Published online Apr 15, 2024. doi: 10.4251/wjgo.v16.i4.1374
Peer-review started: September 17, 2023
First decision: December 1, 2023
Revised: December 16, 2023
Accepted: February 1, 2024
Article in press: February 1, 2024
Published online: April 15, 2024
Despite advances in detection and treatments, biliary tract cancers continue to have poor survival outcomes. Currently, there is limited data investigating the significance of socioeconomic status, race/ethnicity, and environmental factors in biliary tract cancer survival.
To investigate how socioeconomic status and race/ethnicity are associated with survival.
Data from the Surveillance, Epidemiology, and End Results database for biliary and gallbladder adenocarcinomas were extracted from 1975 to 2016. Socioe
Our study included 15883 gallbladder, 11466 intrahepatic biliary, 12869 extrahepatic biliary and 7268 ampulla of Vater adenocarcinoma cases. When analyzing county-specific demographics, patients from counties with higher incomes were associated with higher survival rates [hazard ratio (HR) = 0.97, P <0.05]. Similarly, counties with a higher percentage of patients with a college level education and counties with a higher urban population had higher 5-year survival rates (HR = 0.96, P = 0.002 and HR = 0.97, P = 0.004, respectively).
Worse survival outcomes were observed in lower income counties while higher income and education level were associated with higher 5-year overall survival among gallbladder and biliary malignancies.
Core Tip: Biliary tract cancers exhibit poor survival outcomes despite advances in treatment. There is a paucity of data addressing the role of socioeconomic factors on biliary tract adenocarcinoma survival. This study has a large sample size of hepatobiliary cancers despite low incidence rates due to the utilization of the Surveillance, Epidemiology, and End Results national database. Study findings are unique and significant for a higher 5-year survival among those with a college education and in the urban population.
- Citation: Sahyoun L, Chen K, Tsay C, Chen G, Protiva P. Clinical and socioeconomic determinants of survival in biliary tract adenocarcinomas. World J Gastrointest Oncol 2024; 16(4): 1374-1383
- URL: https://www.wjgnet.com/1948-5204/full/v16/i4/1374.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i4.1374
Biliary tract cancers, which encompass cholangiocarcinomas (CCAs), gallbladder cancers, and ampullary cancers, are associated with poor survival outcomes[1,2]. The risk of these cancers increases with the presence of chronic liver disease, such as primary sclerosing cholangitis or liver cirrhosis from any etiology, as well as factors that lead to ongoing liver injury, like alcohol consumption or parasitic and viral infections[3,4]. CCAs are the second most common primary hepatobiliary malignancy and account for 20% of hepatobiliary cancer deaths[5,6]. Although outcomes vary by intra
In addition, the mortality rate for CCA has increased over time[9]. Gallbladder cancers account for 1.7% of all global cancer deaths with a 5-year survival of 18% in the United States, in part due to the advanced stage at time of diagnosis[11]. Overall, the incidence of gallbladder carcinomas has decreased in women but remained stable in men. However, in some subgroups, notably Blacks and women under 45-years-old, the incidence has increased[12]. Carcinomas located at the ampulla of Vater have a higher reported survival rate, in part due to presenting features of jaundice and symptomatic biliary occlusion, with an overall 5-year survival rate of 40%[13-15]. These rates are highly dependent on the extent of lymph node involvement, the presence of lymphatic vessel invasion and pre-existing comorbidities[14]. Additionally, some evidence has suggested that sex differences not only impact survival but treatment response, with one meta-analysis reporting slightly higher overall survival in male patients receiving single-agent immunotherapy[16]. This is an important finding, as neoadjuvant therapies can improve the success of resection rates in certain subsets of biliary tract cancer patients and possibly reduce the rates of disease relapse[17-19].
Given the mortality associated with these malignancies, previous studies have analyzed the impact of several factors such as age, race, and ethnicity on survival outcomes to identify those individuals that carry a higher mortality risk. Current existing studies focus on CCAs; higher mortality rates have been observed in patients who are older than 65 years of age, male, or of non-Hispanic Asian descent. Non-Hispanic Asians have a higher mortality with ampullary cancers[20]. The role of race/ethnicity is less clear in gallbladder cancers. One review reported no difference in stage at time of diagnosis among different races/ethnicities[21]. However, Henley et al[12] discovered higher mortality in American Indian and Alaska native patients. Additionally, there are limited studies on the significance of socioeconomic status and environmental factors in biliary tract cancer survival. This study aims to investigate how socioeconomic and environmental factors are associated with survival among biliary tract adenocarcinomas.
Using SEER*Stat software, we extracted United States survival data from the SEER Database for biliary and gallbladder adenocarcinomas for the period between 1975 to 2016. This registry is an authoritative source of cancer statistics in the United States and is supported by the Surveillance Research Program in the National Cancer Institute Division of Cancer Control and Population Sciences. SEER has 16 central cancer registries as of 2020, comprising 35% of the United States population and over 9 million cancer cases since 1973[22].
The study sample included individuals aged > 30 years with biliary, ampullary and gallbladder adenocarcinomas. Carcinomas were defined according to International Classification of Diseases codes: ICC bile duct adenocarcinoma (C22.1), ECC bile duct adenocarcinoma (C24.0), gallbladder adenocarcinoma (C23.9), and ampullary adenocarcinoma (C24.1). Demographic, clinical, and county-level socioeconomic data were extracted using SEER*Stat software. We excluded cases with missing cancer stage or grade and cases with multiple tumors or more than one primary malignancy. Malignancies that were not adenocarcinomas were also excluded.
The demographic data included age, sex, race/ethnicity (American Indian/Alaska Native, Asian/Pacific Islander, Hispanic, Black, or White), and marital status (married, divorced, separated, never married, domestic partner, or widowed). Clinical information included year of diagnosis, primary cancer site, tumor grade (well-differentiated, Grade I; moderately differentiated, Grade II; poorly differentiated, Grade III; and undifferentiated, anaplastic, Grade IV), survival time, and type of treatment received, if any (chemotherapy, surgery, and/or radiation). Tumor location, as defined by SEER database, was also analyzed (local, or confined to organ of origin; regional, or direct extension from organ of origin and/or regional lymph nodes; distant)[22]. We also included relevant county-level socioeconomic data, such as percentage of current smokers, colorectal cancer screening adherence, percentage of individuals with college-level education, cost-adjusted household income, and percentage of urban population. The primary endpoint was survival at 5 years after diagnosis.
Survival was compared by calculating relative hazard ratios (HRs) for death in the 5-year period following diagnosis using Cox proportional hazard models, adjusted for all studied covariates. Statistics for county level data were stratified by county medians. Significance level was set at P < 0.05 for two-tailed tests. Data were analyzed using SAS 9.4 software (SAS Institute, Cary, NC) and R software.
This study included 15883 gallbladder, 11466 ICC biliary, 12869 ECC biliary and 7268 ampulla of Vater adenocarcinoma cases between 1975 to 2016 (Table 1). There was a similar distribution of males and females, with a slightly higher percentage of men for all carcinomas except for gallbladder carcinoma (72.1% female vs 27.9% male). Most of the study population was over the age of 60 (74.8%) and were white and non-Hispanic ethnicity (Table 1).
| Characteristics | Ampulla of Vater, n = 7268 | Intrahepatic bile duct, n = 11466 | Extrahepatic bile duct, n = 12869 | Gallbladder, n = 15883 |
| Sex | ||||
| Female | 3347 (46.1) | 5560 (49.4) | 6141 (47.7) | 11446 (72.1) |
| Male | 3921 (53.9) | 5806 (50.6) | 6728 (52.3) | 4437 (27.9) |
| Race | ||||
| White | 5763 (79.3) | 8957 (78.1) | 10092 (78.4) | 12530 (78.9) |
| American Indian/Alaska native | 57 (0.8) | 119 (1) | 120 (0.9) | 269 (1.7) |
| Asian or Pacific Islander | 888 (12.2) | 1513 (13.2) | 1641 (12.8) | 1467 (9.2) |
| Black | 560 (7.7) | 877 (7.7) | 1016 (7.9) | 1617 (10.2) |
| Ethnicity | ||||
| Hispanic | 1174 (16.2) | 1548 (13.5) | 1678 (13.0) | 3071 (19.3) |
| Non-Hispanic | 6094 (83.8) | 9918 (86.5) | 11191 (87.0) | 12812 (80.7) |
| Age | ||||
| ≤ 60 yr | 2040 (28.1) | 3337 (29.1) | 2881 (22.4) | 3694 (23.3) |
| > 60 yr | 5228 (71.9) | 8129 (70.9) | 9988 (77.6) | 12189 (76.7) |
| Stage | ||||
| Local | 738 (10.2) | 1641 (14.3) | 1379 (10.7) | 995 (6.3) |
| Regional | 2522 (34.7) | 2189 (19.1) | 3025 (23.5) | 3594 (22.6) |
| Distant | 824 (11.3) | 2859 (24.9) | 2532 (19.7) | 3607 (22.7) |
| Unknown/un-staged | 3184 (43.8) | 4777 (41.7) | 5933 (46.1) | 7687 (48.4) |
| Grade | ||||
| Well differentiated, I | 1016 (14.0) | 478 (4.2) | 1021 (7.9) | 1805 (11.4) |
| Moderately differentiated, II | 3039 (41.8) | 1688 (14.7) | 2415 (18.8) | 4608 (29.0) |
| Poorly differentiated, III | 1720 (23.7) | 1631 (14.2) | 1926 (15.0) | 4769 (30.0) |
| Undifferentiated; anaplastic, IV | 72 (1.0) | 72 (0.6) | 82 (0.6) | 231 (1.5) |
| Unknown | 1421 (19.5) | 7597 (66.3) | 7425 (57.7) | 4470 (28.1) |
| Marital status | ||||
| Married | 4185 (57.6) | 6449 (56.2) | 7032 (54.6) | 7664 (48.3) |
| Divorced | 604 (8.3) | 1071 (9.3) | 1102 (8.6) | 1399 (8.8) |
| Single, never married | 907 (12.5) | 1452 (12.7) | 1522 (11.8) | 1868 (11.8) |
| Widowed | 1322 (18.2) | 2094 (18.3) | 2764 (21.5) | 4345 (27.4) |
| Unknown | 250 (3.4) | 400 (3.5) | 449 (3.5) | 607 (3.8) |
| Yr of diagnosis | ||||
| 1975-1990 | 796 (11.0) | 684 (6.0) | 1675 (13.0) | 3107 (19.6) |
| 1991-2005 | 2691 (37.0) | 3898 (34.0) | 5513 (34.7) | 5513 (34.7) |
| 2006-2016 | 3781 (52.0) | 6884 (60.0) | 7117 (55.3) | 7263 (45.7) |
| Chemotherapy | ||||
| Yes | 2245 (30.9) | 4445 (38.8) | 4134 (32.1) | 4705 (29.6) |
| No/unknown | 5023 (69.1) | 7021 (61.2) | 8735 (67.9) | 11178 (70.4) |
| Surgery | ||||
| Yes | 1003 (13.8) | 427 (3.7) | 1245 (9.7) | 1787 (11.3) |
| No/unknown | 6265 (86.2) | 11039 (96.3) | 11624 (90.3) | 14096 (88.7) |
| Radiation | ||||
| Yes | 1245 (17.2) | 1604 (14.0) | 2489 (19.4) | 2183 (13.8) |
| No/unknown | 6003 (82.8) | 9831 (86.0) | 10334 (80.6) | 13664 (86.2) |
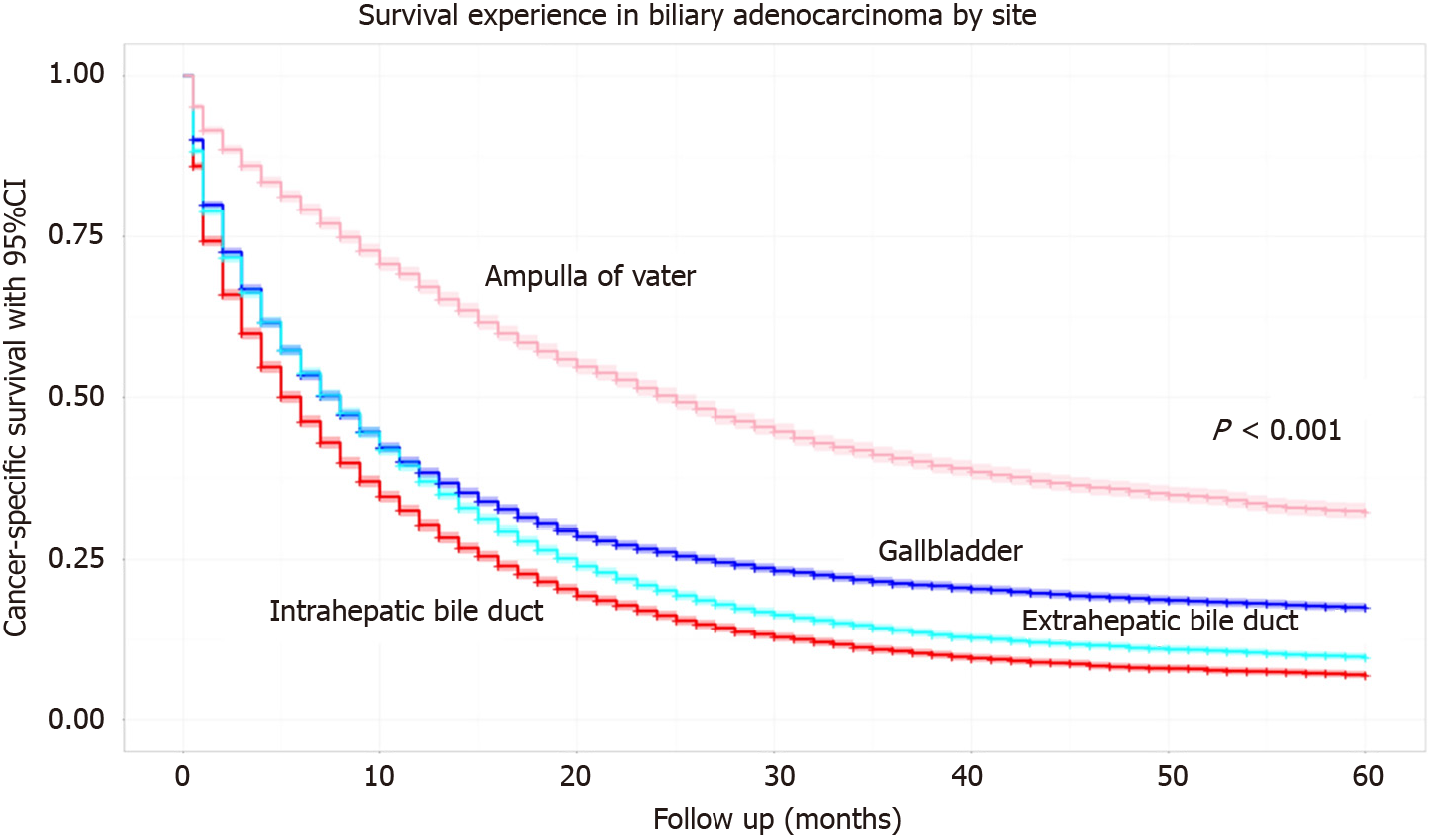
The 5-year survival probability with 95% confidence interval (95%CI) for all four studied malignancies is shown in Figure 1. Ampullary cancers had the highest 5-year survival at 31%. CCAs had the lowest 5-year survival, < 10%, with ICC having slightly worse outcomes compared to ECC when stratified by location. Gallbladder cancers had a 5-year survival of nearly 20%.
Cox proportional hazard ratios for 5-year mortality among the studied parameters are in Table 2. Survival was lower with increased age (> 60-years-old) at time of diagnosis, (HR = 1.31, P < 0.0001). Male patients had a statistically significant higher 5-year mortality (HR = 1.07, P < 0.0001). When stratified according to race/ethnicity, Asians and Pacific Islanders had higher survival (HR = 0.93, P < 0.0001). While Black and American Indian/Alaska Native individuals had lower survival probabilities compared to white individuals, these trends were not statistically significant. Those who were divorced, single or widowed had worse survival outcomes compared to married patients (P < 0.0001).
| Parameter | Hazard ratio | 95%CI | P value |
| Site | |||
| Ampullary carcinoma | 1 | ||
| Intrahepatic bile duct carcinoma | 2.17 | 2.08-2.25 | < 0.0001 |
| Extrahepatic bile duct carcinoma | 1.87 | 1.80-1.94 | < 0.0001 |
| Gallbladder carcinoma | 1.68 | 1.62-1.74 | < 0.0001 |
| Sex | |||
| Female | 1 | ||
| Male | 1.07 | 1.05-1.10 | < 0.0001 |
| Race/ethnicity | |||
| White | 1 | ||
| American Indian/Alaska native | 1.02 | 0.93-1.13 | 0.64 |
| Asian or Pacific Islander | 0.93 | 0.90-0.96 | < 0.0001 |
| Black | 1.05 | 1.01-1.09 | 0.03 |
| Hispanic | 0.97 | 0.93-0.99 | 0.03 |
| Age | |||
| ≤ 60 yr | 1 | ||
| > 60 yr | 1.31 | 1.28-1.35 | < 0.0001 |
| Stage | |||
| Local | 1 | ||
| Regional | 1.29 | 1.42-1.56 | < 0.0001 |
| Distant | 3.02 | 2.88-3.16 | < 0.0001 |
| Unknown | 1.90 | 1.81-2.00 | < 0.0001 |
| Grade | |||
| Well differentiated, I | 1 | ||
| Moderately differentiated, II | 1.39 | 1.33-1.46 | < 0.0001 |
| Poorly differentiated, III | 2.17 | 2.07-2.28 | < 0.0001 |
| Undifferentiated; anaplastic, IV | 2.22 | 1.99-2.48 | < 0.0001 |
| Marital status | |||
| Married | 1 | ||
| Divorced | 1.13 | 1.08-1.17 | < 0.0001 |
| Single, never married | 1.13 | 1.09-1.17 | < 0.0001 |
| Widowed | 1.19 | 1.15-1.22 | < 0.0001 |
| Unknown | 0.99 | 0.93-1.05 | 0.63 |
| Year of Diagnosis | |||
| 1975-1990 | 1 | ||
| 1991-2005 | 0.89 | 0.85-0.92 | < 0.0001 |
| 2006-2016 | 0.88 | 0.83-0.92 | < 0.0001 |
| Chemotherapy | |||
| Yes | 1 | ||
| No/unknown | 1.34 | 1.31-1.38 | < 0.0001 |
| Surgery | |||
| Yes | 1 | ||
| No/unknown | 1.30 | 1.23-1.37 | < 0.0001 |
| Radiation | |||
| Yes | 1 | ||
| No/unknown | 1.02 | 0.98-1.07 | 0.26 |
ICC had the highest 5-year mortality (HR = 2.17, P < 0.0001). A diagnosis of gallbladder or biliary cancer after 1991 had a better 5-year survival outcome compared to a diagnosis between 1975-1990 (P < 0.0001). A higher grade of malignancy at the time of diagnosis was associated with a higher 5-year mortality, with Grade IV having the highest HR (2.22, P < 0.0001). Not receiving chemotherapy or surgery as treatment was associated with higher mortality (HR = 1.34, P < 0.0001 and HR = 1.30, P < 0.0001, respectively). The addition of radiation to treatment regimens was not significantly associated with survival outcomes (Table 2).
When analyzing county-specific demographics, patients from counties with higher incomes were associated with higher survival rates (HR = 0.97, P < 0.05). Similarly, counties with a higher percentage of individuals with a college level education and counties with a higher urban population had higher 5-year survival rates (HR = 0.96, P = 0.002 and HR = 0.97, P = 0.004, respectively). There was no statistical difference in survival outcomes among county percentages of current smokers (Table 3).
| County parameter | Hazard ratio | 95%CI | P value | |
| Urban population, % | Below median | 1 | ||
| Above median | 0.97 | 0.94-0.99 | 0.004 | |
| College education level, % | Below median | 1 | ||
| Above median | 0.96 | 0.93-0.99 | 0.002 | |
| Colorectal cancer screening rates, % | Below median | 1 | ||
| Above median | 0.95 | 0.93-0.97 | < 0.0001 | |
| Income level, per $ 10 | Below median | 1 | ||
| Above median | 0.97 | 0.94-0.99 | 0.04 | |
| Smokers, % | Below median | 1 | ||
| Above median | 1.01 | 0.99-1.04 | 0.42 | |
This is the largest study thus far that provides a comprehensive analysis of socioeconomic factors in relation to biliary tract cancer survival. While clinical and pathologic factors are well-known and studied in association with cancer survival outcomes, our study sought to evaluate other contributors to mortality. The study findings confirm the low 5-year survival associated with biliary cancers, which has been previously reported[8,11,23,24]. Additionally, higher cancer grade at time of diagnosis was associated with higher mortality, as expected. Male sex and advanced age were also associated with poorer cancer survival outcomes, which was previously demonstrated among CCAs[25]. However, this study also revealed certain patient and socioeconomic characteristics that were associated with a lower risk for mortality. Higher income and education level as well as Asian/Pacific Islander race were associated with a higher 5-year overall survival among gallbladder and biliary malignancies, in contrast to other studies reporting increased mortality[20]. After adjusting for grade/stage of cancer, these socioeconomic factors remained independently associated with longer survival.
In this study, worse survival outcomes were observed in lower income and rural counties. Poverty has long been associated with worse cancer outcomes, thought to be secondary to higher rates of tobacco use, obesity, and food insecurity that worsen healthcare disparities[26]. This can be further explained by decreased accessibility to medical care or limited ability to be treated at tertiary centers, as rural counties were also found to have worse survival outcomes. In fact, rural populations have higher levels of poverty[26,27] and higher rates of unemployment compared to urban regions[28], resulting in lower rates of employer-sponsored healthcare. Additionally, higher rates of hospital closures in rural areas, increased distance to tertiary centers, higher rates of disabilities, and a disparity in the number of physicians practicing in rural areas are all factors that limit access to care[29-31]. These considerations can contribute to the lower survival rates seen in counties with a higher percentage of rural populations.
Conversely, counties with a higher incidence of colorectal cancer screening, which can serve as a marker for access to preventative healthcare[32], were associated with a lower hazard ratio for mortality. Higher rates of college-level education were also associated with better survival outcomes, underscoring the role that health literacy plays in survival. Education level can also be related to income level, which could in turn explain the observed survival benefit. Aside from access to medical care, social support plays an important role in cancer outcomes. Marital status was significantly associated with survival outcomes, with those being married or in a domestic partnership having improved survival. This association, which has also previously been reported in liver and colorectal cancers[33], suggests that having good social/family and economic support positively impacts survival.
This study also showed that cancer diagnosed during the second half of the study period (after 1990) was associated with improved survival outcomes. However, this finding could be confounded by lead time bias, as the increased availability and accuracy of diagnostic studies and imaging could result in an earlier diagnosis without impacting survival outcomes. Alternatively, it is possible that this earlier diagnosis of malignancy can equate to a less advanced cancer stage and perhaps a wider range of treatment options. Recognizing patients at a higher risk for morbidity and mortality is important as we can consider the addition of neoadjuvant therapies and other treatment modalities to improve long term survival outcomes.
An understanding of the modifiable demographic and socioeconomic factors that affect survival is key to the development of healthcare policy. There are still significant knowledge gaps around why non-modifiable risk factors, such as sex or race/ethnicity may influence survival. The rise of precision medicine may be important to understanding the impact of genetics, environmental and lifestyle influences, and the microbiome on survival.
A strength of this study is the use of national data reflective of the United States cancer population, including comprehensive demographic information and survival statistics. SEER is the largest cancer database in the United States and allows for a large sample size of cancer cases with lower incidence rates, as was the case in this study. Additionally, given the nationwide distribution of cancer centers in the SEER program, the data is generalizable to the national population and helps mitigate regional variability in medical treatment.
Limitations include the retrospective nature of the study. Given its cross-sectional design, causal relationships between the studied variables and mortality outcomes cannot be determined. As this study occurred over an extended period, there have been advances in diagnostic tests and treatments available, which further impacts morbidity and mortality within certain populations. Additionally, the SEER database does not report information regarding underlying patient comorbidities and functional status as well as baseline liver function, which play an important role in cancer mortality outcomes.
Future studies targeting these socioeconomic characteristics associated with poorer survival outcomes are warranted to determine potential etiologies and develop strategies for improving outcomes in these groups. Additionally, by identifying at-risk populations, more aggressive screening or treatment strategies can be considered in those known to have poorer outcomes.
Despite advances in detection and treatment, biliary tract cancers continue to exhibit poor survival outcomes.
Currently, limited data exist on the significance of socioeconomic status, race/ethnicity, and environmental factors in biliary tract cancer survival.
Our objective was to comprehensively evaluate the impact of socioeconomic factors on survival in biliary tract cancer.
This study compared survival outcomes among patients with hepatobiliary adenocarcinomas based on socioeconomic indicators, including smoking status, poverty level, level of education, adjusted household income, percentage of urban population, and foreign-born individuals.
This study provided a large study sample size of hepatobiliary cancer despite low incidence rates. We found that college-level education and urban population was associated with higher overall 5-year survival.
To the best of our knowledge, this is the largest study to assess the role of socioeconomic factors in hepatobiliary cancer survival outcomes. By utilizing national data representative of the United States cancer population, we achieved a large sample size of hepatobiliary cancer cases, despite their low incidence rates to identify other factors that impact overall survival.
Future studies targeting socioeconomic characteristics associated with poorer survival outcomes are warranted to determine potential etiologies and develop treatment strategies for improving outcomes in these groups.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Rizzo A, Italy S-Editor: Lin C L-Editor: Filipodia P-Editor: Yu HG
| 1. | Tariq NU, McNamara MG, Valle JW. Biliary tract cancers: current knowledge, clinical candidates and future challenges. Cancer Manag Res. 2019;11:2623-2642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 63] [Article Influence: 12.6] [Reference Citation Analysis (1)] |
| 2. | Benavides M, Antón A, Gallego J, Gómez MA, Jiménez-Gordo A, La Casta A, Laquente B, Macarulla T, Rodríguez-Mowbray JR, Maurel J. Biliary tract cancers: SEOM clinical guidelines. Clin Transl Oncol. 2015;17:982-987. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology. 2011;54:173-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 594] [Cited by in F6Publishing: 614] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 4. | Marcano-Bonilla L, Mohamed EA, Mounajjed T, Roberts LR. Biliary tract cancers: epidemiology, molecular pathogenesis and genetic risk associations. Chin Clin Oncol. 2016;5:61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 5. | Plentz RR, Malek NP. Clinical presentation, risk factors and staging systems of cholangiocarcinoma. Best Pract Res Clin Gastroenterol. 2015;29:245-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Patel N, Benipal B. Incidence of Cholangiocarcinoma in the USA from 2001 to 2015: A US Cancer Statistics Analysis of 50 States. Cureus. 2019;11:e3962. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Bergquist A, von Seth E. Epidemiology of cholangiocarcinoma. Best Pract Res Clin Gastroenterol. 2015;29:221-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 264] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 8. | Mukkamalla SKR, Naseri HM, Kim BM, Katz SC, Armenio VA. Trends in Incidence and Factors Affecting Survival of Patients With Cholangiocarcinoma in the United States. J Natl Compr Canc Netw. 2018;16:370-376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 9. | Saha SK, Zhu AX, Fuchs CS, Brooks GA. Forty-Year Trends in Cholangiocarcinoma Incidence in the U.S.: Intrahepatic Disease on the Rise. Oncologist. 2016;21:594-599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 364] [Cited by in F6Publishing: 462] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 10. | Shaib YH, Davila JA, McGlynn K, El-Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40:472-477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 514] [Cited by in F6Publishing: 517] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 11. | Rawla P, Sunkara T, Thandra KC, Barsouk A. Epidemiology of gallbladder cancer. Clin Exp Hepatol. 2019;5:93-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 12. | Henley SJ, Weir HK, Jim MA, Watson M, Richardson LC. Gallbladder Cancer Incidence and Mortality, United States 1999-2011. Cancer Epidemiol Biomarkers Prev. 2015;24:1319-1326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 13. | Yeo CJ, Sohn TA, Cameron JL, Hruban RH, Lillemoe KD, Pitt HA. Periampullary adenocarcinoma: analysis of 5-year survivors. Ann Surg. 1998;227:821-831. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 303] [Cited by in F6Publishing: 297] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 14. | Klein F, Jacob D, Bahra M, Pelzer U, Puhl G, Krannich A, Andreou A, Gül S, Guckelberger O. Prognostic factors for long-term survival in patients with ampullary carcinoma: the results of a 15-year observation period after pancreaticoduodenectomy. HPB Surg. 2014;2014:970234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Miedema BW, Sarr MG, van Heerden JA, Nagorney DM, McIlrath DC, Ilstrup D. Complications following pancreaticoduodenectomy. Current management. Arch Surg. 1992;127:945-9; discussion 949. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 267] [Cited by in F6Publishing: 299] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 16. | Santoni M, Rizzo A, Mollica V, Matrana MR, Rosellini M, Faloppi L, Marchetti A, Battelli N, Massari F. The impact of gender on The efficacy of immune checkpoint inhibitors in cancer patients: The MOUSEION-01 study. Crit Rev Oncol Hematol. 2022;170:103596. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 89] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 17. | Rizzo A, Brandi G. Neoadjuvant therapy for cholangiocarcinoma: A comprehensive literature review. Cancer Treat Res Commun. 2021;27:100354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 18. | Rizzo A, Brandi G. Pitfalls, challenges, and updates in adjuvant systemic treatment for resected biliary tract cancer. Expert Rev Gastroenterol Hepatol. 2021;15:547-554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 19. | Ricci AD, Rizzo A, Brandi G. Immunotherapy in Biliary Tract Cancer: Worthy of a Second Look. Cancer Control. 2020;27:1073274820948047. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 20. | Kim D, Konyn P, Cholankeril G, Bonham CA, Ahmed A. Trends in the Mortality of Biliary Tract Cancers Based on Their Anatomical Site in the United States From 2009 to 2018. Am J Gastroenterol. 2021;116:1053-1062. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Jaruvongvanich V, Assavapongpaiboon B, Wong L. Racial/ethnic disparities in gallbladder cancer receipt of treatments. J Gastrointest Oncol. 2018;9:348-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program. [cited 12 July 2023]. Available from: https://surveillance.cancer.gov/. [Cited in This Article: ] |
| 23. | Unger JM, Stires H, Levit LA, Stewart M, McKelvey BA, Canin B, Dressler E, Flaherty K, Fredette P, Jones L, McCann P, Miller T, Onitilo AA, Palmieri F, Patel T, Paul R, Smith GL, Bruinooge SS, Garrett-Mayer E, Lei XJ, Alva A, Schenkel C. Sponsor Perspectives on the Impact of the COVID-19 Pandemic on Interventional Cancer Clinical Trial Protocols and Data Quality. JCO Oncol Pract. 2023;19:907-916. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Kim BW, Oh CM, Choi HY, Park JW, Cho H, Ki M. Incidence and Overall Survival of Biliary Tract Cancers in South Korea from 2006 to 2015: Using the National Health Information Database. Gut Liver. 2019;13:104-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 25. | Turati F, Bertuccio P, Negri E, Vecchia CL. Epidemiology of cholangiocarcinoma. Hepatoma Res. 2022;8:19. [DOI] [Cited in This Article: ] |
| 26. | O'Connor JM, Sedghi T, Dhodapkar M, Kane MJ, Gross CP. Factors Associated With Cancer Disparities Among Low-, Medium-, and High-Income US Counties. JAMA Netw Open. 2018;1:e183146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 27. | Blake KD, Moss JL, Gaysynsky A, Srinivasan S, Croyle RT. Making the Case for Investment in Rural Cancer Control: An Analysis of Rural Cancer Incidence, Mortality, and Funding Trends. Cancer Epidemiol Biomarkers Prev. 2017;26:992-997. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 241] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 28. | U.S. Department of Agriculture. Ag and Food Statistics. Charting the Essentials. [cited 12 July 2023]. Available from: https://www.ers.usda.gov/data-products/ag-and-food-statistics-charting-the-essentials/. [Cited in This Article: ] |
| 29. | Zhao G, Okoro CA, Hsia J, Garvin WS, Town M. Prevalence of Disability and Disability Types by Urban-Rural County Classification-U.S., 2016. Am J Prev Med. 2019;57:749-756. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 30. | Medicaid and CHIP Payment and Access Commission. Medicaid and Rural Health. [cited 12 July 2023]. Available from: https://www.macpac.gov/publication/medicaid-and-rural-health/. [Cited in This Article: ] |
| 31. | National Institutes of Health. Health in Rural America: Connecting to Care. Mar 7, 2022. [cited 12 July 2023]. Available from: http://www.advancingstates.org/node/73717. [Cited in This Article: ] |
| 32. | Patel A, Gantz O, Zagadailov P, Merchant AM. The role of socioeconomic disparity in colorectal cancer stage at presentation. Updates Surg. 2019;71:523-531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Aizer AA, Chen MH, McCarthy EP, Mendu ML, Koo S, Wilhite TJ, Graham PL, Choueiri TK, Hoffman KE, Martin NE, Hu JC, Nguyen PL. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31:3869-3876. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 576] [Cited by in F6Publishing: 703] [Article Influence: 63.9] [Reference Citation Analysis (0)] |