2497. [PubMed] |
| 17. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6807] [Cited by in RCA: 8234] [Article Influence: 411.7] [Reference Citation Analysis (5)] |
| 18. | Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467-2474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2702] [Cited by in RCA: 2886] [Article Influence: 111.0] [Reference Citation Analysis (0)] |
| 19. | Stuart KA, Fletcher LM, Clouston AD, Lynch SV, Purdie DM, Kerlin P, Crawford DH. Increased hepatic iron and cirrhosis: no evidence for an adverse effect on patient outcome following liver transplantation. Hepatology. 2000;32:1200-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Kusminski CM, Holland WL, Sun K, Park J, Spurgin SB, Lin Y, Askew GR, Simcox JA, McClain DA, Li C. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat Med. 2012;18:1539-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 383] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 21. | Chung JH, Manganiello V, Dyck JR. Resveratrol as a calorie restriction mimetic: therapeutic implications. Trends Cell Biol. 2012;22:546-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 153] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 22. | Zingg JM, Hasan ST, Meydani M. Molecular mechanisms of hypolipidemic effects of curcumin. Biofactors. 2013;39:101-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 23. | Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 968] [Cited by in RCA: 1057] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 24. | Handa P, Maliken BD, Nelson JE, Morgan-Stevenson V, Messner DJ, Dhillon BK, Klintworth HM, Beauchamp M, Yeh MM, Elfers CT. Reduced adiponectin signaling due to weight gain results in nonalcoholic steatohepatitis through impaired mitochondrial biogenesis. Hepatology. 2014;60:133-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 25. | Messarah M, Amamra W, Boumendjel A, Barkat L, Bouasla I, Abdennour C, Boulakoud MS, Feki AE. Ameliorating effects of curcumin and vitamin E on diazinon-induced oxidative damage in rat liver and erythrocytes. Toxicol Ind Health. 2013;29:77-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Subudhi U, Chainy GB. Curcumin and vitamin E modulate hepatic antioxidant gene expression in PTU-induced hypothyroid rats. Mol Biol Rep. 2012;39:9849-9861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Jones AW, Yao Z, Vicencio JM, Karkucinska-Wieckowska A, Szabadkai G. PGC-1 family coactivators and cell fate: roles in cancer, neurodegeneration, cardiovascular disease and retrograde mitochondria-nucleus signalling. Mitochondrion. 2012;12:86-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 28. | Kuo JJ, Chang HH, Tsai TH, Lee TY. Curcumin ameliorates mitochondrial dysfunction associated with inhibition of gluconeogenesis in free fatty acid-mediated hepatic lipoapoptosis. Int J Mol Med. 2012;30:643-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Li B, Wang L, Lu Q, Da W. Liver injury attenuation by curcumin in a rat NASH model: an Nrf2 activation-mediated effect? Ir J Med Sci. 2016;185:93-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Paddock ML, Wiley SE, Axelrod HL, Cohen AE, Roy M, Abresch EC, Capraro D, Murphy AN, Nechushtai R, Dixon JE. MitoNEET is a uniquely folded 2Fe 2S outer mitochondrial membrane protein stabilized by pioglitazone. Proc Natl Acad Sci USA. 2007;104:14342-14347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 232] [Article Influence: 12.9] [Reference Citation Analysis (0)] |