INTRODUCTION
Calcific aortic valve disease (CAVD) affects 3% of the general population above 75 years of age and is the first indication for valvular replacement worldwide[1,2]. The formation of calcific lesions on the valve leaflets involves active processes including inflammation[3,4], extracellular matrix (ECM) remodeling[5-7] and osteogenesis[8-10]. Calcified valves typically exhibit increased expression of cytokines such as transforming growth factor-beta1 (TGF-β1)[11] and bone morphogenic protein-4 (BMP-4)[12]. While genetic defects[13] and conventional cardiovascular risk factors[2] have been identified as potential triggers of CAVD, blood flow abnormalities have emerged as a potential concomitant contributor[14-16]. Aging, hypertension and anatomical valve defects such as the bicuspid aortic valve, which are risk factors for CAVD[17-21], generate hemodynamic alterations that result in an abnormal friction force or “fluid shear stress” (FSS) on both sides of the leaflets[22-24].
To date, the evidence of causality between hemodynamic abnormalities and valvular pathogenesis has been provided ex vivo, using sophisticated bioreactors aimed at replicating the characteristics of the leaflet FSS environment[25,26]. Due to the challenge to replicate the native side-specific leaflet FSS (i.e., unidirectional on the ventricularis, oscillatory on the fibrosa), early studies investigated the role played by FSS abnormalities in valvular pathogenesis by subjecting only one leaflet surface at a time to flow. Studies conducted using this simplified model demonstrated the capability of combined alterations in FSS magnitude and pulsatility to stimulate inflammation on the leaflet fibrosa in a TGF-β1- and BMP-4-dependent manner[27], and the capability of elevated FSS to activate the fibrosa endothelium via synergies between BMP-4 and TGF-β1 pathways[28]. Recent advances in bioreactor design have enabled the replication of the native side-specific leaflet FSS in the laboratory setting. Simultaneous exposure of both leaflet surfaces to abnormalities in FSS magnitude and/or frequency has revealed the high sensitivity of the leaflet tissue to elevated FSS magnitude or frequency, and the ability of FSS abnormalities to promote paracrine signaling and ECM degradation[29].
The clear involvement of BMP-4 and TGF-β1 in hemodynamically induced CAVD provides a rationale for considering those molecules for targeted cell-based therapies aimed at attenuating or blocking the downstream pathological cascade. However, the upstream role of and potential synergies between TGF-β1 and BMP-4 in the transduction of FSS abnormalities have not been evidenced in the native (i.e., side-specific) leaflet FSS environment. Supported by our previous results, the present study addresses the hypothesis that TGF-β1 and BMP-4 synergistically interact to regulate valvular pathogenesis in response to side-specific alterations in FSS magnitude or frequency. This hypothesis was tested ex vivo by exploring the effects of each cytokine on the downstream FSS-induced pathological response. This dependence was characterized by measuring endothelial activation, paracrine signaling and ECM remodeling events secondary to FSS alterations after silencing or promoting pharmacologically the expression of each molecule.
MATERIALS AND METHODS
Experimental conditions
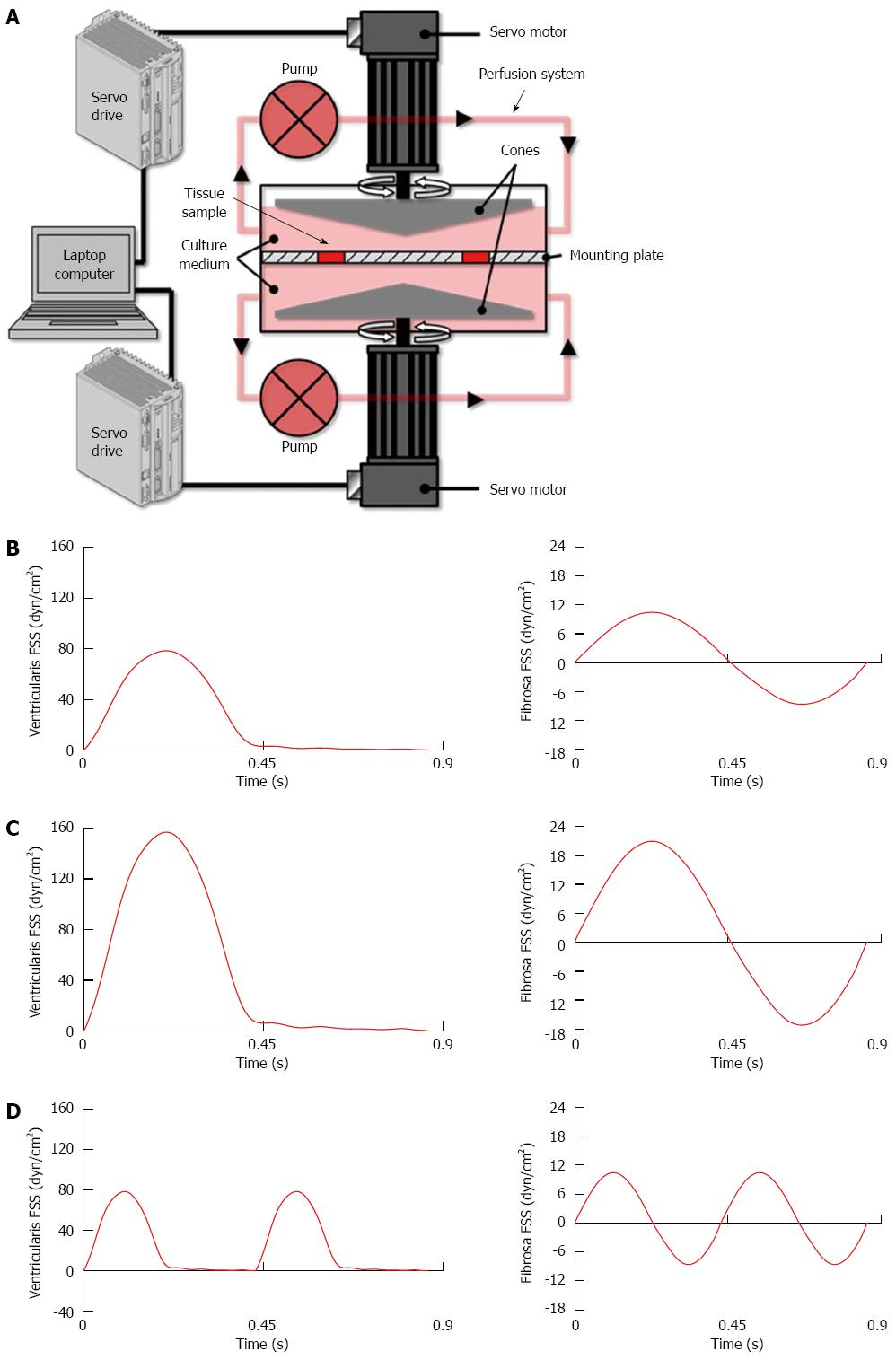
Porcine aortic valve leaflets were subjected to physiologic and supra-physiologic FSS environments ex vivo using our double-sided cone-and-plate bioreactor (Figure 1A)[26]. This device has been previously validated mechanically and biologically and implemented in different ex vivo studies to subject simultaneously but independently both leaflet surfaces to desired side-specific and time-varying FSS[29,30]. Fresh porcine valves (6-12 mo) were obtained from a local abattoir (Martin’s Custom Butchering, Wakarusa, IN), immediately transported to the laboratory in ice-cold phosphate buffered saline. A circular section of 7 mm in diameter was excised from the base of each leaflet. Two samples from each valve were used as experimental samples while the third sample served as fresh control. Six experimental samples were mounted in the bioreactor, exposing both their aortic and ventricular sides to FSS. All experiments were conducted for 48 h, a duration sufficient for valve leaflets to transduce FSS abnormalities into a pathological response[29].
Figure 1 Tissue conditioning methodology and fluid shear stress environments.
A: Schematic of the double-sided cone-and-plate bioreactor; B: Physiologic fluid shear stress (FSS); C: Supra-physiologic FSS magnitude at physiologic frequency; D: Supra-physiologic FSS frequency at physiologic magnitude.
Consistent with our previous studies[27-29], the physiologic FSS environment consisted of a unidirectional FSS varying between 0 and 80 dyn/cm2 on the ventricularis (leaflet surface facing the ventricle) and a reciprocal FSS varying between -8 and +10 dyn/cm2 on the fibrosa (leaflet surface facing the aorta; Figure 1B). The two supra-physiologic FSS environments consisted of supra-physiologic FSS magnitude (i.e., twice the physiologic level) at physiologic frequency (Figure 1C) and supra-physiologic FSS frequency (i.e., twice the physiologic frequency) at physiologic magnitude (Figure 1D). Those abnormal FSS environments were selected based on their demonstrated ability to stimulate acute CAVD mechanisms[29].
In order to isolate the possible synergies between BMP-4 and TGF-β1, the experiments were conducted using standard culture medium (Dulbecco’s Modified Eagle Medium, Sigma) as well as four additional culture medium variations. The downstream action of BMP-4 was blocked by supplementing the standard culture medium with noggin, a well-known BMP antagonist[31-33], while TGF-β1 signaling was blocked by supplementing the medium with SB-431542, a small molecule inhibitor specifically targeting the TGF-β type-I receptor[34,35]. The inhibitor concentrations used in this study (noggin: 100 ng/mL; SB-431542: 1 μmol/L) have been shown to effectively inhibit BMP- and TGF-β1 signaling in response to stretch and FSS abnormalities ex vivo[27,28,36]. Conversely, BMP-4 and TGF-β1 signaling were promoted by supplementing the standard culture medium with recombinant BMP-4 and TGF-β1, respectively, using concentrations (BMP-4: 10 ng/mL; TGF-β1: 10 ng/mL) previously established to effectively enhance paracrine signaling processes in valvular tissue[27,28]. Fresh porcine leaflets were used as controls.
Biological characterization
Endothelial activation was assessed in terms of intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1). Paracrine signaling events were characterized in terms of the cytokines BMP-4 and TGF-β1. ECM remodeling and degradation were quantified in terms of matrix metalloproteinases (MMP-2 and -9) and cathepsins (cathepsin L and S). Detailed immunostaining and immunoblotting protocols are described in Supplementary Material.
Statistical analysis
Each experimental group consisted of six leaflet samples. Data from each group were quantified as mean ± SD error and then normalized to the values measured in the fresh control. Following this procedure, all biomarker expressions were expressed in terms of a normalized mean value ± normalized standard error. Data from all experiments were tested for normality by the Anderson-Darling method, then analyzed using ANOVA followed by the Bonferroni post-hoc test. A P-value of less than 0.05 was used as a measure of statistical significance. The statistical review of the study was performed by a biomedical statistician (Dr. Jun Li, Department of Applied and Computational Mathematics and Statistics, University of Notre Dame, Notre Dame, IN, United States).
RESULTS
BMP-4 supplementation promotes endothelial activation in response to supra-physiologic FSS magnitude
Immunostaining was performed to examine endothelial activation in response to all three FSS environments using standard, pro- and anti-osteogenic culture media. Tissue conditioned under physiologic FSS did not exhibit any positive staining for ICAM-1 or VCAM-1, regardless of the culture medium (Figure 2A). Exposure of leaflet tissue to supra-physiologic FSS magnitude exhibited a similar trend except when BMP-4 was added to the culture medium, which resulted in ICAM-1 expression on the endothelial lining of the fibrosa (Figure 2B). Similarly to the results obtained under physiologic FSS, supra-physiologic FSS frequency did not promote cell adhesion molecule expression with any culture medium (Figure 2C).
Figure 2 Intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 immunostaining.
Tissue exposed for 48 h to: (A) physiologic fluid shear stress (FSS), (B) supra-physiologic FSS magnitude, and (C) supra-physiologic FSS frequency in standard medium and medium supplemented with BMP-4 [+(BMP-4)], noggin [-(BMP-4)], TGF-β1 [+(TGF-β1)] or SB-431542 [-(TGF-β1)] (F: Fibrosa; green: Positively stained cells; blue: Cell nucleus). ICAM-1: Intercellular adhesion molecule-1; VCAM-1: Vascular cell adhesion molecule-1; TGF-β1: Transforming growth factor-beta1; BMP-4: Bone morphogenic protein-4.
Synergistic effects of BMP-4 and TGF-β1
Potential synergies between BMP-4 and TGF-β1 signaling in response to FSS abnormalities were investigated by quantifying the expression of one cytokine following the pharmacological inhibition or supplementation of the other. Western blot results indicate that under physiologic FSS, medium supplementation with BMP-4 or noggin had no significant effect on TGF-β1 expression, which remained statistically similar to the levels measured in fresh controls and in tissue conditioned using the standard medium (Figure 3A). Similarly, no significant difference in BMP-4 expression was detected between any culture medium treatment groups. In contrast, analysis of tissue conditioned to supra-physiologic FSS magnitude (Figure 3B) revealed a significant 4.7-fold, 6.2-fold and 4.1-fold increase in TGF-β1 expression using standard medium, medium supplemented with BMP-4 and medium supplemented with noggin, respectively, relative to the fresh controls (467% ± 22%, 624% ± 100%, 405% ± 38%, respectively, vs 100% ± 6%; P < 0.05). However, no statistical difference in TGF-β1 expression was detected between the standard medium, BMP-4 treatment and noggin treatment groups. While supra-physiologic FSS also resulted in a significant 2.6-fold, 2.2-fold and 2.1-fold increase in BMP-4 expression using standard medium, medium supplemented with TGF-β1 and medium supplemented with SB-431542, respectively, relative to the fresh controls (258% ± 22%, 219% ± 33%, 207% ± 13%, respectively, vs 100% ± 4%; P < 0.05), no statistical difference in BMP-4 expression was detected between those three culture medium groups. Lastly, exposure of leaflet tissue to supra-physiologic FSS frequency using the five culture media (Figure 3C) produced results similar to those obtained under physiologic FSS, in which no significant difference in TGF-β1 or BMP-4 expression was detected between the fresh controls, the standard medium group, the pro-osteogenic medium groups (BMP-4 or TGF-β1 treatment) and the anti-osteogenic medium groups (noggin or SB-431542 treatment).
Figure 3 Transforming growth factor-beta1 and bone morphogenic protein-4 immunoblotting.
Tissue exposed for 48 h to: (A) physiologic fluid shear stress (FSS), (B) supra-physiologic FSS magnitude, and (C) supra-physiologic FSS frequency standard medium and medium supplemented with BMP-4 [+(BMP-4)], noggin [-(BMP-4)], TGF-β1 [+(TGF-β1)] or SB-431542 [-(TGF-β1)] (aP < 0.05 vs fresh control). TGF-β1: Transforming growth factor-beta1; BMP-4: Bone morphogenic protein-4.
TGF-β1 silencing reduces MMP-9 expression in response to FSS abnormalities
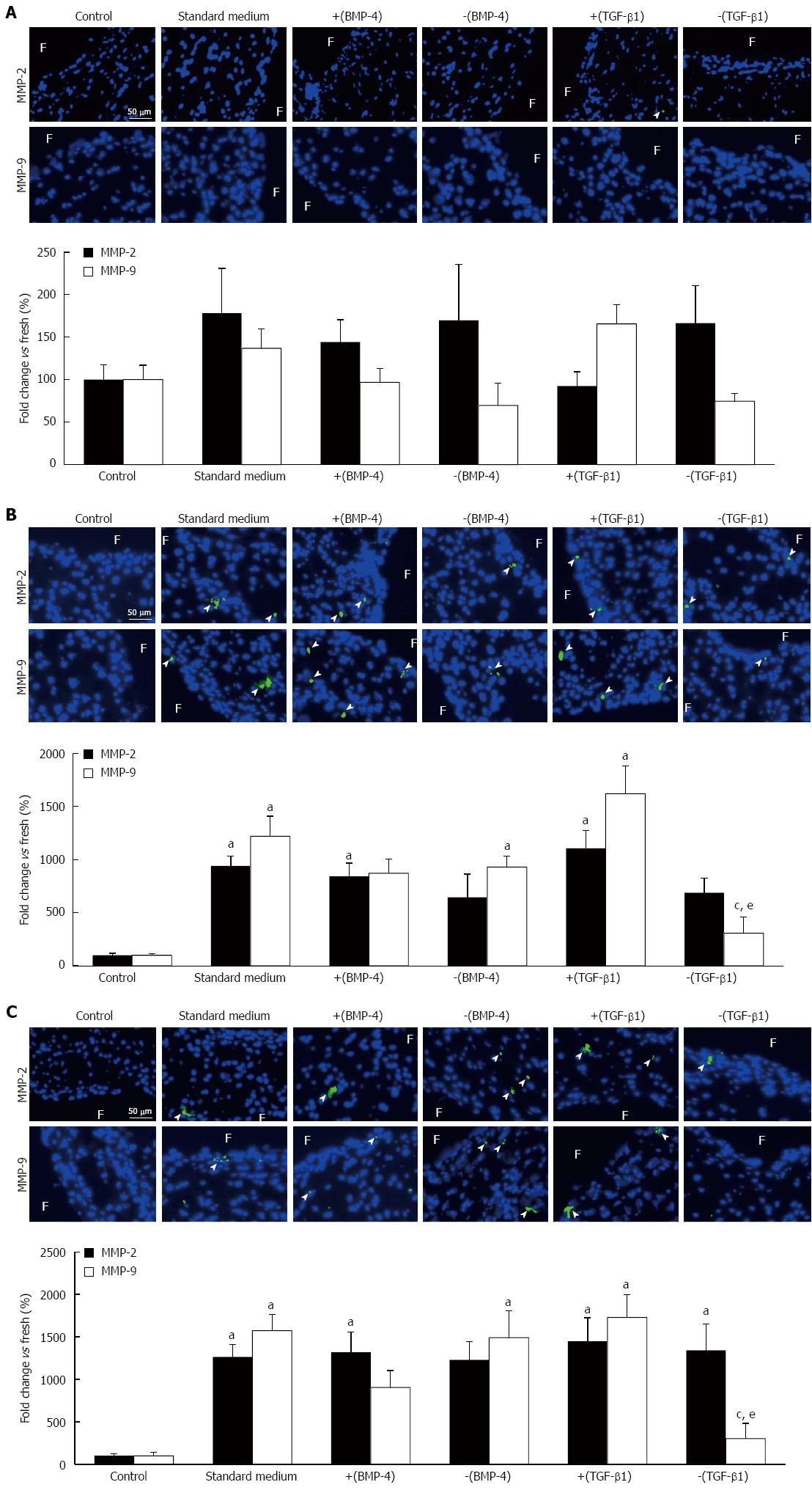
MMP-2 and MMP-9 immunostaining was performed in leaflet tissue exposed to physiologic and supra-physiologic FSS using the five culture media to characterize the downstream action of BMP-4 and TGF-β1 on ECM degradation. No significant difference in MMP-2 and MMP-9 expression was detected between any culture medium treatment group and the fresh controls in leaflets subjected to physiologic FSS (Figure 4A). Leaflet exposure to supra-physiologic FSS magnitude resulted in a significant 9.4-fold, 8.4-fold and 11.1-fold increase in MMP-2 expression using standard medium, medium supplemented with BMP-4 and medium supplemented with TGF-β1, respectively, relative to the fresh controls (941% ± 90%, 842% ± 126%, 1108% ± 170%, respectively, vs 100% ± 19%; P < 0.05; Figure 4B) but no difference in expression was detected between the five culture media. A significant 12.2-fold, 9.3-fold and 16.2-fold increase in MMP-9 expression was also observed with the standard medium, noggin and TGF-β1 treatment groups, respectively, relative to the fresh controls (1219% ± 190%, 931% ± 104%, 1621% ± 261%, respectively, vs 100% ± 16%; P < 0.05), with no significant difference in MMP-9 expression between the five culture media. In contrast, TGF-β1 silencing resulted in a significant 75% and 81% reduction in MMP-9 expression relative to the standard culture medium and TGF-β1 treatment group, respectively, and resulted in a MMP-9 expression level statistically similar to that measured in fresh controls. Lastly, supra-physiologic FSS frequency resulted in a significant 12.6-fold, 13.2-fold, 14.5-fold and 13.4-fold increase in MMP-2 expression using standard medium, medium supplemented with BMP-4, medium supplemented with TGF-β1 and medium supplemented with SB-431542, respectively, relative to the fresh controls (1264% ± 145%, 1318% ± 239%, 1447% ± 278%, 1339% ± 314%, respectively, vs 100% ± 21%; P < 0.05) without any significant difference between any culture medium groups (Figure 4C). This FSS environment also resulted in a significant 15.7-fold, 14.9-fold and 17.3-fold increase in MMP-9 expression in the standard culture medium, noggin and TGF-β1 treatment groups, respectively, relative to the fresh controls (1571% ± 191%, 1488% ± 316%, 1728% ± 268%, respectively, vs 100% ± 42%; P < 0.05). TGF-β1 silencing resulted in a significant 81% and 83% reduction in MMP-9 expression relative to the standard culture medium and TGF-β1 treatment group, respectively (302% ± 182% vs 1571% ± 191% and 1728% ± 268%, respectively; P < 0.05), and resulted in a MMP-9 expression level statistically similar to that measured in fresh controls.
Figure 4 Metalloproteinases-2 and metalloproteinases-9 immunostaining.
Tissue exposed for 48 h to: (A) physiologic fluid shear stress (FSS), (B) supra-physiologic FSS magnitude, and (C) supra-physiologic FSS frequency standard medium and medium supplemented with BMP-4 [+(BMP-4)], noggin [-(BMP-4)], TGF-β1 [+(TGF-β1)] or SB-431542 [-(TGF-β1)] (F: Fibrosa; green: Positively stained cells; blue: Cell nucleus; aP < 0.05 vs fresh control; cP < 0.05 vs standard culture medium; eP < 0.05 vs standard medium supplemented with TGF-β1). MMP: Metalloproteinases; TGF-β1: Transforming growth factor-beta1; BMP-4: Bone morphogenic protein-4.
BMP-4 and TGF-β1 do not synergistically regulate cathepsin expression in response to FSS
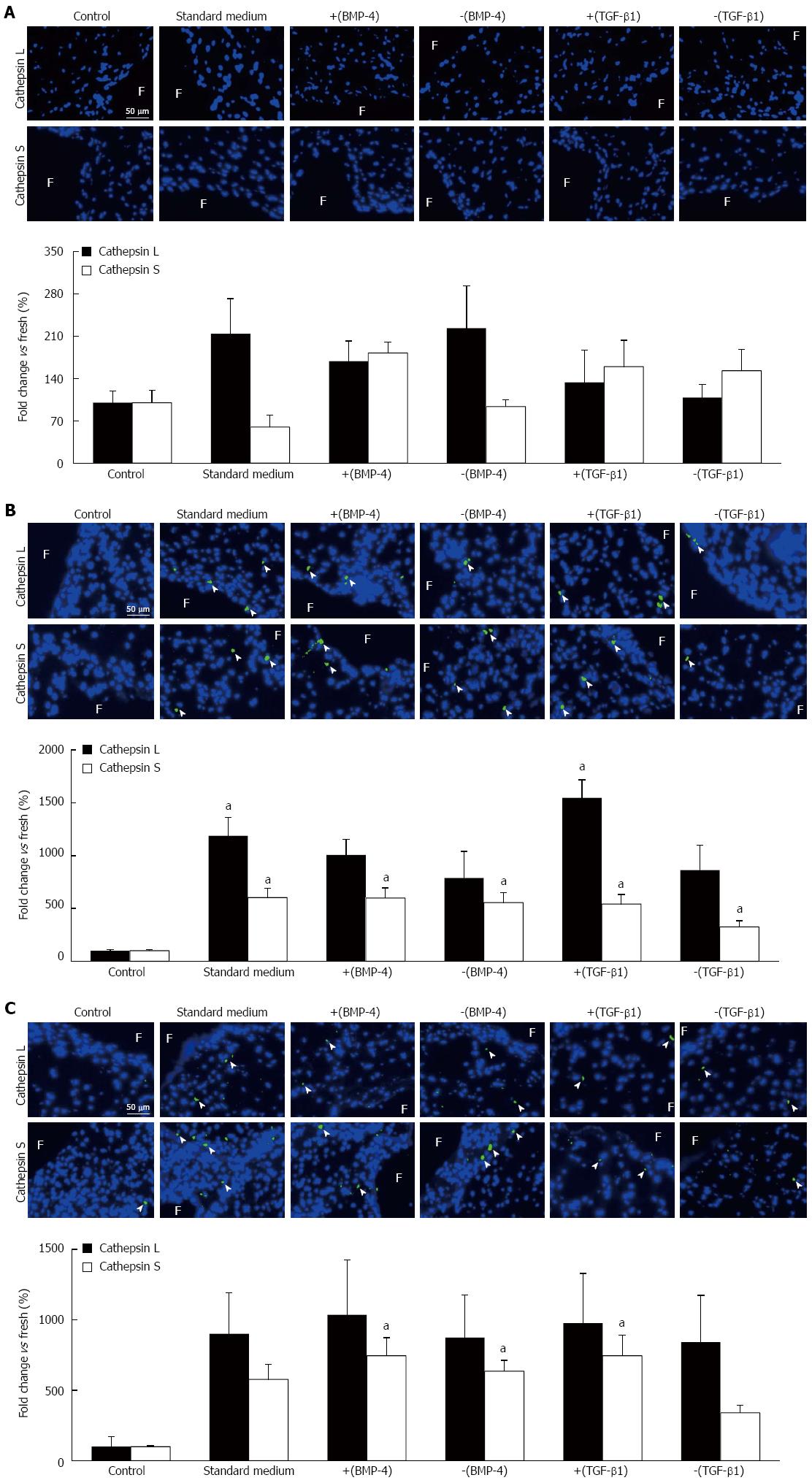
The synergistic effects of BMP-4 and TGF-β1 on FSS-mediated protease expression were characterized via cathepsin L and cathepsin S immunostaining. Under physiologic FSS, no significant difference in cathepsin L and cathepsin S expression was detected between any culture medium treatment group and the fresh controls (Figure 5A). Tissue exposure to supra-physiologic FSS magnitude resulted in a significant 11.9-fold and 15.5-fold increase in cathepsin L expression using the standard medium and medium supplemented with TGF-β1, respectively, relative to the fresh controls (1187% ± 175%, 1546% ± 171%, respectively, vs 100% ± 12%; P < 0.05; Figure 5B). The same FSS environment resulted in a significant 6.0-fold, 6.0-fold, 5.5-fold, 5.4-fold and 3.3-fold increase in cathepsin S expression using the standard medium, BMP-4, noggin, TGF-β1 and SB-431542 treatment groups, respectively, relative to the fresh controls (603% ± 88%, 598% ± 96%, 554% ± 94%, 541% ± 92%, 325% ± 57%, respectively, vs 100% ± 13%; P < 0.05). However, the effects of BMP-4 and TGF-β1 on protease expression remained limited as indicated by the absence of significant difference in cathepsin L and S expression between the different medium treatment groups. Lastly, supra-physiologic FSS frequency did not promote cathepsin L expression, regardless of the culture medium treatment group (Figure 5C). In contrast, the same mechanical treatment resulted in a significant 7.4-fold, 6.4-fold and 7.4-fold increase in cathepsin S expression in the BMP-4, noggin and TGF-β1 treatment groups, respectively, relative to the fresh controls (744% ± 129%, 635% ± 76%, 744% ± 144%, respectively, vs 100% ± 7%; P < 0.05).
Figure 5 Cathepsin L and cathepsin S immunostaining.
Tissue exposed for 48 h to: (A) physiologic fluid shear stress (FSS), (B) supra-physiologic FSS magnitude, and (C) supra-physiologic FSS frequency standard medium and medium supplemented with BMP-4 [+(BMP-4)], noggin [-(BMP-4)], TGF-β1 [+(TGF-β1)] or SB-431542 [-(TGF-β1)] (F: Fibrosa; green: Positively stained cells; blue: Cell nucleus; aP < 0.05 vs fresh control). TGF-β1: Transforming growth factor-beta1; BMP-4: Bone morphogenic protein-4.
DISCUSSION
In this ex vivo study, we investigated the role of the cytokines BMP-4 and TGF-β1 in the acute pathological response of porcine valve leaflets exposed to FSS abnormalities. We demonstrated that: (1) valvular endothelial activation is weakly regulated by BMP-4 in response to FSS abnormalities; (2) TGF-β1 silencing attenuates FSS-induced ECM degradation via MMP-9 downregulation; and (3) BMP-4 and TGF-β1 do not synergistically interact in response to FSS abnormalities.
This study first confirms the key role played by FSS in the maintenance of valvular homeostasis. In fact, exposure of leaflet tissue to its native FSS environment did not stimulate any pathological event and resulted in biomarker expressions similar to those measured in fresh tissue, regardless of the culture medium. Valvular tissue has been shown to be sensitive to the forces present in its hemodynamic environment[37,38]. As compared to stretch and pressure which propagate throughout the leaflet and stimulate both valvular endothelial cells (VECs) and interstitial cells (VICs), FSS is an interfacial stress sensed primarily by VECs. Therefore, the ability of FSS alone to maintain leaflet homeostasis in the absence of any other mechanical signal demonstrates the key role played by the leaflet endothelium in valvular function. In contrast, leaflet exposure to supra-physiologic FSS resulted in increased paracrine signaling and ECM degradation. Those results confirm those of previous ex vivo studies on the effects of normal and abnormal FSS on valvular biology[27-29] and on the role played by FSS in bicuspid aortic valve calcification[30].
This study is the first to report the dependence of FSS-mediated valvular ECM degradation on TGF-β1 signaling, as suggested by the dramatic decrease in MMP-9 expression in response to FSS abnormalities following TGF-β1 inhibition. The potential involvement of TGF-β1 in valvular ECM degradation is consistent with previous reports suggesting the modulation of MMP-9 expression by several growth factors and inflammatory cytokines in sheep VICs[39,40]. The ability of the small molecule inhibitor SB-431542 to reduce MMP-9 expression to the level measured in fresh leaflets also suggests the possible use of this molecule in drug-based therapies aimed at attenuating ECM degradation.
Interestingly, this study did not reveal the existence of clear synergies between BMP-4 and TGF-β1 signaling in response to FSS abnormalities, as shown by the inability of the pro- and anti-osteogenic media to alter the expression of those cytokines. This result differs from our previous ex vivo results on the effects of single-sided FSS magnitude and/or pulsatility abnormalities on the leaflet fibrosa[27,28], which suggested a downregulation of FSS-induced BMP-4 expression following TGF-β1 inhibition. One possible explanation for this discrepancy is the difference in mechanical environment considered in those studies. While those earlier ex vivo studies subjected only one leaflet surface to FSS abnormalities, the present experiments were performed using a more realistic side-specific FSS environment, which potentially attenuated the severity of the pathological response.
Lastly, the interpretation of the results should be put in the perspective of two important considerations. First, noggin is a BMP antagonist that not only binds BMP-4 but also BMP-2, -5, -6 and -7[31,32]. Therefore, the observed biological response following noggin supplementation may be the result of the inhibition of other BMP members. Second, while the study only focuses on the acute biological response, the results may still be relevant to the long-term processes leading to CAVD as valve leaflets respond to FSS alterations in periods as short as 48 h and continued mechanical conditioning for up to 72 h has been shown not to alter that initial response[29].
In summary, this study provides further evidence of the key role played by BMP-4 and TGF-β1 in valvular FSS mechanotransduction and the specific involvement of TGF-β1 in FSS-induced ECM degradation. While inhibition of those cytokines is not sufficient to completely block the FSS-induced pathological response, the TGF-β1 inhibitor SB-431542 emerges as a potential candidate molecule for attenuating the adverse effects of FSS abnormalities on ECM degradation.
COMMENTS
Background
Calcific aortic valve disease (CAVD) is driven by inflammatory, remodeling and osteogenic processes triggered by cardiovascular risk factors and hemodynamic cues. In particular, supra-physiologic fluid shear stress (FSS) environments, which may result from hypertension, aging and valvular defects, have been shown to stimulate early CAVD signaling in a bone morphogenetic protein-4 (BMP-4) and transforming growth factor-beta1 (TGF-β1)-dependent manner. While the demonstrated involvement of BMP-4 and TGF-β1 in early CAVD provides a rationale for considering those molecules in targeted cell-based therapies aimed at attenuating or blocking the downstream pathological cascade, the synergies and modes of action of those molecules in response to FSS abnormalities have not been defined yet.
Research frontiers
The current modality to treat CAVD consists of the complete replacement of the valve by an artificial implant, which only partially restores valvular function and does not address the root cause of the disease. The development of non-invasive pharmacological approaches requires more insights into the basic biology of CAVD. Therefore, the characterization of the signaling pathways involved in the disease and the interacting mechanisms of cardiovascular calcification, micro-scale mechano-transduction and macro-scale hemodynamics are current hotspots in valvular research.
Innovations and breakthroughs
Their previous characterization of the contribution of side-specific FSS magnitude and/or frequency abnormalities to early valvular pathogenesis revealed the sensitivity of the leaflet tissue to elevated FSS magnitude or frequency and the ability of FSS abnormalities to promote paracrine signaling via BMP-4- and TGF-β1-dependent pathways. The present study is a logical extension of our previous work as it investigates the upstream roles of BMP-4 and TGF-β1 in the FSS-mediated valvular response. Here, the authors programmed their FSS bioreactor to generate the most unfavorable FSS environment for valvular tissue (i.e., side-specific supra-physiologic FSS magnitude or side-specific supra-physiologic FSS frequency) and the authors aimed at isolating the mechanistic role played by BMP-4 and TGF-β1 in the FSS-mediated pathological response by either promoting or inhibiting pharmacologically the downstream action of those molecules. The results demonstrate for the first time the mechano-sensitivity of BMP-4 and TGF-β1 to alterations in the native valvular FSS environment and their respective role in FSS-mediated pathogenesis. While BMP-4 promotes valvular endothelial activation in response to supra-physiologic FSS, TGF-β1 mediates extracellular matrix (ECM) degradation.
Applications
The ability of the TGF-β1 inhibitor SB-431542 to reduce flow-mediated ECM degradation suggests the possible use of this molecule in non-invasive drug-based therapies aimed at attenuating flow-induced aortic valve pathogenesis.
Terminology
CAVD is the most common valvular disease and is characterized by the formation of calcific lesions on the valve leaflets. FSS is the frictional fluid force resulting from the relative motion between the valve leaflets and the surrounding blood flow.
Peer-review
This is a very well conducted study.