Published online Aug 26, 2015. doi: 10.4331/wjbc.v6.i3.83
Peer-review started: May 13, 2015
First decision: June 24, 2015
Revised: July 1, 2015
Accepted: July 24, 2015
Article in press: July 27, 2015
Published online: August 26, 2015
The retrovirus integrase (IN) is responsible for integration of the reverse transcribed linear cDNA into the host DNA genome. First, IN cleaves a dinucleotide from the 3’ OH blunt ends of the viral DNA exposing the highly conserved CA sequence in the recessed ends. IN utilizes the 3’ OH ends to catalyze the concerted integration of the two ends into opposite strands of the cellular DNA producing 4 to 6 bp staggered insertions, depending on the retrovirus species. The staggered ends are repaired by host cell machinery that results in a permanent copy of the viral DNA in the cellular genome. Besides integration, IN performs other functions in the replication cycle of several studied retroviruses. The proper organization of IN within the viral internal core is essential for the correct maturation of the virus. IN plays a major role in reverse transcription by interacting directly with the reverse transcriptase and by binding to the viral capsid protein and a cellular protein. Recruitment of several other host proteins into the viral particle are also promoted by IN. IN assists with the nuclear transport of the preintegration complex across the nuclear membrane. With several retroviruses, IN specifically interacts with different host protein factors that guide the preintegration complex to preferentially integrate the viral genome into specific regions of the host chromosomal target. Human gene therapy using retrovirus vectors is directly affected by the interactions of IN with these host factors. Inhibitors directed against the human immunodeficiency virus (HIV) IN bind within the active site of IN containing viral DNA ends thus preventing integration and subsequent HIV/AIDS.
Core tip: This review examines the multifunctional properties of retrovirus integrase (IN) besides its key function of integrating the viral DNA into host chromosomes. IN has a major role in the maturation of the virus, reverse transcription and nuclear transport of the preintegration complex. IN binds to cellular cofactors for uncoating of the core and to other cellular proteins that guide the preintegration complex to preferred regions on the host genome for integration. Understanding these IN functions has resulted in the production of clinical IN strand transfer inhibitors to prevent human immunodeficiency virus (HIV/AIDS) and development of retrovirus vectors for human gene therapy.
- Citation: Grandgenett DP, Pandey KK, Bera S, Aihara H. Multifunctional facets of retrovirus integrase. World J Biol Chem 2015; 6(3): 83-94
- URL: https://www.wjgnet.com/1949-8454/full/v6/i3/83.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v6.i3.83
Retroviruses are ubiquitous in nature and are found in yeast to insects and to the animal and plant kingdoms. In retroviruses, the single stranded viral RNA genome is reverse transcribed by the viral reverse transcriptase into a double strand DNA copy that is subsequently integrated into the host genome. The integration process that inserts the viral DNA into the host DNA is mediated by the viral encoded integrase (IN). Retroviruses are mobile elements that distinguish themselves from other viruses because their DNA genomes become an integral part of the host genome, an essential requirement in their replication cycle. There are slight variations in this integration process in the above mentioned biological systems, some of which are called Ty1/copia and Ty3/gypsy elements or LTR retrotransposons. This review will focus on IN found in alpharetroviruses, gammaretroviruses, lentiviruses which includes human immunodeficiency virus (HIV), and spumaviruses. The purpose of the review will be to briefly highlight the most current salient features of the many functional facets of the retrovirus IN and identify critical papers and reviews that provide in-depth information for a specific subject.
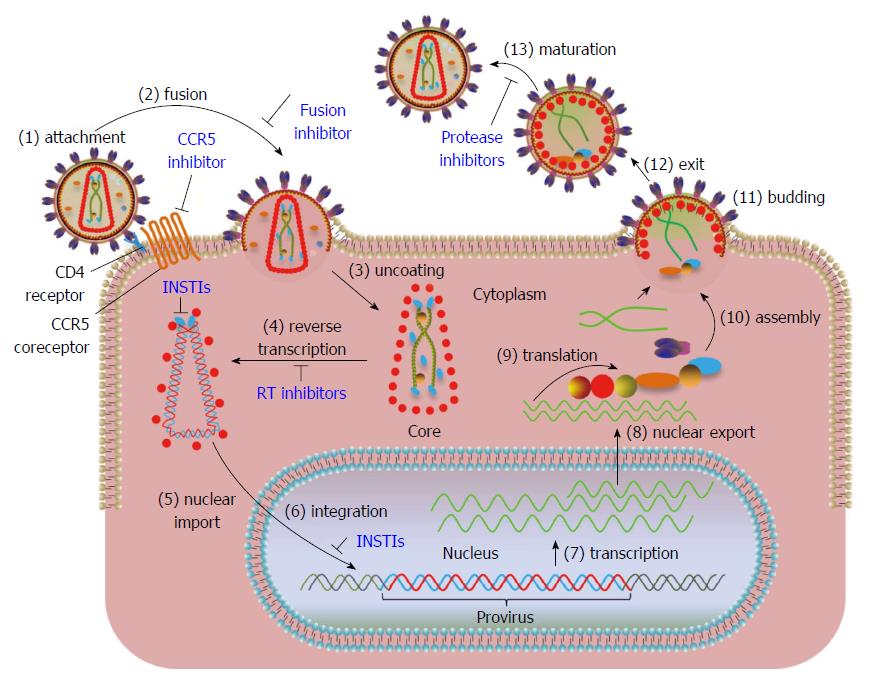
HIV is the most studied retrovirus with regards to its biology, biochemistry and pathogenesis. A general retrovirus replication cycle is depicted in Figure 1 along with location of steps for FDA approved HIV drug therapies. Each step from the binding of the virus particle to a cellular receptor to the release of a new virus particle is described in the legend. In Figure 1, IN plays a role in step 3: uncoating of the internalized viral core, step 4: reverse transcription of the viral RNA, step 5: nuclear transport of the preintegration complex (PIC), step 6: integration of viral DNA into the host genome, and step 13: maturation.
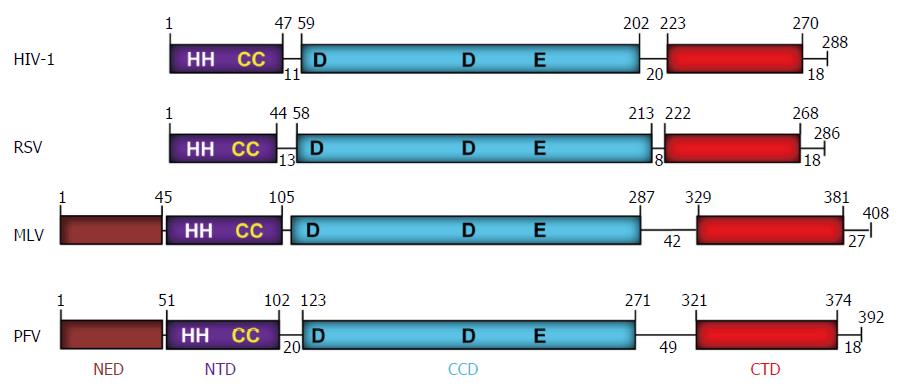
The retrovirus IN is located at the C-terminal end of the Pol protein that is part of a larger precursor polyprotein (Gag-Pol) encapsulated within the virus particle. The retrovirus IN was first identified and purified from an alpharetrovirus[1] and genetically shown to be necessary for integration[2-5]. The avian retrovirus or Rous sarcoma virus (RSV) and HIV IN proteins are 286 and 288 residues in length, respectively, while the prototype foamy virus (PFV) IN is 392 residues[6] (Figure 2). RSV IN possesses three structural domains comprising an N-terminal domain (NTD) (residues 1-44) that contains a zinc-binding HHCC motif, a catalytic core domain (CCD) (residues 58-213) and a C-terminal domain (CTD) (residues 222-268). HIV IN has similar size domains and linkers as RSV IN. Both PFV and murine leukemia virus (MLV) IN (408 residues) possess an additional N-terminal extension domain (NED) (approximately 50 residues) attached adjacent to the NTD of IN.
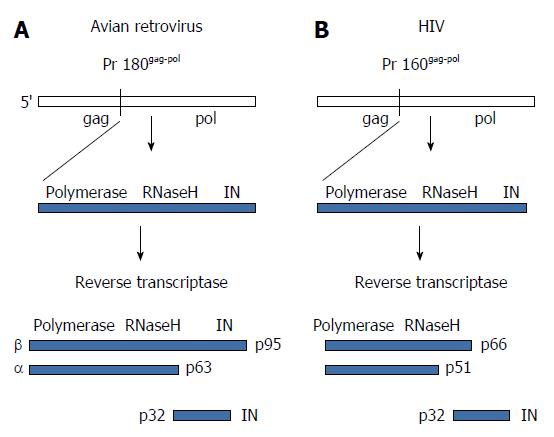
The retrovirus structural Gag proteins are approximately 80% of the total mass of the viral proteins. The reverse transcriptase (RT) and IN are derived from a separate Gag-Pol precursor polyprotein produced by translational frameshifts. This precursor polyprotein accounts for approximately 2% to 3% of the total viral protein mass (Figure 3). Despite this lower level of IN in virus particles, studies using site-directed mutagenesis of HIV IN highly suggest that deletion and missense mutations affect HIV assembly and release[7]. The mechanisms associated with these defects are not well understood but may include activation of the viral protease located within Gag-Pol and dimerization of this precursor polyprotein.
The recent discovery of inhibitors that do not interact within the catalytic active site of HIV IN has provided unforeseen information on a structural role for IN in virus particle maturation. These inhibitors called LEDGINS[8] or ALLINIS (allosteric IN inhibitors)[9] prevent interactions of IN with a cellular chromatin binding protein lens epithelium-derived growth factor (LEDGF/p75), a transcriptional cofactor that guides the HIV PIC (Figure 1) to active transcription sites for integration (see host factors). In addition to inhibiting integration, significant structural defects are caused by these inhibitors resulting in abnormal formation of electron dense HIV virus cores thus producing non-infectious particles upon egress[10-12] (Figure 1). Other studies have shown that these inhibitors significantly induce HIV IN monomers to produce inactive oligomers in vitro[10,13,14]. These results highly suggest that IN plays a critical role in virus maturation (Figure 1).
RT and IN are linked together covalently at the C-terminal end of Gag-Pol precursor polyprotein (Figure 3). The processing of the precursor protein to RT and IN by the viral protease in the virus particle is different between RSV and HIV. For RSV, the Pol product from the polyprotein is a 95 kDa polypeptide termed β that forms dimers (Figure 3). The viral protease cleaves the dimers into active RT, termed αβ, which causes the release and activation of the 32 kDa IN. The αβ polypeptide still contains a single copy of IN residues that facilitates a processive mechanism for reverse transcription and its associated RNaseH activity. In contrast, HIV IN is released entirely from the polyprotein producing RT (p66/p51) (Figure 3), similarly to MLV IN (not shown).
Site-specific mutations introduced into the cleaved HIV IN result in reverse transcription defects but not integration[15]. The exact mechanisms associated with these RT defects are mostly unknown. HIV IN (Figure 3) appears to have a physical interaction with RT (p66/p51) that is necessary for normal viral DNA synthesis[16-19]. In normal HIV infected cells, IN interacts with the major structural viral capsid protein (CA) whose interactions affect the normal uncoating process of the core and thus reverse transcription[20]. Following the above theme that viral core uncoating affects reverse transcription, recent results suggest that the cellular protein DYNLL1 is required for early viral DNA synthesis and uncoating via a physical interaction of DYNLL1 with HIV IN[21]. As these authors suggested, IN-DYNLL1 interactions may be a potential target for future inhibitors.
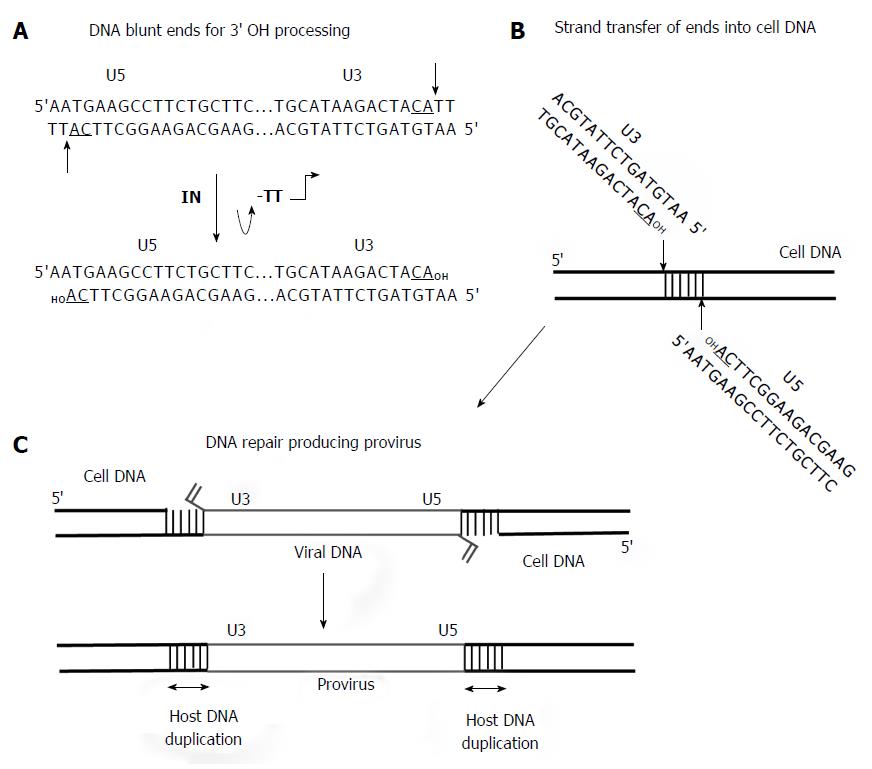
Although IN has many roles in the retrovirus replication cycle, IN is the catalytic component necessary for concerted integration of the viral DNA ends into the host genome (Figures 4 and 5). A pivotal study clearly demonstrated that linear MLV DNA acts as the substrate for integration in vivo[22]. The high molecular weight PIC containing a copy of the linear viral DNA was isolated from MLV infected cells (Figures 1 and 4). This complex is capable of integrating the viral ends in a concerted fashion into a target substrate in vitro. DNA sequence analysis of the integrated viral DNA demonstrated that IN inserted the 3’ OH ends into opposite strands of the target DNA producing the 4 bp host site duplication found associated with the integrated MLV provirus in vivo or a 6 bp duplication with RSV (Figure 5). The host site duplication for HIV is 5 bp.
There are numerous publications (many not indicated here) describing the integration capabilities of the PIC for MLV[22,23] and later for HIV[24-27] at the biochemical, genetic and inhibition of strand transfer by IN inhibitors (Figure 1). The PIC is composed of linear DNA, viral proteins IN, RT, CA, matrix (MA), viral protein R (Vpr) and several cellular associated proteins (Figure 4). In the cytoplasm, IN cleaves a dinucleotide from the 3’ OH ends of blunt DNA before transport of the PIC into the nucleus (Figure 5). HIV IN also appears to play a role in the nuclear transport of the PIC along with other viral and cellular proteins[28,29]. After transport, IN inserts the 3’ OH recessed DNA ends into the cellular DNA genome. As described later, the HIV IN strand transfer inhibitors (STIs) interact within the active site of IN and the 3’ processed LTR ends in the cytoplasmic PIC, thus inactivating the complex preventing integration into cellular DNA[27]. The retrovirus PIC has provided major insights into retrovirus integration but has a severe limitation for providing sufficient quantities necessary for biochemical studies.
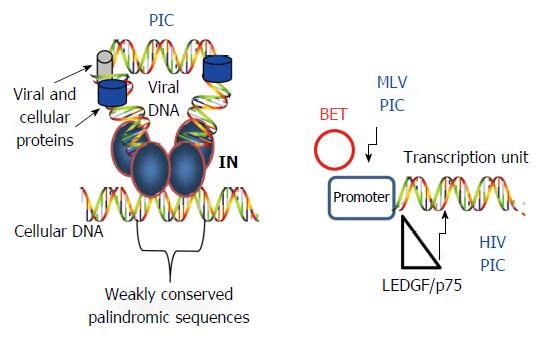
IN is the dominant factor for selection of weakly conserved palindromic sequences at the target site in vivo[30-32] (Figure 4). Whole genomic sequencing of DNA target sites demonstrates that HIV IN prefers to integrate its viral DNA into active transcriptional units[30] while MLV IN favors integration sites near the promoter region of genes and not into transcriptional units[33]. Further studies demonstrated that RSV IN essentially integrates its DNA into the host genome at random sites[34,35]. These results suggested that HIV and MLV IN interact with specific host factors that direct IN to preferred sites in the host genome.
Major studies have centered on the ability of LEDGF/p75, a transcriptional co-activator, to guide the HIV PIC to integrate its DNA into active transcriptional sites[36-41] (Figure 4). Besides LEDGF/p75, cellular Nup153 is indispensable for the integration of HIV DNA into the peripheral edge of the nuclear DNA suggesting that nuclear topography is also an important for HIV integration site selection[42]. For MLV IN, recent studies demonstrated that IN interacts with the chromatin BET (bromo- and extra-terminal domain) proteins (BRD2, BRD3 and BRD4) directing it to the promoter region of genes[39,43-45] (Figure 4). To date, there are no reports that have identified cellular proteins involved in host site selection by avian retroviruses. In summary, IN is capable of integrating its viral DNA into numerous segments of the host genome but is preferentially directed to specific regions by host factors.
Biochemical and biophysical studies of retrovirus IN has been extensive over the last 37 years. Most studies have used recombinant RSV, HIV, MLV and PFV IN. Only the avian retrovirus IN has been purified from virus particles[1,46]. As stated above, IN cleaves a dinucleotide from both 3’ OH blunt ends of the viral DNA and subsequently inserts the recessed ends into a target DNA by a transesterification reaction (Figure 5). The biochemistry of these reactions and other structural properties of IN were recently reviewed[47].
The subunit structure of IN is varied and highly dependent on the purification process and different salt requirements to maintain solubility for each expressed recombinant IN. PFV IN is soluble (10-15 mg/mL) in 0.2 mol/L NaCl[48,49] while RSV IN is highly soluble (30 mg/mL) in 0.2 mol/L (NH4)2SO4[50]. PFV IN is monomeric[49] while RSV IN is a dimer in solution[50-52]. Recombinant HIV IN has been purified as a monomer, dimer and tetramer[47,53,54]. Recent studies suggest that the active form of HIV IN is a monomer that is responsible for assembly of the synaptic complex (SC) or intasome capable of efficient concerted integration of two oligonucleotides into a target substrate in vitro[53]. The HIV IN monomer purified in the presence of EDTA is converted into a tetramer in the presence of Zn2+ without the formation of a dimer intermediate[53,54]. Studies have demonstrated that a tetramer of IN is associated with HIV, PFV and RSV SC capable of concerted integration[50,55-57].
The oligomeric state of retrovirus IN play key roles in virus maturation upon release of the virus particle, enzymatic activities for 3’ OH processing and concerted integration of viral DNA and possibly for reverse transcription in the cytoplasmic PIC.
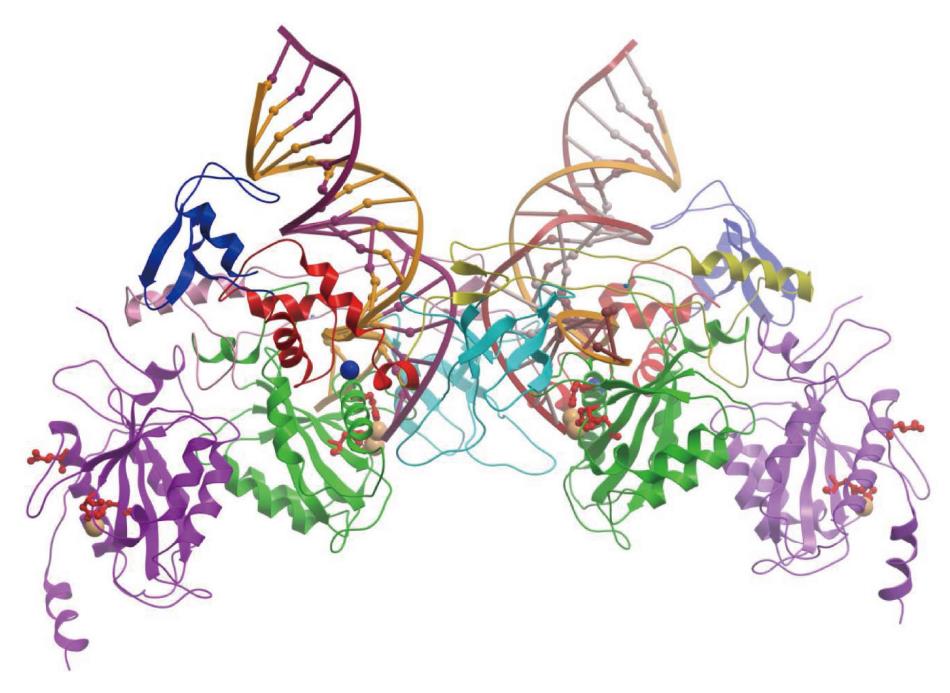
The retrovirus IN superfamily comprises proteins that are involved in a variety of activities like replication and repair of DNA, V(D)J recombination, and DNA transposition. The DDE domain of retrovirus IN (Figure 2) is highly conserved in proteins capable of similar catalytic reactions including MuA transposase, human RAG1 recombinase, the RNaseH domain on the retrovirus RT (Figure 3) and other bacterial and human endonucleases[58,59]. Structures of many of the above protein have been resolved at the atomic level in complex with DNA. However, among the retrovirus INs only the four-domain PFV intasome (PFV IN-DNA complex) has been resolved (Figure 6).
The crystallographic study of the full-length PFV IN with bound viral DNA revealed, for the first time, the architecture of retrovirus intasome assembly and atomic details of the IN-DNA interactions[57,60] (Figure 6). The PFV intasome contains a tetramer of IN, which consists of a dimer of the “inner” subunits that make extensive protein-protein and protein-DNA interactions, and a pair of the “outer” subunits that appear to play structural roles. All four domains of the IN subunits and the inter-domain linkers interact extensively with DNA to bridge between the two viral DNA ends and capture the target DNA. The engagement of the viral DNA sequence and catalysis in trans is a feature shared with related transposase systems[59,61].
The determination of the atomic structure of the PFV intasome greatly enhanced our understanding of IN interactions with 3’ OH recessed ends in the active site and showing how a tetramer of IN catalyzes the concerted integration reaction[57]. Other structural studies have shown how PFV IN is bound to the viral DNA in its ground state prior to 3’ OH processing or strand transfer[62]. Lastly, the PFV intasome in complex with target DNA after insertion of the 3’ OH ends (strand transfer complex) demonstrated how severely the target DNA is bent upon insertion of the viral DNA ends[60,63].
The requirement for integration of the viral DNA into the host genome for replication renders IN as a natural target for development of inhibitors. Numerous studies in the 1990s to identify relevant HIV IN inhibitors culminated with a study demonstrating that IN STI prevent the integration of the viral DNA into the cell genome resulting in inhibition of HIV replication in cells[27]. Further studies established that the STI competed with target DNA binding to IN suggesting a unique conformation of IN for the strand transfer reaction[64]. The diketo acid pharmacophore of the STI also bound to the divalent metal ion in the active site of IN[65].
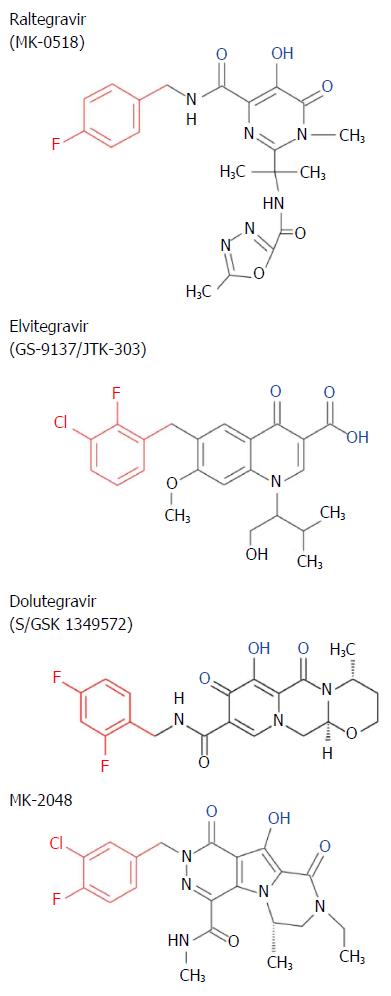
Crystallization studies of the four-domain PFV IN intasome without (Figure 6) and with STIs demonstrated the interactions of these inhibitors within the active site of IN[57,62,66,67] (see Figures 7 and 8 for brief description of STIs and their mechanism). There are currently three clinical STIs approved by the FDA for treatment of HIV/AIDS (Figure 7). They are Raltegravir (RAL), Elvitegravir (EVG) and Dolutegravir (DTG). The HIV IN STIs are interfacial inhibitors whose mechanisms are similar to other compounds that target bacterial DNA gyrase and topoisomerase IV as well as anticancer drugs targeting human topoisomerases[68,69]. Interfacial inhibitors, like the HIV STIs, bind to a site produced at the interface of two (or more) molecules bound together resulting in a functional complex. In the case of STIs, the interfaces are formed by the viral terminal CA nucleotides and specific IN residues in the PFV intasome[57,67] (Figure 8). A recent book[70] and several reviews of HIV IN inhibitors provide an extensive overview of the discovery of STIs and their mechanisms[47,71-73].
The atomic structures of the three-domain HIV and RSV IN (Figure 2) in complex with viral DNA are unknown. Crystallization of these INs in complex with their viral DNA substrates at high IN concentrations has not been successful for numerous biochemical reasons including aggregation of HIV IN and limited stability of the complexes.
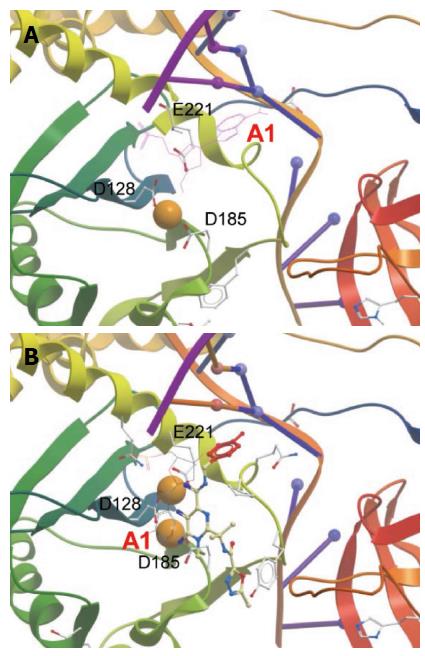
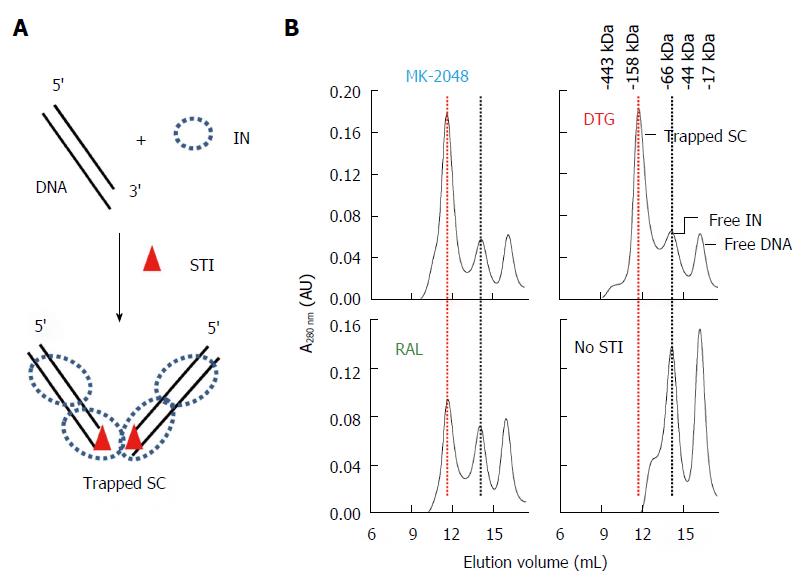
The HIV SC at low nM concentrations can be physically trapped in the presence of HIV IN STIs as analyzed by native agarose gel electrophoresis[74,75]. There are strong structural similarities in the CCD between HIV, PFV and RSV IN (Figure 2)[47,51,52,57,67,76,77] suggesting that under appropriate conditions the highly soluble RSV IN could also be trapped in the presence of the appropriate oligonucleotide (ODN) substrate and STIs. Assembly of a trapped RSV SC at high IN concentrations (1.5 mg/mL; 45 μmol/L) in solution required the presence of 3’ OH recessed viral ODN ranging in sizes (16/18R to 18/20R) and DTG, RAL or MK-2048[50] (Figure 9). DTG and MK-2048 (an investigational inhibitor) possess a significantly longer dissociative half-life than RAL within an HIV IN-DNA complex allowing these STIs to capture and stabilize the IN-DNA complex[78,79]. Without the STI presence, the transient RSV SC produced in the concerted integration pathway could not be isolated highly suggesting the STI-trapped SC is kinetically stabilized. Efforts are ongoing to resolve the atomic structure of this kinetically stabilized STI-trapped SC and the RSV strand transfer complex.
The previously described three clinical STIs (Figure 7) are the newest class of inhibitors to treat HIV/AIDS that must be used in combinational drug therapy with reverse transcriptase, protease and entry inhibitors. RAL and EVG were FDA approved for clinical use in 2007 and 2012, respectively, while DTG was approved in 2013. IN STIs are superior to other regimens in first-line therapies[80,81]. All three IN STIs have a high safety record and display strong efficacy[81].
HIV has a high mutational rate, approximately 3 × 10-5 mutations/base pair per cycle[82]. Thus, like with RT and protease inhibitors, the use of IN STIs has resulted in the emergence of drug-resistant viral strains that causes a loss of drug effectiveness. Both RAL and EVG possess a low genetic barrier giving rise to drug-resistant IN mutants (G140S/Q148H, N155S, Q148K, F121Y, T66I/S153Y) in tissue culture as well as in treated patients[72,83]. In contrast, DTG has a high genetic barrier and to date significant resistant IN mutants have been nearly absent in treatment-naïve patients[81,84,85]. The higher genetic barrier of DTG in patients may be due to its longer dissociative half-life from the HIV IN-DNA complex suggesting that DTG extended binding may be a significant factor for prevention of drug-resistance[86]; also shown to be a factor in producing kinetically stabilized RSV SC (Figure 9). HIV carrying the rare signature IN R263K mutation (others are H51Y and E138K) observed with DTG apparently cannot co-exist in combination with many of the above classical IN resistance mutations[87]. HIV is apparently unable to compensate for these mutations which induce a fitness cost that prevents the virus from evading inhibitor pressure.
Taken these demonstrated properties of DTG, investigators have slightly modified its structure to formulate a long-acting injectable nanoparticle (200 nm) suitable for clinical administration on a quarterly basis[88]. In these preclinical studies, GSK744 provided high-level protection against repeated simian/human immunodeficiency virus challenge in rhesus macaques. This study suggests that GSK744 in combination with a similar injectable RT inhibitor could decrease adherence problems associated with pre-exposure prophylaxis.
Retroviruses are natural vectors for transfer of genetic information between cells. For example, the insertion of an oncogene derived from one cell by a retrovirus into another cell occurs naturally[82]. Numerous commercial and investigative retroviral vectors have been developed to accomplish a wide variety of experimental protocols as well as the study of human diseases[89]. This permanent transfer of genetic information by retroviruses has led to an explosion of ideas for human gene therapies using gammaretroviral (MLV) and lentiviral (HIV) vectors.
The selection of DNA target sites by IN in cells is highly influenced by the interactions of IN with host cofactors and nuclear import properties of the PIC (Figure 4, see above). The selective property of MLV IN to preferentially integrate its viral DNA in the promoter regions of genes had placed a hold on human gene therapy using MLV vectors to treat SCID-X1 (x-linked severe combined immunodeficiency) in boys, which was successful[90-92]. Insertional mutagenesis lead to T-cell acute lymphoblastic leukemia in 5 of the 20 patients caused by transactivation of LMO2 or CCND2 proto-oncogenes[93,94]. Recently, a highly modified self-inactivating (SIN) MLV vector with deleted viral U3 enhancer sequences provided the same efficacy as the original MLV vectors and with no leukemogenesis produced after 33 mo[95]. Future observations of these patients using this modified MLV vector will determine further use of this kind of SIN vector. SIN HIV vectors have been used in human trials and have the advantage that they can effectively transfect resting cells due to their nuclear import properties[89,96]. Finally, SIN avian retrovirus vectors have been developed but have not been used in human gene therapy[97]. Consistent with the observation that avian retroviruses integrate randomly into cellular DNA[34,35], genome-wide analysis of integration sites by a SIN avian retrovirus vector in human hematopoietic stem/progenitor cells demonstrated that integration is random and not near hotspots containing proto-oncogenes like LMO2[98].
This review has outlined the multiple functions that the retrovirus IN has in the life cycle of retroviruses besides the key function of DNA integration. The development of the three clinical STIs (Figure 7) directed against HIV IN has provided a strong premise for supporting basic scientific research[73]. The future development of advanced STIs for pre-exposure prophylaxis, the search for new IN inhibitors and the discovery other IN functions are currently underway. The potential utilization of retrovirus vectors for human gene therapy only adds to this future spectrum for scientific investigation of the retrovirus IN.
P- Reviewer: Martinez-Costa OH, O’Connor TR S- Editor: Ji FF L- Editor: A E- Editor: Wang CH
| 1. | Grandgenett DP, Vora AC, Schiff RD. A 32,000-dalton nucleic acid-binding protein from avian retrovirus cores possesses DNA endonuclease activity. Virology. 1978;89:119-132. [PubMed] [Cited in This Article: ] |
| 2. | Donehower LA, Varmus HE. A mutant murine leukemia virus with a single missense codon in pol is defective in a function affecting integration. Proc Natl Acad Sci USA. 1984;81:6461-6465. [PubMed] [Cited in This Article: ] |
| 3. | Panganiban AT, Temin HM. The retrovirus pol gene encodes a product required for DNA integration: identification of a retrovirus int locus. Proc Natl Acad Sci USA. 1984;81:7885-7889. [PubMed] [Cited in This Article: ] |
| 4. | Schwartzberg P, Colicelli J, Goff SP. Construction and analysis of deletion mutations in the pol gene of Moloney murine leukemia virus: a new viral function required for productive infection. Cell. 1984;37:1043-1052. [PubMed] [Cited in This Article: ] |
| 5. | Quinn TP, Grandgenett DP. Genetic evidence that the avian retrovirus DNA endonuclease domain of pol is necessary for viral integration. J Virol. 1988;62:2307-2312. [PubMed] [Cited in This Article: ] |
| 6. | Li X, Krishnan L, Cherepanov P, Engelman A. Structural biology of retroviral DNA integration. Virology. 2011;411:194-205. [PubMed] [Cited in This Article: ] |
| 7. | Engelman A. Neamati N, editor. Pleiotrophic nature of HIV-1 integrase mutations, Chapter 6, in HIV-1 Integrase, Mechanism and Inhibitor Design. New Jersey: John Wiley and Sons 2011; 67-81. [Cited in This Article: ] |
| 8. | Christ F, Voet A, Marchand A, Nicolet S, Desimmie BA, Marchand D, Bardiot D, Van der Veken NJ, Van Remoortel B, Strelkov SV. Rational design of small-molecule inhibitors of the LEDGF/p75-integrase interaction and HIV replication. Nat Chem Biol. 2010;6:442-448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 358] [Cited by in F6Publishing: 359] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 9. | Jurado KA, Engelman A. Multimodal mechanism of action of allosteric HIV-1 integrase inhibitors. Expert Rev Mol Med. 2013;15:e14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Jurado KA, Wang H, Slaughter A, Feng L, Kessl JJ, Koh Y, Wang W, Ballandras-Colas A, Patel PA, Fuchs JR. Allosteric integrase inhibitor potency is determined through the inhibition of HIV-1 particle maturation. Proc Natl Acad Sci USA. 2013;110:8690-8695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 157] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 11. | Balakrishnan M, Yant SR, Tsai L, O’Sullivan C, Bam RA, Tsai A, Niedziela-Majka A, Stray KM, Sakowicz R, Cihlar T. Non-catalytic site HIV-1 integrase inhibitors disrupt core maturation and induce a reverse transcription block in target cells. PLoS One. 2013;8:e74163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 12. | Feng L, Larue RC, Slaughter A, Kessl JJ, Kvaratskhelia M. HIV-1 integrase multimerization as a therapeutic target. Curr Top Microbiol Immunol. 2015;389:93-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Desimmie BA, Schrijvers R, Demeulemeester J, Borrenberghs D, Weydert C, Thys W, Vets S, Van Remoortel B, Hofkens J, De Rijck J. LEDGINs inhibit late stage HIV-1 replication by modulating integrase multimerization in the virions. Retrovirology. 2013;10:57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 14. | Feng L, Sharma A, Slaughter A, Jena N, Koh Y, Shkriabai N, Larue RC, Patel PA, Mitsuya H, Kessl JJ. The A128T resistance mutation reveals aberrant protein multimerization as the primary mechanism of action of allosteric HIV-1 integrase inhibitors. J Biol Chem. 2013;288:15813-15820. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Engelman A. In vivo analysis of retroviral integrase structure and function. Adv Virus Res. 1999;52:411-426. [PubMed] [Cited in This Article: ] |
| 16. | Zhu K, Dobard C, Chow SA. Requirement for integrase during reverse transcription of human immunodeficiency virus type 1 and the effect of cysteine mutations of integrase on its interactions with reverse transcriptase. J Virol. 2004;78:5045-5055. [PubMed] [Cited in This Article: ] |
| 17. | Wu X, Liu H, Xiao H, Conway JA, Hehl E, Kalpana GV, Prasad V, Kappes JC. Human immunodeficiency virus type 1 integrase protein promotes reverse transcription through specific interactions with the nucleoprotein reverse transcription complex. J Virol. 1999;73:2126-2135. [PubMed] [Cited in This Article: ] |
| 18. | Dobard CW, Briones MS, Chow SA. Molecular mechanisms by which human immunodeficiency virus type 1 integrase stimulates the early steps of reverse transcription. J Virol. 2007;81:10037-10046. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Wilkinson TA, Chow S. Neamati N, editor.Functional interaction between human immunodeficiency virus type 1 reverse transcriptase and integrase, in HIV-1 Integrase, Mechanism and Inhibitor Design. New Jersey: John Wiley and Sons, Inc 2011; 95-103. [Cited in This Article: ] |
| 20. | Briones MS, Dobard CW, Chow SA. Role of human immunodeficiency virus type 1 integrase in uncoating of the viral core. J Virol. 2010;84:5181-5190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Jayappa KD, Ao Z, Wang X, Mouland AJ, Shekhar S, Yang X, Yao X. Human immunodeficiency virus type 1 employs the cellular dynein light chain 1 protein for reverse transcription through interaction with its integrase protein. J Virol. 2015;89:3497-3511. [PubMed] [Cited in This Article: ] |
| 22. | Brown PO, Bowerman B, Varmus HE, Bishop JM. Correct integration of retroviral DNA in vitro. Cell. 1987;49:347-356. [PubMed] [Cited in This Article: ] |
| 23. | Wei SQ, Mizuuchi K, Craigie R. Footprints on the viral DNA ends in moloney murine leukemia virus preintegration complexes reflect a specific association with integrase. Proc Natl Acad Sci USA. 1998;95:10535-10540. [PubMed] [Cited in This Article: ] |
| 24. | Farnet CM, Haseltine WA. Integration of human immunodeficiency virus type 1 DNA in vitro. Proc Natl Acad Sci USA. 1990;87:4164-4168. [PubMed] [Cited in This Article: ] |
| 25. | Chen H, Wei SQ, Engelman A. Multiple integrase functions are required to form the native structure of the human immunodeficiency virus type I intasome. J Biol Chem. 1999;274:17358-17364. [PubMed] [Cited in This Article: ] |
| 26. | Chen H, Engelman A. Asymmetric processing of human immunodeficiency virus type 1 cDNA in vivo: implications for functional end coupling during the chemical steps of DNA transposition. Mol Cell Biol. 2001;21:6758-6767. [PubMed] [Cited in This Article: ] |
| 27. | Hazuda DJ, Felock P, Witmer M, Wolfe A, Stillmock K, Grobler JA, Espeseth A, Gabryelski L, Schleif W, Blau C. Inhibitors of strand transfer that prevent integration and inhibit HIV-1 replication in cells. Science. 2000;287:646-650. [PubMed] [Cited in This Article: ] |
| 28. | Ao Z, Huang G, Yao H, Xu Z, Labine M, Cochrane AW, Yao X. Interaction of human immunodeficiency virus type 1 integrase with cellular nuclear import receptor importin 7 and its impact on viral replication. J Biol Chem. 2007;282:13456-13467. [PubMed] [Cited in This Article: ] |
| 29. | Christ F, Thys W, De Rijck J, Gijsbers R, Albanese A, Arosio D, Emiliani S, Rain JC, Benarous R, Cereseto A. Transportin-SR2 imports HIV into the nucleus. Curr Biol. 2008;18:1192-1202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 210] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 30. | Schröder AR, Shinn P, Chen H, Berry C, Ecker JR, Bushman F. HIV-1 integration in the human genome favors active genes and local hotspots. Cell. 2002;110:521-529. [PubMed] [Cited in This Article: ] |
| 31. | Wu X, Li Y, Crise B, Burgess SM, Munroe DJ. Weak palindromic consensus sequences are a common feature found at the integration target sites of many retroviruses. J Virol. 2005;79:5211-5214. [PubMed] [Cited in This Article: ] |
| 32. | Serrao E, Ballandras-Colas A, Cherepanov P, Maertens GN, Engelman AN. Key determinants of target DNA recognition by retroviral intasomes. Retrovirology. 2015;12:39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 33. | Wu X, Li Y, Crise B, Burgess SM. Transcription start regions in the human genome are favored targets for MLV integration. Science. 2003;300:1749-1751. [PubMed] [Cited in This Article: ] |
| 34. | Mitchell RS, Beitzel BF, Schroder AR, Shinn P, Chen H, Berry CC, Ecker JR, Bushman FD. Retroviral DNA integration: ASLV, HIV, and MLV show distinct target site preferences. PLoS Biol. 2004;2:E234. [PubMed] [Cited in This Article: ] |
| 35. | Narezkina A, Taganov KD, Litwin S, Stoyanova R, Hayashi J, Seeger C, Skalka AM, Katz RA. Genome-wide analyses of avian sarcoma virus integration sites. J Virol. 2004;78:11656-11663. [PubMed] [Cited in This Article: ] |
| 36. | Bushman FD. Integration site selection by lentiviruses: biology and possible control. Curr Top Microbiol Immunol. 2002;261:165-177. [PubMed] [Cited in This Article: ] |
| 37. | Bushman F, Lewinski M, Ciuffi A, Barr S, Leipzig J, Hannenhalli S, Hoffmann C. Genome-wide analysis of retroviral DNA integration. Nat Rev Microbiol. 2005;3:848-858. [PubMed] [Cited in This Article: ] |
| 38. | Christ F, Debyser Z. The LEDGF/p75 integrase interaction, a novel target for anti-HIV therapy. Virology. 2013;435:102-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 39. | Kvaratskhelia M, Sharma A, Larue RC, Serrao E, Engelman A. Molecular mechanisms of retroviral integration site selection. Nucleic Acids Res. 2014;42:10209-10225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 40. | Ciuffi A, Llano M, Poeschla E, Hoffmann C, Leipzig J, Shinn P, Ecker JR, Bushman F. A role for LEDGF/p75 in targeting HIV DNA integration. Nat Med. 2005;11:1287-1289. [PubMed] [Cited in This Article: ] |
| 41. | Desimmie BA, Weydert C, Schrijvers R, Vets S, Demeulemeester J, Proost P, Paron I, De Rijck J, Mast J, Bannert N. HIV-1 IN/Pol recruits LEDGF/p75 into viral particles. Retrovirology. 2015;12:16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Marini B, Kertesz-Farkas A, Ali H, Lucic B, Lisek K, Manganaro L, Pongor S, Luzzati R, Recchia A, Mavilio F. Nuclear architecture dictates HIV-1 integration site selection. Nature. 2015;521:227-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 228] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 43. | Studamire B, Goff SP. Host proteins interacting with the Moloney murine leukemia virus integrase: multiple transcriptional regulators and chromatin binding factors. Retrovirology. 2008;5:48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 44. | De Rijck J, de Kogel C, Demeulemeester J, Vets S, El Ashkar S, Malani N, Bushman FD, Landuyt B, Husson SJ, Busschots K. The BET family of proteins targets moloney murine leukemia virus integration near transcription start sites. Cell Rep. 2013;5:886-894. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 45. | Sharma A, Larue RC, Plumb MR, Malani N, Male F, Slaughter A, Kessl JJ, Shkriabai N, Coward E, Aiyer SS. BET proteins promote efficient murine leukemia virus integration at transcription start sites. Proc Natl Acad Sci USA. 2013;110:12036-12041. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 149] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 46. | Knaus RJ, Hippenmeyer PJ, Misra TK, Grandgenett DP, Müller UR, Fitch WM. Avian retrovirus pp32 DNA binding protein. Preferential binding to the promoter region of long terminal repeat DNA. Biochemistry. 1984;23:350-359. [PubMed] [Cited in This Article: ] |
| 47. | Engelman A, Cherepanov P. Retroviral Integrase Structure and DNA Recombination Mechanism. Microbiol Spectr. 2014;2:1-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 48. | Valkov E, Gupta SS, Hare S, Helander A, Roversi P, McClure M, Cherepanov P. Functional and structural characterization of the integrase from the prototype foamy virus. Nucleic Acids Res. 2009;37:243-255. [PubMed] [Cited in This Article: ] |
| 49. | Gupta K, Curtis JE, Krueger S, Hwang Y, Cherepanov P, Bushman FD, Van Duyne GD. Solution conformations of prototype foamy virus integrase and its stable synaptic complex with U5 viral DNA. Structure. 2012;20:1918-1928. [PubMed] [Cited in This Article: ] |
| 50. | Pandey KK, Bera S, Korolev S, Campbell M, Yin Z, Aihara H, Grandgenett DP. Rous sarcoma virus synaptic complex capable of concerted integration is kinetically trapped by human immunodeficiency virus integrase strand transfer inhibitors. J Biol Chem. 2014;289:19648-19658. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 51. | Shi K, Pandey KK, Bera S, Vora AC, Grandgenett DP, Aihara H. A possible role for the asymmetric C-terminal domain dimer of Rous sarcoma virus integrase in viral DNA binding. PLoS One. 2013;8:e56892. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 52. | Bojja RS, Andrake MD, Weigand S, Merkel G, Yarychkivska O, Henderson A, Kummerling M, Skalka AM. Architecture of a full-length retroviral integrase monomer and dimer, revealed by small angle X-ray scattering and chemical cross-linking. J Biol Chem. 2011;286:17047-17059. [PubMed] [Cited in This Article: ] |
| 53. | Pandey KK, Bera S, Grandgenett DP. The HIV-1 integrase monomer induces a specific interaction with LTR DNA for concerted integration. Biochemistry. 2011;50:9788-9796. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 54. | Lee SP, Xiao J, Knutson JR, Lewis MS, Han MK. Zn2+ promotes the self-association of human immunodeficiency virus type-1 integrase in vitro. Biochemistry. 1997;36:173-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 55. | Li M, Craigie R. Processing of viral DNA ends channels the HIV-1 integration reaction to concerted integration. J Biol Chem. 2005;280:29334-29339. [PubMed] [Cited in This Article: ] |
| 56. | Bera S, Pandey KK, Vora AC, Grandgenett DP. Molecular Interactions between HIV-1 integrase and the two viral DNA ends within the synaptic complex that mediates concerted integration. J Mol Biol. 2009;389:183-198. [PubMed] [Cited in This Article: ] |
| 57. | Hare S, Gupta SS, Valkov E, Engelman A, Cherepanov P. Retroviral intasome assembly and inhibition of DNA strand transfer. Nature. 2010;464:232-236. [PubMed] [Cited in This Article: ] |
| 58. | Nowotny M. Retroviral integrase superfamily: the structural perspective. EMBO Rep. 2009;10:144-151. [PubMed] [Cited in This Article: ] |
| 59. | Montaño SP, Pigli YZ, Rice PA. The μ transpososome structure sheds light on DDE recombinase evolution. Nature. 2012;491:413-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 60. | Maertens GN, Hare S, Cherepanov P. The mechanism of retroviral integration from X-ray structures of its key intermediates. Nature. 2010;468:326-329. [PubMed] [Cited in This Article: ] |
| 61. | Davies DR, Goryshin IY, Reznikoff WS, Rayment I. Three-dimensional structure of the Tn5 synaptic complex transposition intermediate. Science. 2000;289:77-85. [PubMed] [Cited in This Article: ] |
| 62. | Hare S, Maertens GN, Cherepanov P. 3’-processing and strand transfer catalysed by retroviral integrase in crystallo. EMBO J. 2012;31:3020-3028. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 63. | Yin Z, Lapkouski M, Yang W, Craigie R. Assembly of prototype foamy virus strand transfer complexes on product DNA bypassing catalysis of integration. Protein Sci. 2012;21:1849-1857. [PubMed] [Cited in This Article: ] |
| 64. | Espeseth AS, Felock P, Wolfe A, Witmer M, Grobler J, Anthony N, Egbertson M, Melamed JY, Young S, Hamill T. HIV-1 integrase inhibitors that compete with the target DNA substrate define a unique strand transfer conformation for integrase. Proc Natl Acad Sci USA. 2000;97:11244-11249. [PubMed] [Cited in This Article: ] |
| 65. | Grobler JA, Stillmock K, Hu B, Witmer M, Felock P, Espeseth AS, Wolfe A, Egbertson M, Bourgeois M, Melamed J. Diketo acid inhibitor mechanism and HIV-1 integrase: implications for metal binding in the active site of phosphotransferase enzymes. Proc Natl Acad Sci USA. 2002;99:6661-6666. [PubMed] [Cited in This Article: ] |
| 66. | Hare S, Vos AM, Clayton RF, Thuring JW, Cummings MD, Cherepanov P. Molecular mechanisms of retroviral integrase inhibition and the evolution of viral resistance. Proc Natl Acad Sci USA. 2010;107:20057-20062. [PubMed] [Cited in This Article: ] |
| 67. | Cherepanov P, Maertens GN, Hare S. Structural insights into the retroviral DNA integration apparatus. Curr Opin Struct Biol. 2011;21:249-256. [PubMed] [Cited in This Article: ] |
| 68. | Pommier Y, Johnson AA, Marchand C. Integrase inhibitors to treat HIV/AIDS. Nat Rev Drug Discov. 2005;4:236-248. [PubMed] [Cited in This Article: ] |
| 69. | Pommier Y, Marchand C. Interfacial inhibitors: targeting macromolecular complexes. Nat Rev Drug Discov. 2012;11:25-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 177] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 70. | Neamati N. HIV-1 integrase inhibitor design: Overview and historial perspectives. In HIV-1 Integrase, Mechanism and Inhibitor Design. New Jersey: John Wiley & Sons, Inc 2011; 165-196. [Cited in This Article: ] |
| 71. | Métifiot M, Marchand C, Pommier Y. HIV integrase inhibitors: 20-year landmark and challenges. Adv Pharmacol. 2013;67:75-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 72. | Di Santo R. Inhibiting the HIV integration process: past, present, and the future. J Med Chem. 2014;57:539-566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 73. | Craigie R. The road to HIV-1 integrase inhibitors: the case for supporting basic research. Future Virol. 2014;9:899-903. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 74. | Pandey KK, Bera S, Vora AC, Grandgenett DP. Physical trapping of HIV-1 synaptic complex by different structural classes of integrase strand transfer inhibitors. Biochemistry. 2010;49:8376-8387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 75. | Bera S, Pandey KK, Vora AC, Grandgenett DP. HIV-1 integrase strand transfer inhibitors stabilize an integrase-single blunt-ended DNA complex. J Mol Biol. 2011;410:831-846. [PubMed] [Cited in This Article: ] |
| 76. | Wang JY, Ling H, Yang W, Craigie R. Structure of a two-domain fragment of HIV-1 integrase: implications for domain organization in the intact protein. EMBO J. 2001;20:7333-7343. [PubMed] [Cited in This Article: ] |
| 77. | Yang ZN, Mueser TC, Bushman FD, Hyde CC. Crystal structure of an active two-domain derivative of Rous sarcoma virus integrase. J Mol Biol. 2000;296:535-548. [PubMed] [Cited in This Article: ] |
| 78. | Hightower KE, Wang R, Deanda F, Johns BA, Weaver K, Shen Y, Tomberlin GH, Carter HL, Broderick T, Sigethy S. Dolutegravir (S/GSK1349572) exhibits significantly slower dissociation than raltegravir and elvitegravir from wild-type and integrase inhibitor-resistant HIV-1 integrase-DNA complexes. Antimicrob Agents Chemother. 2011;55:4552-4559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 174] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 79. | Grobler J, McKemma PM, Ly S, Stillmock KA, Bahnck CM, Danovich RM, Dornadula G, Hazuda D, Miller MD. HIV integrase inhibitor dissociation rates correlate with efficacy in vitro. Antiviral Ther. 2009;14 Suppl 1:A27. [Cited in This Article: ] |
| 80. | Messiaen P, Wensing AM, Fun A, Nijhuis M, Brusselaers N, Vandekerckhove L. Clinical use of HIV integrase inhibitors: a systematic review and meta-analysis. PLoS One. 2013;8:e52562. [PubMed] [Cited in This Article: ] |
| 81. | White KL, Raffi F, Miller MD. Resistance analyses of integrase strand transfer inhibitors within phase 3 clinical trials of treatment-naive patients. Viruses. 2014;6:2858-2879. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 82. | Rawson JM, Mansky LM. Retroviral vectors for analysis of viral mutagenesis and recombination. Viruses. 2014;6:3612-3642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 83. | Llibre JM, Pulido F, García F, García Deltoro M, Blanco JL, Delgado R. Genetic barrier to resistance for dolutegravir. AIDS Rev. 2015;17:56-64. [PubMed] [Cited in This Article: ] |
| 84. | Mesplède T, Wainberg MA. Is resistance to dolutegravir possible when this drug is used in first-line therapy? Viruses. 2014;6:3377-3385. [PubMed] [Cited in This Article: ] |
| 85. | Miller MM, Liedtke MD, Lockhart SM, Rathbun RC. The role of dolutegravir in the management of HIV infection. Infect Drug Resist. 2015;8:19-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 86. | DeAnda F, Hightower KE, Nolte RT, Hattori K, Yoshinaga T, Kawasuji T, Underwood MR. Dolutegravir interactions with HIV-1 integrase-DNA: structural rationale for drug resistance and dissociation kinetics. PLoS One. 2013;8:e77448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 87. | Anstett K, Mesplede T, Oliveira M, Cutillas V, Wainberg MA. Dolutegravir resistance mutation R263K cannot coexist in combination with many classical integrase inhibitor resistance substitutions. J Virol. 2015;89:4681-4684. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 88. | Andrews CD, Spreen WR, Mohri H, Moss L, Ford S, Gettie A, Russell-Lodrigue K, Bohm RP, Cheng-Mayer C, Hong Z. Long-acting integrase inhibitor protects macaques from intrarectal simian/human immunodeficiency virus. Science. 2014;343:1151-1154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 89. | Niederer HA, Bangham CR. Integration site and clonal expansion in human chronic retroviral infection and gene therapy. Viruses. 2014;6:4140-4164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 90. | Antoine C, Müller S, Cant A, Cavazzana-Calvo M, Veys P, Vossen J, Fasth A, Heilmann C, Wulffraat N, Seger R. Long-term survival and transplantation of haemopoietic stem cells for immunodeficiencies: report of the European experience 1968-99. Lancet. 2003;361:553-560. [PubMed] [Cited in This Article: ] |
| 91. | Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P, Selz F, Hue C, Certain S, Casanova JL. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669-672. [PubMed] [Cited in This Article: ] |
| 92. | Gaspar HB, Parsley KL, Howe S, King D, Gilmour KC, Sinclair J, Brouns G, Schmidt M, Von Kalle C, Barington T. Gene therapy of X-linked severe combined immunodeficiency by use of a pseudotyped gammaretroviral vector. Lancet. 2004;364:2181-2187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 541] [Cited by in F6Publishing: 540] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 93. | Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, Lim A, Osborne CS, Pawliuk R, Morillon E. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2671] [Cited by in F6Publishing: 2513] [Article Influence: 119.7] [Reference Citation Analysis (0)] |
| 94. | Howe SJ, Mansour MR, Schwarzwaelder K, Bartholomae C, Hubank M, Kempski H, Brugman MH, Pike-Overzet K, Chatters SJ, de Ridder D. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J Clin Invest. 2008;118:3143-3150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 892] [Cited by in F6Publishing: 883] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 95. | Hacein-Bey-Abina S, Pai SY, Gaspar HB, Armant M, Berry CC, Blanche S, Bleesing J, Blondeau J, de Boer H, Buckland KF. A modified γ-retrovirus vector for X-linked severe combined immunodeficiency. N Engl J Med. 2014;371:1407-1417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 317] [Cited by in F6Publishing: 286] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 96. | Sakuma T, Barry MA, Ikeda Y. Lentiviral vectors: basic to translational. Biochem J. 2012;443:603-618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 211] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 97. | Suerth JD, Labenski V, Schambach A. Alpharetroviral vectors: from a cancer-causing agent to a useful tool for human gene therapy. Viruses. 2014;6:4811-4838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 98. | Moiani A, Suerth JD, Gandolfi F, Rizzi E, Severgnini M, De Bellis G, Schambach A, Mavilio F. Genome-wide analysis of alpharetroviral integration in human hematopoietic stem/progenitor cells. Genes (Basel). 2014;5:415-429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 99. | Aiyer S, Rossi P, Malani N, Schneider WM, Chandar A, Bushman FD, Montelione GT, Roth MJ. Structural and sequencing analysis of local target DNA recognition by MLV integrase. Nucleic Acids Res. 2015;43:5647-5663. [PubMed] [Cited in This Article: ] |