Published online Apr 18, 2017. doi: 10.4254/wjh.v9.i11.551
Peer-review started: June 23, 2015
First decision: September 21, 2015
Revised: February 10, 2017
Accepted: March 14, 2017
Article in press: March 17, 2017
Published online: April 18, 2017
Processing time: 316 Days and 2.8 Hours
To evaluate new therapies for hepatitis C virus (HCV), data about real-world outcomes are needed.
Outcomes of 223 patients with genotype 1 HCV who started telaprevir- or boceprevir-based triple therapy (May 2011-March 2012) at the Mount Sinai Medical Center were analyzed. Human immunodeficiency virus-positive patients and patients who received a liver transplant were excluded. Factors associated with sustained virological response (SVR24) and relapse were analyzed by univariable and multivariable logistic regression as well as classification and regression trees. Fast virological response (FVR) was defined as undetectable HCV RNA at week-4 (telaprevir) or week-8 (boceprevir).
The median age was 57 years, 18% were black, 44% had advanced fibrosis/cirrhosis (FIB-4 ≥ 3.25). Only 42% (94/223) of patients achieved SVR24 on an intention-to-treat basis. In a model that included platelets, SVR24 was associated with white race [odds ratio (OR) = 5.92, 95% confidence interval (CI): 2.34-14.96], HCV sub-genotype 1b (OR = 2.81, 95%CI: 1.45-5.44), platelet count (OR = 1.10, per x 104 cells/μL, 95%CI: 1.05-1.16), and IL28B CC genotype (OR = 3.54, 95%CI: 1.19-10.53). Platelet counts > 135 x 103/μL were the strongest predictor of SVR by classification and regression tree. Relapse occurred in 25% (27/104) of patients with an end-of-treatment response and was associated with non-FVR (OR = 4.77, 95%CI: 1.68-13.56), HCV sub-genotype 1a (OR = 5.20; 95%CI: 1.40-18.97), and FIB-4 ≥ 3.25 (OR = 2.77; 95%CI: 1.07-7.22).
The SVR rate was 42% with telaprevir- or boceprevir-based triple therapy in real-world practice. Low platelets and advanced fibrosis were associated with treatment failure and relapse.
Core tip: A cohort of 223 hepatitis C virus (HCV)-infected patients at a tertiary referral center was analyzed. All patients were treated with telaprevir and boceprevir. Using both logistic regression and a machine learning techniques we identified baseline and on-treatment factors associated with sustained virologic response and relapse. We found that both low platelet count and advanced fibrosis or cirrhosis were associated with treatment failure. Information of the effectiveness of these protease inhibitors could be used to inform clinical trials of future HCV direct-acting antivirals.
- Citation: Bichoupan K, Tandon N, Martel-Laferriere V, Patel NM, Sachs D, Ng M, Schonfeld EA, Pappas A, Crismale J, Stivala A, Khaitova V, Gardenier D, Linderman M, Olson W, Perumalswami PV, Schiano TD, Odin JA, Liu LU, Dieterich DT, Branch AD. Factors associated with success of telaprevir- and boceprevir-based triple therapy for hepatitis C virus infection. World J Hepatol 2017; 9(11): 551-561
- URL: https://www.wjgnet.com/1948-5182/full/v9/i11/551.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i11.551
The hepatitis C virus (HCV) infects about 3% of the world’s population. In the United States, HCV chronically infects an estimated 2.7 to 3.9 million people and is a leading cause of liver disease, liver cancer, and liver-related death[1,2]. In 2012, the Centers for Disease Control and Prevention announced a public health initiative to identify HCV-positive individuals and transition them into care[1]. The recommendation for increased screening was affirmed by the United States Preventive Services Task Force[3]. The goal of treatment is to induce a sustained virological response (SVR) and thereby interrupt, and potentially reverse the progression of liver disease, improve the quality of life, and reduce the risk of transmission.
In the era of direct-acting antiviral (DAAs) drugs, treatment for HCV is evolving rapidly. Data about outcomes in real-world clinical practice are needed to evaluate new medications. The first generation protease inhibitors, telaprevir (TVR) and boceprevir (BOC), were approved for treatment of genotype 1 HCV in combination with pegylated-interferon (PEG) and ribavirin (RBV) in 2011. In clinical trials, overall SVR rates ranged from 64% to 75% for TVR-based triple therapy and from 59% to 66% for BOC-based triple therapy[4,5]. These trials enrolled relatively few patients ≥ 65 years of age and few with liver cirrhosis[6-8], although these patients are often in great need of treatment. Triple therapy with TVR or BOC is no longer the standard of care in the United States and is no longer recommended by the American Association for the Study of Liver Disease and the Infectious Disease Society of America[9]; however, the effectiveness of these regimens in real-world practice may offer important information about currently available NS3/4A protease inhibitors. Information about their real-world effectiveness may benefit providers and patients in these regions of the globe.
Past investigations of factors associated with treatment outcome have yielded varied results[4,10]. Among patients receiving TVR-based therapy, the absence of fibrosis or cirrhosis has been associated with SVR[4,10-12]. Younger age was associated with SVR in one study[4], but not in others[12,13]. Among patients receiving BOC-based triple therapy, younger age and lower fibrosis stage were associated with SVR among treatment-naïve patients, but not among treatment-experienced patients[4,7]. A study of veterans receiving either TVR- or BOC-based triple therapy found that failure to achieve SVR was associated with cirrhosis, prior null or partial response, IL-28B CT or TT genotype, high baseline viral load, black race, diabetes, high aspartate transaminase (AST) to platelet ratio index (APRI) and FIB-4 scores, low platelet counts, and low LDL cholesterol[14].
TVR- and BOC-based triple therapies cause side effects and adverse events, including anemia, neutropenia, thrombocytopenia, rash, and liver decompensation[4,13-16], which can lead to dose reductions and treatment discontinuations[4,7,14]. Several deaths have been reported[17]. A key clinical question is which patients should be treated now, and which should be advised to wait for less toxic and more effective therapies. A major concern is that the rate of liver damage may accelerate with age[18]. Modeling studies predict that HCV-related morbidity and mortality will increase sharply in the years ahead[19,20]. The aging of the HCV-positive population and the age-dependent increase in the risk of HCV-related morbidity and mortality create a need to develop and deploy HCV therapies as safely and effectively as possible.
In real-world clinical practice little is known about the factors associated with SVR rates and relapse with DAA’s. This project investigates these factors and provides a benchmark for comparing newer therapeutic agents to the first generation protease inhibitors. We were especially interested in factors that may worsen over time, such liver fibrosis stage and platelet counts.
The study conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the IRB of Mount Sinai (GCO #: 10-0032). The need for informed consent was waived as the work did not alter standard clinical practice. The study group was composed of 223 adults who initiated triple therapy with PEG-IFN/RBV and TVR or BOC (HCV NS3/4A protease inhibitors) at the Mount Sinai Medical Center between May 2011 and March 2012. Providers submitted names of patients and Mount Sinai’s electronic database was queried to capture the complete cohort of patients. The electronic database query identified patients with an ICD-9 code for HCV and a prescription for either TVR or BOC. Medical records were reviewed to verify patients identified through electronic phenotyping. All patients included in the analysis received at least one dose of TVR or BOC. Human immunodeficiency virus-positive patients and patients who previously received a liver transplant were excluded.
Data about the HCV treatment regimen, age, gender, race, body mass index (BMI), IL28B genotype, baseline clinical laboratory values [albumin, hemoglobin, AST, alanine transaminase (ALT), platelets, creatinine, ferritin, information to calculate the estimated glomerular filtration rate (eGFR), and alpha-fetoprotein (AFP)], indicators of liver fibrosis, past medical history, including diabetes (indicated by at least one of the following-prescription for metformin, pioglitazone, insulin, a recorded diagnosis of diabetes, fasting glucose above 125 mg/dL, or hemoglobin A1c above 6.5%), depression (indicated by use of at least one of the following medications-wellbutrin, aripiprazole, bupropion, citalopram, venlafaxine, escitalopram, paroxetine, or sertraline), outcome of prior HCV treatment, HCV sub-genotype and viral load were extracted from medical records. On-treatment changes in HCV viral load, ferritin, estimated GFR using the Chronic Kidney Disease Epidemiology Collaboration formula[21] were calculated and incident anemia (hemoglobin below 9 g/dL) was recorded.
If available, biopsy or transient elastography data were used to classify liver disease as cirrhosis (yes/no). Cirrhosis was defined as a liver biopsy score of 4 using the Batts-Ludwig system or a transient elastography value > 13.5 kPa. In addition, liver fibrosis stage was estimated from the APRI and the FIB-4 score. APRI scores > 1.5 and FIB-4 scores > 3.25 were coded as advanced fibrosis/cirrhosis. APRI and FIB-4 scores were calculated as follows: APRI = AST (U/L)/(upper limit of normal)/[platelet count (109/L) × 100][22-24]; FIB-4 = age (years) × AST (U/L)/[platelet count (109/L) × ALT (U/L)1/2][25,26]. The FIB-4 score is reliable and has been tested and validated in large HCV mono-infected cohorts[27].
The standard treatment regimens are outlined in supporting Figures 1 (TVR) and 2 (BOC). Patients on TVR received 12 wk of TVR in combination with PEG/RBV. After week 12, TVR was discontinued. PEG/RBV dual therapy was continued for an additional 12 or 36 wk depending on prior treatment response, the presence of cirrhosis, and viral kinetics. With the exceptions noted below, patients on BOC-containing regimens began treatment with 4 wk of PEG-IFN/RBV dual therapy. Then BOC was added and patients received triple therapy for 24 to 44 wk depending on prior treatment response, the presence of cirrhosis and viral kinetics. Eight patients did not receive the standard PEG-IFN/RBV lead-in prior to starting BOC: Six did not have a lead-in phase, one received 19 wk of lead-in, and one received 24 wk of lead-in. Seventy-two patients, 63 on TVR and 9 on BOC, met the eligibility criteria for consideration of response guided therapy. Virologic stopping rules were followed.
During treatment, follow-up visits were scheduled for weeks 4, 8, 12, 24, 48, 60 and 72. Anemia was managed at the discretion of the provider. Generally, RBV dose was reduced at hemoglobin levels ≤ 10 g/dL. If hemoglobin levels remained low, erythropoietin was administered. Blood transfusions were used to treat intractable anemia[9]. Eltrombopag was not used.
SVR was defined as the absence of HCV RNA six months after the end-of-treatment (EOT), as measured by a real-time-polymerase chain reaction assay (Roche Cobas/Ampliprep Cobas Taqman version 2.0, Roche Molecular Systems Inc., Branchburg, NJ, United States). An HCV viral load below the lower limit of detection (18 IU/mL) was recorded as undetectable. Relapse was defined as the detection of HCV RNA after the absence of HCV RNA at EOT. A fast virologic response (FVR) was defined as undetectable HCV RNA 4 wk after the initiation of TVR or 8 wk after the initiation of BOC-based treatment.
Subgroups were compared using t-tests or Mann-Whitney tests for continuous variables and χ2 or Fisher-exact tests for categorical variables. Pearson correlation tests were used to assess associations between continuous variables. Univariable and multivariable logistic regression were used to analyze the association between the baseline factors listed above and SVR calculated on an intention-to-treat basis. Models for relapse were built using data from patients who completed the planned treatment regimen, had undetectable HCV RNA at the EOT, and who were not lost to follow-up. Factors with a P-value below 0.15 in univariable models were included in multivariable analyses; variables with a P-value below 0.05 were retained in final models and were considered to be independently associated with the outcome.
When variables were highly correlated with each other, a series of models was built, each with only one member of the pair. In the case of variables that contained common components (e.g., APRI scores and FIB-4 scores) and variables that represent similar disease characteristics (FIB-4 ≥ 3.25 and histologically/elastography-defined cirrhosis) only one was entered into a model at a time. Missing data for HCV sub-genotype and IL28B polymorphism were imputed as 1a or CT/TT, respectively. A sensitivity analysis was conducted to determine whether variables associated with SVR were specific to one of the two protease inhibitors. Regression analyses were conducted on a dataset containing patients on TVR alone.
Classification and regression trees (CARTs) were built to identify baseline factors associated with SVR. Factors in the tree included race, cirrhosis (FIB-4 score), diabetes, HCV sub-genotype, IL28B genotype, history and previous response to HCV treatment (naïve/relapse vs null/intolerant), and platelet count.
The significance level was set to 0.05. Statistical analyses were conducted with SPSS and SAS.
Two complementary approaches were used to identify cases and ensure that the entire cohort of patients meeting the entry criteria were included. The traditional approach (patients identified by their providers) yielded 209 patients. A query of the Mount Sinai data warehouse identified an additional 14 patients, yielding a total of 223 patients whose baseline characteristics are presented in Table 1. One hundred and seventy-two (77%) were treated with TVR and 51 (23%) were treated with BOC. The patients were predominantly male (65%) with a median age of 57 years (range: 22-74) and a BMI of 27.1 kg/m3 (range: 16.3-49.3). Eighteen percent were black. Liver biopsy and/or transient elastography data were available for 202/223 (91%) patients of whom 96/202 (48%) had cirrhosis.
| n | Continuous variables: Median (IQR)/categorical variables: n (%) | Range | |
| Telaprevir | 223 | 172 (77) | - |
| Demographics and anthropometrics | |||
| Age, yr | 223 | 57 (51-61) | 22-74 |
| Gender, male | 223 | 144 (65) | - |
| Race, white | 223 | 182 (82) | - |
| BMI, kg/m3, 17-24: Normal; 25-30: Overweight; > 30: Obesea | 186 | 27.1 (24.5-30.7) | 16.3-49.3 |
| Past medical history | |||
| Diabetes | 223 | 48 (22) | - |
| Depression | 223 | 47 (21) | - |
| IL28B genotype | 223 | ||
| CC | 20 (9) | - | |
| CT | 50 (22) | - | |
| TT | 21 (10) | - | |
| Unknown | 132 (59) | - | |
| Treatment history | 223 | ||
| Naïve | 68 (31) | - | |
| Non-responder | 95 (43) | - | |
| Relapser | 45 (20) | - | |
| Intolerance | 12 (5) | - | |
| Unknown | 3 (1) | - | |
| HCV treatment related characteristics | |||
| HCV viral load, log IU/mL | 221 | 6.31 (5.89-6.66) | 3.07-7.64 |
| Sub-genotype | 223 | ||
| 1a | 118 (53.5) | - | |
| 1b | 72 (32) | - | |
| 1a/1b | 1 (0.5) | - | |
| Unknown | 32 (14) | - | |
| Laboratory tests | |||
| Hemoglobin, g/dL, female:12-15.5 g/dL; male: 13.5-17.5 g/dLa | 220 | 14.3 (13.2-15.3) | 9.2-18.2 |
| AFP, ng/mL, 1.6-4.5 ng/mLa | 158 | 6.85 (3.58-13.83) | 0.80-208.60 |
| Albumin g/dL, 3.5-4.9 g/dLa | 219 | 4.20 (3.90-4.50) | 2.60-5.30 |
| AST, U/L, 1-50 U/La | 221 | 62 (39-106) | 19-324 |
| ALT, U/L, 1-53 U/La | 221 | 67 (44-107) | 15-403 |
| Platelets, × 103/μL, 150-450 × 103/μLa | 223 | 152 (106-195) | 14-365 |
| Creatinine, mg/dL, 0.60-1.40 mg/dLa | 205 | 0.91 (0.81-1.04) | 0.58-10.2 |
| Ferritin, ng/mL, 15-150 ng/mLa | 54 | 184 (113-373) | 22-893 |
| eGFR, mL/min/1.73 m2 < 60 mL/min per 1.73 m2ab | 204 | 88 (75-99) | 6-125 |
| Indication of liver fibrosis | |||
| APRI score | 221 | 0.82 (0.43-1.82) | 0.10-12.48 |
| FIB-4 score | 221 | 2.65 (1.77-5.66) | 0.35-35.30 |
| FIB-4, advanced fibrosis/cirrhosisc | 221 | 98/221 (44) | - |
| Cirrhosis, biopsy | 189 | 85/189 (45) | - |
| Transient elastography score, kPa | 80 | 11.8 (7.2-20.3) | 3.9-55.1 |
| Cirrhosis, transient elastographyd | 80 | 36/80 (45) | - |
| Cirrhosis, transient elastography/biopsy | 202 | 96/202 (48) | - |
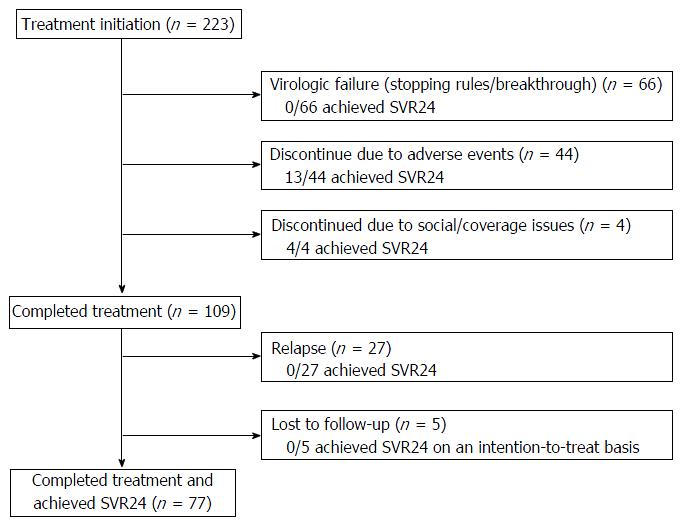
Figure 1 diagrams treatment outcomes. Of the 223 patients who initiate treatment, 66 patients had virologic failure during treatment; 44 discontinued treatment early due to adverse events (13 achieved SVR); four discontinued due to social/coverage issues (four achieved SVR); 109 (49%) completed the intended treatment regimen and were viral load negative at the EOT, but 27 relapsed and five did not return for testing at week-24 post EOT and could not be reached after repeated attempts by providers to contact them. On an intention-to-treat basis, the overall SVR rate was 42%. The overall SVR rate was 45% (77/172) for patients on TVR and 33% (17/51) for patients on BOC (P = 0.15) in an unadjusted analysis that did not correct for baseline differences. The SVR rates for treatment-naïve, prior non-responders, prior-interferon intolerant patients, and prior relapsers were 41%, 35%, 33% and 62%, respectively (P = 0.04).
The baseline characteristics of the SVR and non-SVR groups are presented in Supporting Table 1. The SVR group had a significantly higher proportion of patients with the favorable IL28B CC genotype, with HCV sub-genotype 1b and higher levels of albumin and platelets, and this group had a significantly lower proportion of blacks, patients with diabetes, liver cirrhosis, a history of non-response to PEG/RBV treatment, lower levels of AFP and AST, and lower APRI and FIB-4 scores. Univariable logistic regression analysis of baseline variables showed IL28B CC genotype, higher platelets, higher albumin, HCV sub-genotype 1b, an HCV treatment history that included no prior treatment or relapse were positively associated with SVR and that black race, diabetes, high AST, high AFP, diagnosis of cirrhosis, or and advanced fibrosis/cirrhosis with APRI >1.5 or FIB-4 ≥ 3.25 were negatively associated with SVR (Table 2).
| Univariable | Multivariablea | |||||
| OR | 95%CI | P | OR | 95%CI | P | |
| Protease inhibitor, telaprevir | 1.62 | 0.84-3.12 | 0.15 | - | - | - |
| Age, yr | 0.98 | 0.96-1.01 | 0.23 | - | - | - |
| Gender, male | 1.11 | 0.64-1.94 | 0.71 | - | - | - |
| Race, white | 3.12 | 1.41-6.90 | < 0.01 | 5.92 | 2.34-14.96 | < 0.01 |
| Diabetes | 0.43 | 0.21-0.87 | 0.02 | - | - | - |
| Depression | 1.58 | 0.83-3.02 | 0.17 | - | - | - |
| BMI, kg/m2 | 0.98 | 0.93-1.04 | 0.52 | - | - | - |
| IL28B, CC vs CT/TT | 3.56 | 1.31-9.64 | 0.01 | 3.54 | 1.19-10.53 | 0.02 |
| Treatment history, naïve/relapser | 1.86 | 1.08-3.20 | 0.03 | - | - | - |
| HCV viral load, log IU/mL | 0.79 | 0.54-1.14 | 0.21 | - | - | - |
| Sub-genotype, 1b (vs all other) | 2.06 | 1.17-3.65 | 0.01 | 2.81 | 1.45-5.44 | 0.02 |
| Hemoglobin, g/dL | 1.03 | 0.86-1.23 | 0.78 | - | - | - |
| AFP, ng/mL | 0.95 | 0.92-0.98 | < 0.01 | - | - | - |
| Albumin, g/dL | 2.56 | 1.33-4.92 | < 0.01 | - | - | - |
| AST, U/L | 0.99 | 0.99-0.99 | 0.02 | - | - | - |
| ALT, per U/L | 1.00 | 0.99-1.01 | 0.61 | - | - | - |
| Platelets, per × 104/µL | 1.08 | 1.03-1.13 | < 0.01 | 1.10 | 1.05-1.16 | < 0.01 |
| Creatinine, per mg/dL | 0.8 | 0.42-1.53 | 0.50 | - | - | - |
| Ferritin, per ng/mL | 0.99 | 0.99-1.00 | 0.63 | - | - | - |
| eGFR, per mL/min per 1.73 m2 | 1.00 | 0.99-1.02 | 0.65 | - | - | - |
| APRI | 0.84 | 0.70-1.02 | 0.08 | - | - | - |
| FIB-4 | 0.91 | 0.83-0.99 | 0.02 | - | - | - |
| APRI > 1.5 | 0.44 | 0.24-0.82 | 0.01 | - | - | - |
| FIB-4 ≥ 3.25 | 0.39 | 0.22-0.68 | < 0.01 | - | - | - |
| Cirrhosis transient elastography/biopsy | 0.5 | 0.29-0.87 | 0.01 | - | - | - |
Before building multivariable models, the Pearson’s correlation coefficient for pairs of variables was determined to ensure that two highly correlated variables were not included in the same model. The multivariable logistic regression model shown in Table 2 includes the platelet count and excludes variables highly correlated with the platelet count. SVR was positively associated with IL28B CC genotype [odds ratio (OR) = 3.54, 95% confidence interval (CI): 1.19-10.53], higher platelet counts (OR = 1.10, per x 104 cells/μL, 95%CI: 1.05-1.16), white race (OR = 5.92, 95%CI: 2.34-14.96) and sub-genotype 1b HCV (OR = 2.81, 95%CI: 1.45-5.44). Four additional logistic regression models were built in which the platelet count was excluded and variables that are highly correlated with platelet count were examined individually (Table 2). In all four models white race, sub-genotype 1b, and CC IL28B genotype were positively associated with SVR. Variables indicative of more advanced liver disease - lower albumin, higher FIB-4 score, cirrhosis on biopsy/fibroscan - were significantly associated with treatment failure in individual models. A forest plot showing the interaction between treatment history and various baseline characteristics on outcome is presented in Supporting Figure 3.
An additional multivariable logistic regression analysis was conducted on the subgroup of patients receiving TVR-based triple therapy. Results were similar to those for the entire study group. SVR was positively associated with platelets (OR = 1.07 per × 104 cells/μL; 95%CI: 1.01-1.14), white race (OR = 5.22; 95%CI: 1.76-15.50), and HCV sub-genotype 1b (OR = 3.27; 95%CI: 1.51-7.07).
Table 3 shows the association between SVR and changes that occurred during treatment. As expected, SVR was strongly associated with viral kinetics. Patients whose HCV viral load decreased rapidly after starting the protease inhibitor and who thus had a FVR were significantly more likely to achieve an SVR (P < 0.01). SVR was also significantly associated with a greater decrease in eGFR in the first 4 wk. Treatment discontinuation due to adverse events was less common in the group that achieved SVR than the group that failed therapy (14% vs 24%), however, this difference was not statistically significant, P = 0.06. There was a trend of patients achieving SVR to having higher median changes in ferritin levels than patients who failed to achieve SVR (146 ng/mL vs 62 ng/mL, P = 0.08).
| Total cohort Categorical: n (%) Continous: Median (IQR) | SVR (n = 94) | Fail to achieve SVR (n = 129) | P | |
| Discontinuation due to adverse events | 44/223 (20%) | 13/94 (14%) | 31/94 (24%) | 0.06a |
| Change in ferritin from baseline to week 4 of treatment, ng/mL | 91 (45-212) | 146 (81-331) | 62 (-20-175) | 0.08b |
| Development of severe anemia | 94/223 (42%) | 54/94 (57%) | 66/129 (51%) | 0.35a |
| Change in eGFR from baseline to week 4c, mL/min per 1.73 m2 | -4.41 (-14.87-3.23) | -6.59 (-16.98-0.52) | -1.68 (-13.99-5.00) | 0.04b |
| Fast viral kinetics | 139/223 (62%) | 83/94 (88%) | 56/129 (43%) | < 0.01a |
Overall, 109 patients had an undetectable HCV viral load at the time when they completed the intended therapy. Five were lost to follow-up. Relapse was confirmed in 27/104 (25%) of the patients who had follow-up data. Among the patients who were viral load negative at the time treatment ended and who later became viral load positive, the median time to confirmed relapse was 12 wk after the EOT (IQR = 5-12 wk, range = 2-34 wk); however, in some cases, relapse may have occurred before the time it was detected because of missed visits. Multivariable logistic regression analysis revealed an association between relapse and HCV sub-genotype 1a (OR = 5.15; 95%CI: 1.40-18.97) and advanced fibrosis/cirrhosis (FIB-4 score ≥ 3.25; OR = 2.77; 1.07-7.22) (Table 4). The absence of an FVR (OR = 4.77, 95%CI: 1.68-13.56) was the only on-treatment variable independently associated with relapse.
| Univariable | Multivariable | |||||
| OR | 95%CI | P | OR | 95%CI | P | |
| Protease inhibitor, Telaprevir | 0.41 | 0.15-1.10 | 0.08 | - | - | - |
| Age, yr | 1.00 | 0.96-1.05 | 0.95 | - | - | - |
| Gender, female | 1.36 | 0.53-3.48 | 0.52 | - | - | - |
| Race, black | 1.16 | 0.28-4.71 | 0.84 | - | - | - |
| Diabetes | 1.86 | 0.65-5.28 | 0.25 | - | - | - |
| Depression | 0.54 | 0.17-1.75 | 0.30 | - | - | - |
| BMI, per kg/m2 | 0.98 | 0.88-1.10 | 0.76 | - | - | - |
| IL28B, CC vs CT/TTa | - | - | - | - | - | - |
| Treatment history, naïve/relapser | 1.10 | 0.45-2.72 | 0.84 | - | - | - |
| HCV viral load, log IU/mL | 1.74 | 0.80-3.78 | 0.16 | - | - | - |
| Sub-genotype, 1a (vs all other) | 6.26 | 1.75-22.45 | 0.01 | 5.15 | 1.40-18.97 | 0.01 |
| Hemoglobin, g/dL | 1.24 | 0.88-1.73 | 0.22 | - | - | - |
| AFP, ng/mL | 1.02 | 0.99-1.05 | 0.15 | - | - | - |
| Albumin, g/dL | 1.02 | 0.34-3.08 | 0.97 | - | - | - |
| AST, U/L | 1.01 | 1.00-1.02 | 0.06 | - | - | - |
| ALT, U/L | 1.00 | 0.99-1.01 | 0.23 | - | - | - |
| Platelets, × 104/µL | 0.95 | 0.89-1.02 | 0.19 | - | - | - |
| Creatinine, mg/dL | 1.41 | 0.72-2.74 | 0.31 | - | - | - |
| Ferritin, ng/mL | 1.00 | 0.99-1.01 | 0.26 | - | - | - |
| eGFR, mL/min per 1.73 m2b | 0.99 | 0.97-1.02 | 0.66 | - | - | - |
| APRI score | 1.16 | 0.94-1.43 | 0.17 | - | - | - |
| FIB-4 score | 1.03 | 0.94-1.13 | 0.49 | - | - | - |
| APRI > 1.5 | 2.33 | 0.87-6.23 | 0.09 | - | - | - |
| FiB-4 ≥ 3.25 | 2.77 | 1.12-6.86 | 0.03 | 2.77 | 1.07-7.22 | 0.04 |
| Cirrhosis, transient elastography/biopsy | 2.55 | 1.05-6.19 | 0.04 | - | - | - |
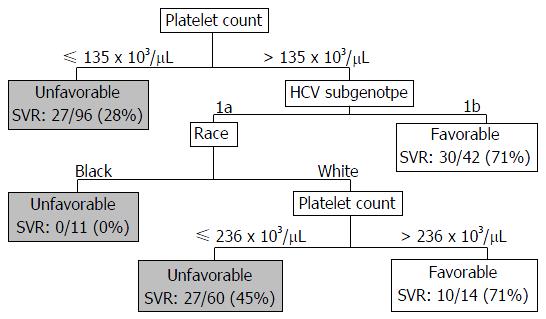
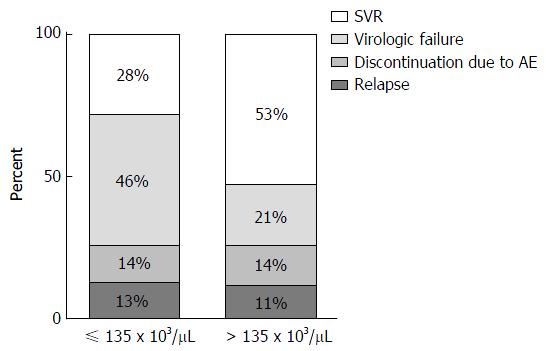
A CART analysis underscored the predictive value of the platelet count and was largely consistent with the results of the logistic regression analysis (Figure 2). The strongest baseline predictor was a platelet count > 135 × 103 cells/μL. Among patients with platelets > 135 × 103 cells/μL, HCV sub-genotype 1b and white race were predictive of SVR. Among patients with platelets > 135 × 103 cells/μL and sub-genotype 1a HCV, platelet counts > 236 × 103/μL were predictive of SVR among whites; however, all of the black patients in this subgroup failed therapy. As illustrated in Figure 3, among patients with platelets > 135 × 103/μL the virologic failure rate was 21% vs 46% in the lower platelet group (P < 0.01), and the SVR rate was 53% vs 28% (P < 0.01).
This study analyzed treatment outcomes in a real-world cohort of patients treated with TVR- and BOC-based triple therapy by experienced hepatologists. The results are important because TVR-based triple therapy is still used in many parts of the world[28]. Our cohort closely resembles the population of HCV-infected individuals in the United States. Twenty-two percent of the United States population is black, 38% have advanced fibrosis/cirrhosis, and the average age is approximately 50 years with 5% above the age of 65 years[28-31]. In our cohort, 18% were black, approximately half had cirrhosis, and 10% were above the age of 65 years. Our most significant findings were the low 42% SVR rate and the association between treatment failure and factors indicative of more advanced liver disease such as low platelets, cirrhosis, high FIB-4 score, high AFP, and low albumin. CART analysis identified platelet counts > 135 × 103/μL as the strongest baseline predictor of SVR. Seventy-two percent of patients with platelet counts < 135 × 103/μL failed therapy.
Our clinical outcomes are consistent with those of the multicenter CUPIC trial, which examined TVR and BOC in 511 patients with compensated cirrhosis[32]. In both studies, non-responders to dual therapy had low SVR rates, whereas prior relapsers had an SVR rate of 62% in our study and 74% in the CUPIC cohort, supporting the use of triple therapy in prior relapsers. Discontinuations due to adverse events and virologic failure were common in both studies. Only 49% of our cohort and 52% of the CUPIC study group completed the planned treatment. The SVR rate in CUPIC was 48%, similar to the 42% in our study (P = 0.15). These results highlight the need for more effective therapies.
The association we observed between high platelets and SVR is consistent with published data. The relationship between thrombocytopenia and failure to achieve SVR was demonstrated in ENABLE-1 and ENABLE-2[33], two phase III multicenter randomized controlled trials in which patients on PEG/RBV were either treated with eltrombopag (a drug that stimulates platelet production) or placebo. In both trials, the SVR rate was significantly higher in the patients receiving eltrombopag: ENABLE-1 (23% vs 14%, P < 0.01) and ENABLE-2 (19% vs 13%, P = 0.02).
We did not find an association between younger age and SVR. This association was previously reported in a study by Frei and colleagues in which patients < 60 years of age or ≥ 60 years of age were matched on gender, cirrhosis, HCV genotype, and prior treatment response[34]. Further studies are needed to clarify the relationship between age and SVR among patients receiving triple therapy.
In our study, relapse occurred in about one-quarter of the patients who completed treatment and had an EOT. Relapse is emerging as the most common cause of non-SVR with newer DAAs. Sofosbuvir is an NS5B inhibitor that recently received FDA approval. In clinical trials, 9% of patients treated with sofosbuvir and PEG/RBV relapsed and 22% of patients treated with sofosbuvir and RBV relapsed[35,36]. Simeprevir is an NS3/4A protease inhibitor that recently received FDA approval. In clinical trials of simeprevir, relapse occurred in 11% of treatment-naïve and 18.5% of treatment-experienced patients[37]. In our study, relapse was related to both viral and host factors. HCV sub-genotype 1a and advanced fibrosis/cirrhosis (FIB-4 ≥ 3.25) were independently associated with relapse. Additionally, the absence of FVR was also associated with relapse, consistent with results of dual therapy[37]. Recent data suggest that viral double-stranded RNA may play a role in relapse[38]. Understanding the molecular basis of relapse may allow the development of drugs that specifically target the processes underlying this type of treatment failure.
The strengths of our study include the complementary methods used to ensure that the entire cohort of patients was included, use of a study group with a high percentage of blacks, older patients and patients with advanced liver disease who were treated in real-world clinical practice, and use of two methods - multivariable logistic regression and CART analysis - to identify factors associated with treatment outcome. The limitations include the relatively small number of patients on BOC, and the observational study design, which limits the ability to compare the two protease inhibitors to each other.
Unless and until treatments are available that greatly reduce the risk of hepatocellular carcinoma in patients who have advanced liver disease, it will be essential to treat patients before extensive liver damage has occurred, as noted by others[36]. Several newly approved DAAs are available for HCV-infected patients including: Simeprevir, sofosbuvir, and the combination of ombitasvir, paritaprevir with ritonavir, and dasabuvir. In clinical trials, these compounds achieved high SVR rates; however, their effectiveness in the real-world is unknown and needs to be determined. This study provides a standard against which new therapies can be objectively evaluated.
Hepatitis C virus (HCV) infects 3% of the world’s population. In the United States, HCV chronically infects an estimated 2.7 to 3.9 million people and is a leading cause of liver disease. Prior to 2011, treatments for HCV involved pegylated-interferon and ribavirin and had low rates of success. New drugs for HCV-infection, called direct-acting antivirals (DAAs), were first approved in 2011 and provided higher rates of success with lower adverse event rates. Understanding the effectiveness of these DAAs bears relevance for future clinical trials and simulation studies. This paper successfully analyzed a real-world cohort of patients treated with telaprevir- and boceprevir-based therapies to provide better information on cohorts using this class of therapies in the future.
NS3/4A protease inhibitors are a relatively new class of therapies available to treat HCV-infection. This paper investigates the effectiveness of the first two FDA-approved protease inhibitors for HCV. Understanding the characteristics associated with successful treatment in HCV-infected patients is critical for the ongoing treatment of the HCV-infected population.
The authors used traditional and machine learning methods to identify factors associated with achieving a sustained virologic response in HCV-treatment. The authors found that factors indicative of more advanced fibrosis or cirrhosis were associated with lower rates of sustained virological response. They also found that specific cut-off points in platelet count that could be used to risk-stratify patients. The results imply that treating patients before they develop more advanced disease is an important message that should be relayed to care-providers.
The results could help improve design of future clinical trials for NS3/4A protease inhibitors for HCV. The population contained individuals with characteristics that were lacking in clinical trials, offering unique information on the effectiveness of NS3/4A protease inhibitors in this population. Currently, numerous protease inhibitors are on the market for HCV. Understanding the risk profile of this class of therapies and the factors associated with their risks and benefits could lead to improvements. Additionally, this real-world study could provide some additional information for future simulation studies to use.
Sustained virologic response - the absence of HCV RNA 12-24 wk after the end-of-treatment. Considered a cure in HCV-treatment. Direct-acting antivirals (DAAs) - a class of therapies that directly target HCV and prevent replication. DAAs are composed of NS3/4A protease inhibitors, nucleos(t)ide and non-nucleos(t)ide NS5B Polymerase Inhibitors, and NS5A inhibitors.
This study will give some useful information to clinicians and provided a successful ways to evaluate the new medications. The manuscript read and organized well, and the data treated were also reasonable.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Long JE S- Editor: Kong JX L- Editor: A E- Editor: Li D
| 1. | Branch AD, Van Natta ML, Vachon ML, Dieterich DT, Meinert CL, Jabs DA. Mortality in hepatitis C virus-infected patients with a diagnosis of AIDS in the era of combination antiretroviral therapy. Clin Infect Dis. 2012;55:137-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 2. | Denniston MM, Jiles RB, Drobeniuc J, Klevens RM, Ward JW, McQuillan GM, Holmberg SD. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 578] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 3. | Moyer VA. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:349-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 360] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 4. | Poordad F, McCone J, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1996] [Cited by in RCA: 1978] [Article Influence: 141.3] [Reference Citation Analysis (0)] |
| 5. | Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405-2416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1905] [Cited by in RCA: 1860] [Article Influence: 132.9] [Reference Citation Analysis (0)] |
| 6. | Davis GL, Albright JE, Cook SF, Rosenberg DM. Projecting future complications of chronic hepatitis C in the United States. Liver Transpl. 2003;9:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 404] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 7. | Poordad F, Bronowicki JP, Gordon SC, Zeuzem S, Jacobson IM, Sulkowski MS, Poynard T, Morgan TR, Molony C, Pedicone LD. Factors that predict response of patients with hepatitis C virus infection to boceprevir. Gastroenterology. 2012;143:608-618.e1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 8. | McAdam-Marx C, McGarry LJ, Hane CA, Biskupiak J, Deniz B, Brixner DI. All-cause and incremental per patient per year cost associated with chronic hepatitis C virus and associated liver complications in the United States: a managed care perspective. J Manag Care Pharm. 2011;17:531-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 9. | Squires JE. Risks of transfusion. South Med J. 2011;104:762-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Kwo PY. Phase III results in Genotype 1 naïve patients: predictors of response with boceprevir and telaprevir combined with pegylated interferon and ribavirin. Liver Int. 2012;32 Suppl 1:39-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Forestier N, Zeuzem S. Triple therapy with telaprevir: results in hepatitis C virus-genotype 1 infected relapsers and non-responders. Liver Int. 2012;32 Suppl 1:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Serfaty L, Forns X, Goeser T, Ferenci P, Nevens F, Carosi G, Drenth JP, Lonjon-Domanec I, DeMasi R, Picchio G. Insulin resistance and response to telaprevir plus peginterferon α and ribavirin in treatment-naive patients infected with HCV genotype 1. Gut. 2012;61:1473-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Chayama K, Hayes CN, Abe H, Miki D, Ochi H, Karino Y, Toyota J, Nakamura Y, Kamatani N, Sezaki H. IL28B but not ITPA polymorphism is predictive of response to pegylated interferon, ribavirin, and telaprevir triple therapy in patients with genotype 1 hepatitis C. J Infect Dis. 2011;204:84-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 14. | Ioannou GN, Beste LA, Green PK. Similar effectiveness of boceprevir and telaprevir treatment regimens for hepatitis C virus infection on the basis of a nationwide study of veterans. Clin Gastroenterol Hepatol. 2014;12:1371-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, Focaccia R, Younossi Z, Foster GR, Horban A. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417-2428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1230] [Cited by in RCA: 1212] [Article Influence: 86.6] [Reference Citation Analysis (0)] |
| 16. | Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, Poordad F, Goodman ZD, Sings HL, Boparai N. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207-1217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1306] [Cited by in RCA: 1307] [Article Influence: 93.4] [Reference Citation Analysis (0)] |
| 17. | Hézode C, Fontaine H, Dorival C, Larrey D, Zoulim F, Canva V, de Ledinghen V, Poynard T, Samuel D, Bourlière M. Triple therapy in treatment-experienced patients with HCV-cirrhosis in a multicentre cohort of the French Early Access Programme (ANRS CO20-CUPIC) - NCT01514890. J Hepatol. 2013;59:434-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 362] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 18. | Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2199] [Cited by in RCA: 2153] [Article Influence: 76.9] [Reference Citation Analysis (0)] |
| 19. | CDC. National Survey 1999-2010 Survey Content National Health and Nutrition Examination Survey, 2011. Available from: http://www.cdc.gov/nchs/data/nhanes/survey_content_99_10.pdf. |
| 20. | CDC. Analytic and reporting guidelines: the National Health and Nutrition Examination Survey (NHANES) 2005. [accessed 2012 Sept 20]. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/nhanes_analytic_guidelines_dec_2005.pdf. |
| 21. | Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604-612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20319] [Cited by in RCA: 19872] [Article Influence: 1242.0] [Reference Citation Analysis (0)] |
| 22. | Snyder N, Nguyen A, Gajula L, Soloway R, Xiao SY, Lau DT, Petersen J. The APRI may be enhanced by the use of the FIBROSpect II in the estimation of fibrosis in chronic hepatitis C. Clin Chim Acta. 2007;381:119-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Snyder N, Gajula L, Xiao SY, Grady J, Luxon B, Lau DT, Soloway R, Petersen J. APRI: an easy and validated predictor of hepatic fibrosis in chronic hepatitis C. J Clin Gastroenterol. 2006;40:535-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Yu ML, Lin SM, Lee CM, Dai CY, Chang WY, Chen SC, Lee LP, Lin ZY, Hsieh MY, Wang LY. A simple noninvasive index for predicting long-term outcome of chronic hepatitis C after interferon-based therapy. Hepatology. 2006;44:1086-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 3484] [Article Influence: 183.4] [Reference Citation Analysis (0)] |
| 26. | Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H, Pol S. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1288] [Cited by in RCA: 1592] [Article Influence: 88.4] [Reference Citation Analysis (0)] |
| 27. | Holmberg SD, Lu M, Rupp LB, Lamerato LE, Moorman AC, Vijayadeva V, Boscarino JA, Henkle EM, Gordon SC. Noninvasive serum fibrosis markers for screening and staging chronic hepatitis C virus patients in a large US cohort. Clin Infect Dis. 2013;57:240-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 28. | Ditah I, Ditah F, Devaki P, Ewelukwa O, Ditah C, Njei B, Luma HN, Charlton M. The changing epidemiology of hepatitis C virus infection in the United States: National Health and Nutrition Examination Survey 2001 through 2010. J Hepatol. 2014;60:691-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 122] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 29. | Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, Kaslow RA, Margolis HS. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1934] [Cited by in RCA: 1900] [Article Influence: 73.1] [Reference Citation Analysis (0)] |
| 30. | Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138:513-521, 521.e1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 665] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 31. | Tandon N, Gunnarsson C, Prabhakar A. Sa1070 Patient Characteristics and Drug Utilization Among Hepatitis C (HCV) Patients in a Large Payer Database. Gastroenterology. 2013;144:S988-S989. [DOI] [Full Text] |
| 32. | Hézode C, Fontaine H, Dorival C, Zoulim F, Larrey D, Canva V, De Ledinghen V, Poynard T, Samuel D, Bourliere M. Effectiveness of telaprevir or boceprevir in treatment-experienced patients with HCV genotype 1 infection and cirrhosis. Gastroenterology. 2014;147:132-142.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 201] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 33. | Afdhal NH, Dusheiko GM, Giannini EG, Chen PJ, Han KH, Mohsin A, Rodriguez-Torres M, Rugina S, Bakulin I, Lawitz E. Eltrombopag increases platelet numbers in thrombocytopenic patients with HCV infection and cirrhosis, allowing for effective antiviral therapy. Gastroenterology. 2014;146:442-452.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 34. | Frei P, Leucht AK, Held U, Kofmehl R, Manser CN, Schmitt J, Mertens J, Rau M, Baur K, Gerlach T. Elderly age is not a negative predictive factor for virological response to therapy with pegylated interferon-α and ribavirin in chronic hepatitis C virus patients. Liver Int. 2014;34:551-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, Schultz M, Davis MN, Kayali Z, Reddy KR. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1322] [Cited by in RCA: 1324] [Article Influence: 110.3] [Reference Citation Analysis (0)] |
| 36. | George SL, Bacon BR, Brunt EM, Mihindukulasuriya KL, Hoffmann J, Di Bisceglie AM. Clinical, virologic, histologic, and biochemical outcomes after successful HCV therapy: a 5-year follow-up of 150 patients. Hepatology. 2009;49:729-738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 291] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 37. | Brochot E, Riachi G, Plantier JC, Guillemard C, Vabret A, Mathurin P, Nguyen-Khac E, Duverlie G. Kinetics of relapse after pegylated interferon and ribavirin therapy for chronic hepatitis C. J Med Virol. 2013;85:1191-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 38. | Klepper AL, Eng FJ, Rahman A, Weeks B, El-Shamy A, Fiel MI, Carrasco G, Roayaie S, Bansal M, Schiano TD. Identification of Viral Double-stranded RNA as a Substrate for Hepatitis C Relapse and as a Target for Ribavirin. 64th Annual Meeting of the American Association for the Study of Liver Disease; Washington, DC, November 1-5, 2013. . |