INTRODUCTION
Biliary atresia (BA) is a neonatal cholangiopathy of unknown pathogenesis characterized by fibrosclerosis and obliteration of the biliary ducts which leads to cholestasis, progressive fibrosis and ultimately cirrhosis and death if untreated. The condition appears after birth with jaundice. Diagnosis is usually suspected on ultrasound[1,2], hepatobiliary scintigraphy and liver biopsy and confirmed by surgical cholangiography. Primary treatment of BA is the hepato-portoenterostomy (Kasai procedure) to reestablish bile flow, delaying fibrosis and biliary cirrhosis. The Kasai procedure, especially if performed before 2 mo of age, both reduces and delays the need for liver transplantation (LT). However, the Kasai procedure is often not curative because liver function usually worsens progressively, leading to continuing cholangitis. LT, however, becomes the only therapeutic option in patients with long-standing BA.
BA represents the most common indication for LT in pediatric patients[3-5].
The multi-detector computed tomography (MDCT) scan and magnetic resonance (MR) of the abdomen play a key role in the work-up to LT by identifying congenital anomalies or cirrhosis-related modifications, conditions that can require changes in surgical technique. Moreover, the MDCT and MR scans allow identification of cirrhotic liver hepatic masses (often not easily identified with ultrasound in a cirrhotic liver), extrahepatic porto-systemic shunts, eventual thrombosis of portal system and radiological signs of portal hypertension associated with BA[6-9].
The aim of this paper is to review multimodality diagnostic imaging and interventional radiology procedures performed to evaluate pediatric patients with end-stage liver disease secondary to BA who are candidates for LT.
PORTAL HYPERTENSION
Portal hypertension is an almost unavoidable consequence of BA with a wide variety of complications including ascites and bleeding from gastroesophageal varices, which represent the leading causes for LT and death in BA children with end-stage liver disease. The risk of death or need for LT after an initial episode of esophageal variceal hemorrhage in patients with BA is 50% at 6 years with a variable prognosis related to serum bilirubin concentration at the time of the episode[10]. In the long term, less then 18% of patients with BA, treated with corrective surgery, can avoid LT but require, in any case, assiduous lifelong care; in these patients clinical, ultrasound or endoscopic signs of portal hypertension are frequently present and gastrointestinal bleeding has been reported in 50% of cases, indicating that portal hypertension is also a major problem in patients in whom the Kasai procedure restores a sufficient bile flow[11].
While there are evidence-based approaches to the management of adults with portal hypertension, these are not available for children, hence making it difficult for pediatric hepatologists to determine whether recent advances in the management of portal hypertension in adults can be extrapolated to pediatric patients[12,13].
Portal pressure, in chronic liver diseases, is commonly measured by the hepatic venous pressure gradient (HVPG), defined as the difference between wedged (occluded) and free hepatic venous pressures with normal values ranging between 1-5 mmHg in adult patients. In adult patients with cirrhosis this measurement has been shown to be reproducible and the best predictor of the complications of portal hypertension[14-16]. It is well known that varices, variceal bleeding, portal hypertensive gastropathy and ascites do not occur until the HVPG increases above 10 mmHg, a pressure threshold defining clinically significant portal hypertension. Actually, although hepatic vein catheterization has been used in children undergoing TIPS procedure or transjugular liver biopsy[17,18], there are no reports of measurements of HVPG in pediatric series; there are no data regarding the normal values of portal pressure in healthy children, and no data regarding the use of HVPG in the evaluation of portal hypertension, nor in assessing response to therapy, although this has been recommended in international consensus conferences[12,13].
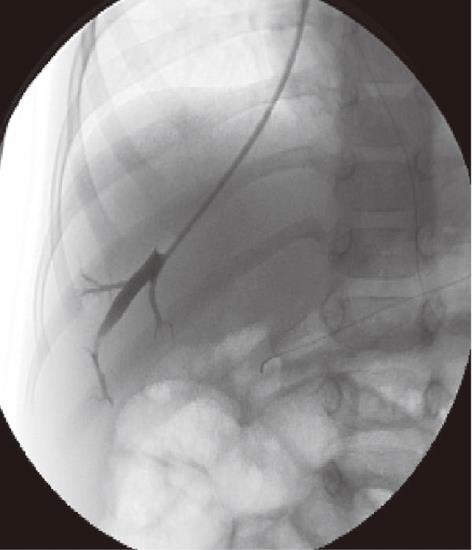
Our experience has showed HVPG to be a feasible and safe procedure in BA patients with advanced liver diseases and patent portal vein. The scenarios where HVPG may be useful in children with BA are the diagnosis of portal hypertension and the evaluation of the effects of preventive pharmacological therapy. In our experience, HVPG measurement in pediatric patients is performed after overnight fast in the angiographic suite under monitored anesthesia care with spontaneous respiration and local anesthesia, or under general anesthesia. The right internal jugular vein is punctured under ultrasound guidance. The hepatic vein, right or middle, is catheterized under fluoroscopic guidance with a 5F or 4F Cobra 2 angiographic catheter and a hydrophilic wire. The hepatic venogram is performed by hand with gentle injections of a small amount (2 to 6 cc) of iso-osmolar contrast medium with the catheter tip positioned in the mid/distal portion of the vein. The angiographic catheter is then exchanged with a standard 5F occlusion balloon catheter. Wedged (occluded) hepatic vein pressure (WHVP) and free hepatic vein pressure (FHVP) are obtained by inflating and releasing the balloon. The HVPG is estimated from the difference between WHPV and FHVP (Figure 1). Unexpectedly, intrahepatic venous-venous shunts (IVVS) are frequently detected in BA patients (Figure 2A). This is relevant with regards to the use of HVPG as a measurement of portal pressure. Obviously when the presence of IVVS precludes the total occlusion of the outflow from a hepatic vein, the pressure measured no longer equilibrates with the portal pressure, resulting in a gross underestimation of portal pressure as it occurs in patients with presinusoidal intrahepatic portal hypertension. In our patients with BA and multiple IVVS, the WHVP had to be measured distally in the hepatic vein to avoid the shunt precluding an effective occlusion of the hepatic vein outflow, hence measuring the WHVP of a small vascular territory (Figure 2B). Veno-venous shunts are usually detected, with MDCT, in the portal-venous phase near the liver surface in the axial plane and can be confirmed in 3D reconstructions (Figures 3 and 4). BA patients frequently also show portal-venous shunts (Figure 4); only a few cases of communication between the portal and hepatic venous systems have been described in pediatric patients[19-21].
Figure 1 A 3-year-old male child post-Kasai.
Fluoroscopic image of normal wedged venogram, hepatic venous pressure gradient 15.5 mmHg.
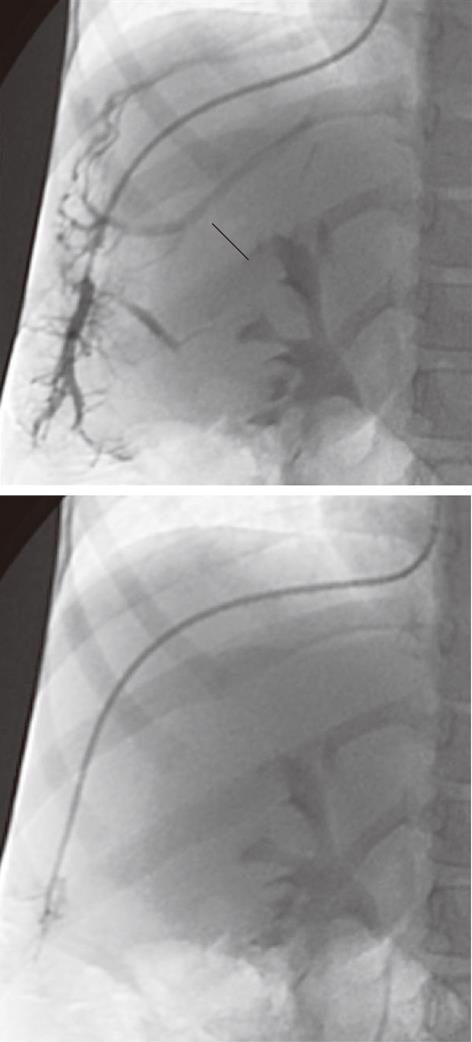
Figure 2 A 5-year-old female child post-Kasai.
Fluoroscopic image. A: Wedged venogram showing multiple peripheral venovenous communications (arrows); B: 5F occlusion balloon catheter advanced distally in the hepatic vein, no venous communications visualized; Wedged (occluded) hepatic vein pressure obtained, hepatic venous pressure gradient 15 mmHg.
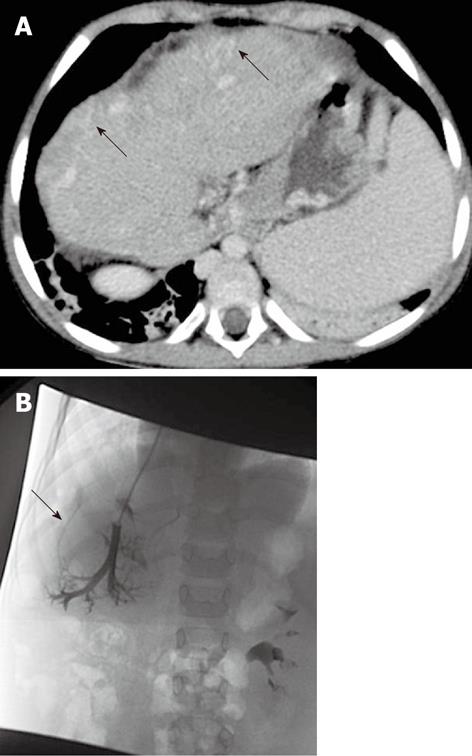
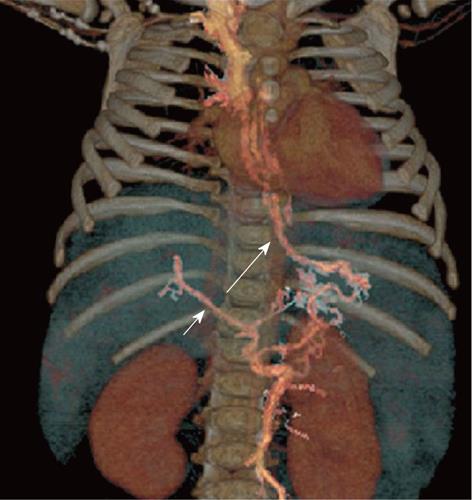
Figure 3 A 5-year-old female child post-Kasai.
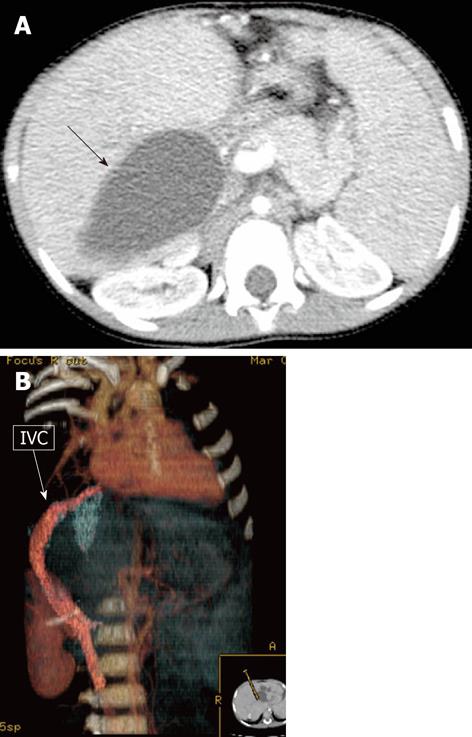
A: Multi-detector computed tomography shows peripheral venous-venous communications (arrows). Of note: Azygos vein dilatation secondary to retro-hepatic interruption of inferior vena cava; B: Fluoroscopic image: wedged venogram confirms peripheral venovenous communications (arrow).
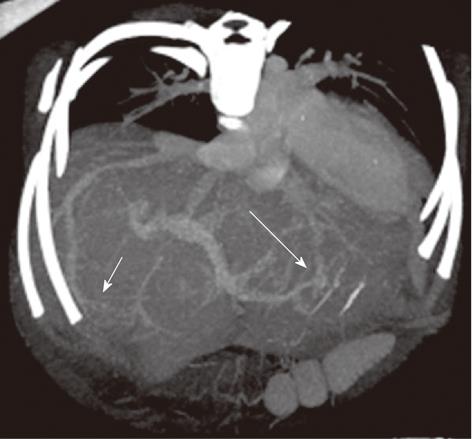
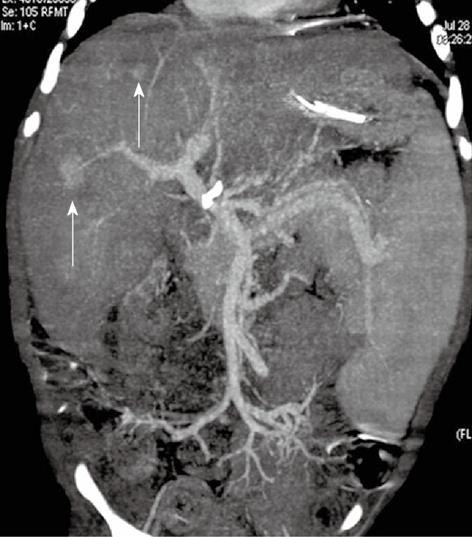
Figure 4 A 9-mo-old boy post-Kasai.
Multi-detector computed tomography: Paraxial multi intensity projection reconstruction shows peripheral venous-venous communication (short arrow) and portal venous shunts (long arrow).
Because the severity and progression of portal hypertension influence the timing of LT, MDCT and MR imaging of portal hypertension (gastro-esophageal varices, perisplenic-perigastric varices, spontaneous spleno-renal shunt, ascites, splenomegaly), as an adjunct to endoscopic and clinical evaluations, have acquired an important role in patient follow-up.
VASCULAR ANOMALIES
In BA patients, anatomical variants of the hepatic artery, portal vein hypoplasia or thrombosis of the main portal vein (MPV) can require changes of surgical technique. Thus, preoperative depiction of any vascular anomalies offers the possibility of precise surgical planning reducing the risk of operative and postoperative complications.
For the very good morphologic and vascular imaging that can determine changes in surgical planning, MDCT is considered, in our hospital, the most important diagnostic tool in pediatric LT work-up. Compared with MR, MDCT offers the advantage of a better spatial and contrast resolution with a short examination time and consequently less time with the patient under sedation. Radiation exposure is a relative disadvantage of MDCT; for this reason the use of pediatric parameters tailored on an individual basis is mandatory for maximal dose reduction obtaining satisfactory diagnostic images. Also, the need for intravenous iodinated contrast media is a relative disadvantage of MDCT although the occurrence of significant adverse effects in children is very rare[22]. In our experience, all the anatomical arterial variants and/or portal vein thrombosis or hypoplastic MPV cases were confirmed in the transplanted patients, confirming the high accuracy of the technique.
HEPATIC ARTERY
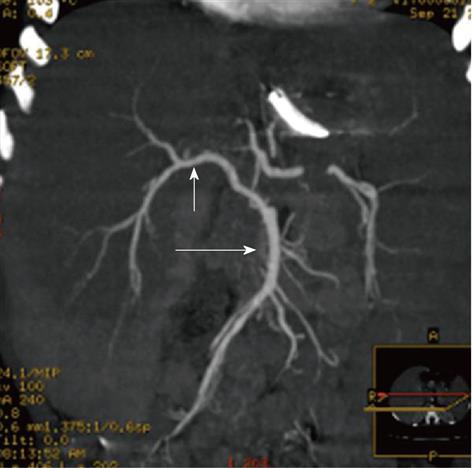
Anatomic and pathologic variation of the liver vasculature are frequent in BA patients[8,23]. In our experience, anatomical variants of the hepatic artery were found in 46% of cases (Figures 5 and 6); the most common variant was the common hepatic artery from the superior mesenteric artery. We also detected early bifurcation, right hepatic artery from the superior mesenteric artery, left hepatic artery from the left gastric artery, celiac-mesenteric common trunk, gastro-duodenal artery from segment 4 artery, accessory right hepatic artery from common hepatic artery, and left hepatic artery from the celiac artery. Occasionally, hepatic artery with intimal dissection or occluded hepatic artery have been reported in BA patients, which may be due to recurrent cholangitis or result from intraoperative injuries during Kasai procedures[7].
Figure 5 A 2-year-old female child post-Kasai.
Multi-detector computed tomography: Multiple Intensity Projection reconstruction, right hepatic artery (short arrow) off from superior mesenteric artery (long arrow).
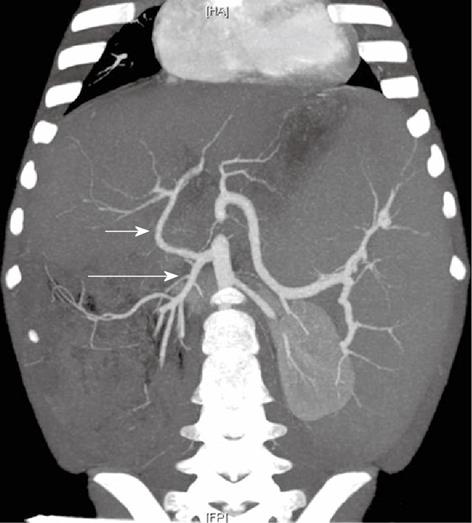
Figure 6 A 5-year-old female child post-Kasai.
Multi-detector computed tomography: Multiple Intensity Projection reconstruction, common hepatic artery (short arrow) arising from superior mesenteric artery (long arrow). Of note: large splenomegaly.
Hepatic artery variants can be easily managed by experienced surgeons in whole liver deceased donor LT because of the sufficient length of the graft hepatic artery for the anastomosis; however, a short hepatic artery can be a problem in partial LT, living related or from deceased donor.
The arterial axis of the graft usually includes the proper and common hepatic artery, in continuity with the celiac artery, and a patch of the aorta. The level of the anastomosis is chosen at any place along the recipient’s
arterial axis, and the two vessels are trimmed to obtain a similar section and an adequate length. An end-to-end anastomosis is usually performed. If the recipient’s arterial axis is deemed inadequate, the aorta can be clamped at the origin of the celiac artery or just below the renal arteries, and an end-to-side anastomosis can be performed at one of these sites. In the latter case, the interposition of an arterial graft from the donor, usually represented by an iliac artery, may be necessary. For those reasons a correct recipient hepatic artery evaluation is considered mandatory.
Frequently, BA patients show enlargement of the hepatic artery[1,2,7,24,25]. In a study performed in BA patients, at the diagnosis, mean hepatic artery diameter was 2.2 mm ± 0.59 mm[1]. Interestingly enough, in our experience, the mean diameter of the hepatic artery evaluated with MDCT in end-stage patients was 4.0 mm ± 1.2 mm[26]; this finding correlated well with the results of Humphrey et al[1] and could be the result of the hyperdynamic circulation secondary to cirrhotic changes of the liver, also reflecting, as a buffering effect, the reduction of portal flow in patients with thrombosis of the portal vein or the small portal vein.
PORTAL VEIN
Hypoplasia or thrombosis of the MPV in BA recipients can require changes of surgical technique during LT. Portal vein anastomosis during LT is usually performed in an end-to-end fashion. If the portal vein is small and sclerotic, it has to be dissected proximally to the confluence of the splenic and superior mesenteric vein, dividing the coronary vein of the stomach. The portal vein anastomosis will then be performed by means of a donor interposition vein graft. Patients with BA show higher risk of portal vein thrombosis than other pediatric recipients of LT[27] and the portal vein diameter of small recipients is strongly linked to the risk of thrombosis[28]. For this reason, surgeons have to know exactly the anatomy of the portal vein before LT (Figures 7 and 8). In our experience, thrombosis of the MPV in BA patients has an incidence of 15% while small portal vein, defined as diameter of the MPV < 3 mm, has an incidence of 26%. Although rare, congenital absence of portal vein has also been reported in BA patients[29,30] (Figure 9).
Figure 7 A 15-mo-old male child post-Kasai.
Multi-detector computed tomography: Multiple Intensity Projection reconstruction. Good caliper of main portal vein, significant splenomegaly. Two hepatic hypervascular nodules coexist (arrows).
Figure 8 A 8-mo-old female child post-Kasai.
Multi-detector computed tomography: volume rendering reconstruction shows small main portal vein (short arrow), patent coronary vein (long arrow) with filling of esophageal varices.
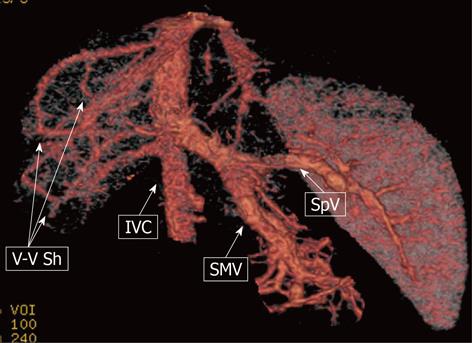
Figure 9 A 9-mo-old male child.
Multi-detector computed tomography: volume rendering reconstruction shows the superior mesenteric vein (SMV) and the splenic vein (SpV) joining to form a short common trunk that directly enters into the suprarenal inferior vena cava (IVC); Venous-venous intrahepatic shunting (V-V Sh) coexist.
VENA CAVA
Retro-hepatic interruption of the inferior vena cava, with azygeal continuation (Figure 3), is a rare vascular alteration described in BA patients, sometimes in association with polysplenia syndrome[9,31,32]. Although rare, its presence should be evaluated before LT because of possible complications regarding outflow reconstructions.
INTRAHEPATIC BILIARY CYSTS
Intrahepatic biliary cysts (IBC) are frequent in BA patients and reported in up to 30% of cases[33-37]. IBC are more frequent in post-Kasai patients. The mechanisms of cyst formation are unclear, although the extrahepatic as well as the intrahepatic duct fibro-obliterative process, inflammatory process and cirrhotic changes in the intralobular spaces are thought to be primary causes. Two types of IBC can be detected: solitary cysts (Figure 10A), which are actually bile lakes, associated with poor prognosis[36] and likely determined by recurrent cholangitis; and continuous beaded cysts (Figures 11 and 12), which are dilated intrahepatic bile ducts and may be reversed. IBC are usually well detected with sonography. MDCT and MR both show good results in cyst detection and show advantages, compared to ultrasound, mainly in cases of large cysts as they can determine compression and/or dislodgment of vascular structures such as hepatic artery, portal vein and inferior vena cava (Figure 10B).
Figure 10 A 12-mo-old female child post-Kasai.
A: Multi-detector computed tomography (MDCT), isolated large biliary cyst in the right lobe (arrow); B: MDCT, volume rendering reconstruction shows inferior vena cava (IVC) compressed and displaced by the large intrahepatic biliary cyst.
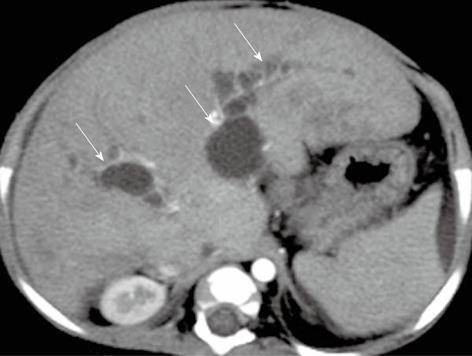
Figure 11 A 8-mo-old female child post-Kasai.
Multi-detector computed tomography shows multiple intrahepatic biliary cysts after Kasai operation (arrows).
Figure 12 A 5-mo-old female child post-Kasai.
Colangio magnetic resonance shows multiple intrahepatic biliary cysts (arrows).
INTRAHEPATIC NODULES
Kasai surgery increases the survival of patients with BA[11]. The survival benefit of the Kasai procedure can delay subsequent LT, as primary LT in very young patients has a high morbidity and mortality rate. Postponing LT, however, leads to an increase in complication rate in the long-term survivor group having a native liver with evidence of various intrahepatic nodules. A pseudotumor, giant regenerative nodule, or macroregenerative nodule, like hepatocellular carcinoma, may be correlated with liver cirrhosis. Other tumors usually not associated with cirrhosis, such as focal nodular hyperplasia, regenerative nodular hyperplasia, hepatic cholangiocarcinoma and hepatic adenoma, have also been reported[3,38-46].
Lesion biopsy is considered the gold standard for the diagnosis of liver hepatic nodules, especially for hepatocellular carcinoma. However, this procedure has several limitations, including possible bleeding, a significant false negative rate, high interobserver variability in the differentiation between high-grade dysplastic nodules and well-differentiated hepatocellular carcinoma, as well as the possibility that the bioptic sample may not be representative of the entire lesion[39]. Furthermore, biopsy may not be technically possible because of lesion localization. In BA cirrhotic patients, liver biopsy may be contraindicated also because of the presence of ascites or impaired coagulation test and, most importantly, it is not uncommon to find that multiple hepatic nodules coexist in these patients. In this scenario, imaging with MR and/or MDCT plays a key role in liver nodule diagnosis of BA patients avoiding, when possible, liver biopsy. Although rare, hepatocellular carcinoma can occur in children with BA. In these patients serum α-fetoprotein level can be within normal range; for this reason there is the necessity for repeated sequential MR or MDCT imaging to monitor possible malignant transformation of liver nodules in patients with BA after Kasai operation. The prevalence of hepatocellular carcinoma in explanted livers of patients transplanted for BA has been reported to be 0.73%-2.44%[43-45]. Although the presence of hepatocellular carcinoma is not a contraindication to LT in BA patients, a delayed diagnosis can impair the post-transplant prognosis because node involvement, vascular invasion and tumor size are risk factors for recurrence of hepatocellular carcinoma after LT.
Imaging of hepatocellular carcinoma in children with BA has been reported as similar to hepatocellular carcinoma in adult patients with cirrhosis, with enhancement of the tumor in the arterial phase after contrast administration followed by hypointensity/hypodensity compared with the liver in the delayed phase indicating washout. Delayed enhancement of a fibrous tumor capsule and areas with intratumoral fatty infiltration has also been reported[41].
In our experience, we have not found hepatocellular carcinoma in BA patients before LT with MDCT and/or MR or at liver explants. Regenerative nodular hyperplasia nodules can be detected in BA patients. They are characterized by hyperplastic nodules composed of cells resembling normal hepatocytes. The nodules range in size from smaller than a hepatic acinus to conglomerate nodules forming large masses (Figure 13)[46]. Large nodules are hyperplastic lesions measuring between 5 mm and 5 cm in diameter that are larger than most cirrhotic nodules located in a liver that is otherwise abnormal[47,48]. Such lesions might be caused by an abnormal liver cell response to an altered portal flow. These benign nodules typically appear as iso-hyperintense in T1 images, isointense in T2 images, hypervascular on contrast-enhanced, cross-sectional imaging and have the potential to be misdiagnosed as hepatocellular carcinoma as they appear as hypervascular masses within a chronically diseased liver. Differently from hepatocellular carcinoma in portal venous and/or delayed phase, they have no washout. Regenerative nodular hyperplasia nodules have been described in association with congenital absence of portal vein[48] and also in Budd-Chiari syndrome. In Budd-Chiari patients, a 4% incidence of hepatocellular carcinoma has also been reported[49] and in several cases hepatocellular carcinoma showed no typical washout on portal venous phase. In these patients increased α-fetoprotein level, increased size on successive imaging and the lack of a central scar were helpful for the diagnosis, although liver biopsy was performed to confirm hepatocellular carcinoma.
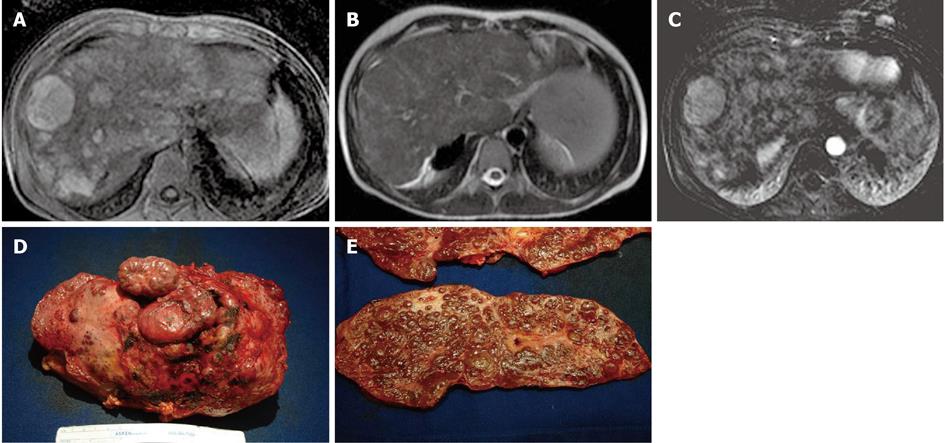
Figure 13 A 13-year-old male post-Kasai who underwent surgical spleno-renal shunt for gastrointestinal bleeding.
A: Axial T1 Fat Sat weighted acquisition shows multiple hyperintense nodules, the largest of these in S8; B: Axial T2 weighted acquisition: the nodules are isointense; C: Axial T1 Fat Sat weighted acquisition obtained with digital subtraction between the arterial phase and the unenhanced phase shows the vascular enhancement of the nodules; D: Explanted liver shows an irregular and multilobulated surface; E: Gross examination of sectioned liver confirms the presence of multiple regenerative nodular hyperplasia nodules.
Macroregenerative nodule is occasionally encountered in BA cirrhotic livers[39,49]. A macroregenerative nodule is defined as a hepatocellular nodule containing one or more portal tracts located in a liver that is otherwise abnormal due to either cirrhosis or other severe disease[48]. These nodules typically appear as iso-hyperintense in T1 images and iso-hypointense in T2 images. The blood supply of a regenerative nodule comes largely from the portal vein, with minimal contribution from the hepatic artery. This vascular supply dynamic explains why there is no enhancement during the hepatic arterial phase on MR or MDCT images, with iso-hypointensity on portal venous-delayed phases (Figure 14).
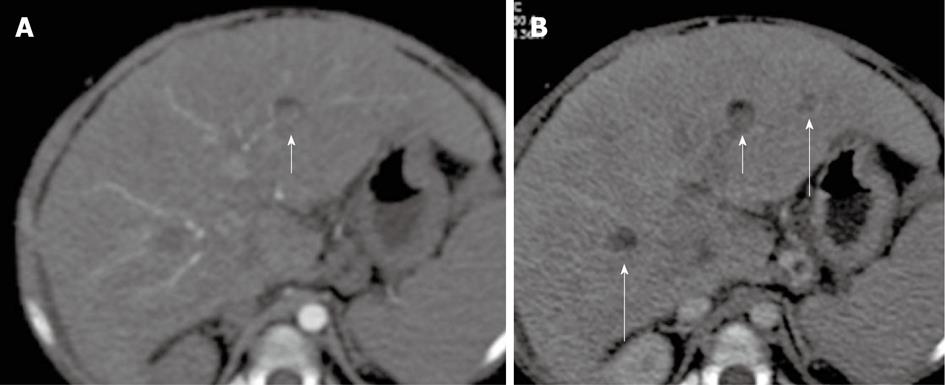
Figure 14 A 20-mo-old female without previous Kasai operation.
Multi-detector computed tomography: In S3, nodule (short arrow) that shows in anterior portion a mild amount of fat. The nodule appears slightly hyperdense in arterial phase (A) and hypoattenuating in portal/venous phase; B: Regenerative nodule was found at liver explant. Of note: other hypoattenuating regenerative nodules in S6 and S3 (long arrows).
In conclusion, we suggest following solid nodules with imaging in BA patients, proposing liver biopsy only for those with suspect morphological changes or in cases with increased α-fetoprotein level.
Advances in the field of MR, MDCT and in percutaneous minimally invasive techniques have increased the importance of radiology in the management of pediatric patients with BA who are candidates for LT.
Peer reviewer: Frank Pilleul, MD, PhD, Professor, Department of Radiology, Hospices Civils de Lyon, CHU E. Herriot, Pavillon H, Department of Gastrointestinal Imaging, Place d’Arsonval, 69003 Lyon, Cedex, France
S- Editor Cheng JX L- Editor Logan S E- Editor Zheng XM