Published online Feb 26, 2020. doi: 10.4252/wjsc.v12.i2.123
Peer-review started: July 17, 2019
First decision: August 23, 2019
Revised: December 3, 2019
Accepted: December 23, 2019
Article in press: December 23, 2019
Published online: February 26, 2020
Endothelial colony-forming cells (ECFCs) have been implicated in the process of vascularization, which includes vasculogenesis and angiogenesis. Vasculogenesis is a de novo formation of blood vessels, and is an essential physiological process that occurs during embryonic development and tissue regeneration. Angiogenesis is the growth of new capillaries from pre-existing blood vessels, which is observed both prenatally and postnatally. The placenta is an organ composed of a variety of fetal-derived cells, including ECFCs, and therefore has significant potential as a source of fetal ECFCs for tissue engineering.
To investigate the possibility of isolating clonal ECFCs from human early gestation chorionic villi (CV-ECFCs) of the placenta, and assess their potential for tissue engineering.
The early gestation chorionic villus tissue was dissociated by enzyme digestion. Cells expressing CD31 were selected using magnetic-activated cell sorting, and plated in endothelial-specific growth medium. After 2-3 wks in culture, colonies displaying cobblestone-like morphology were manually picked using cloning cylinders. We characterized CV-ECFCs by flow cytometry, immunophenotyping, tube formation assay, and Dil-Ac-LDL uptake assay. Viral transduction of CV-ECFCs was performed using a Luciferase/tdTomato-containing lentiviral vector, and transduction efficiency was tested by fluorescent microscopy and flow cytometry. Compatibility of CV-ECFCs with a delivery vehicle was determined using an FDA approved, small intestinal submucosa extracellular matrix scaffold.
After four passages in 6-8 wks of culture, we obtained a total number of 1.8 × 107 CV-ECFCs using 100 mg of early gestational chorionic villus tissue. Immunophenotypic analyses by flow cytometry demonstrated that CV-ECFCs highly expressed the endothelial markers CD31, CD144, CD146, CD105, CD309, only partially expressed CD34, and did not express CD45 and CD90. CV-ECFCs were capable of acetylated low-density lipoprotein uptake and tube formation, similar to cord blood-derived ECFCs (CB-ECFCs). CV-ECFCs can be transduced with a Luciferase/tdTomato-containing lentiviral vector at a transduction efficiency of 85.1%. Seeding CV-ECFCs on a small intestinal submucosa extracellular matrix scaffold confirmed that CV-ECFCs were compatible with the biomaterial scaffold.
In summary, we established a magnetic sorting-assisted clonal isolation approach to derive CV-ECFCs. A substantial number of CV-ECFCs can be obtained within a short time frame, representing a promising novel source of ECFCs for fetal treatments.
Core tip: We established a magnetic sorting-assisted clonal isolation protocol to derive chorionic villus endothelial colony-forming cells (CV-ECFCs) from early gestation placentas. Using our protocol, a substantial number of CV-ECFCs can be obtained from chorionic villus sampling specimens within a short time frame, making it feasible for autologous fetal treatment. CV-ECFCs are comparable to umbilical cord blood-derived ECFCs in terms of surface marker expression, tube formation capability, transducibility, and compatibility with biomaterial delivery vehicles. CV-ECFCs represent a novel autologous source of cells for fetal or postnatal treatment of congenital anomalies or defects.
- Citation: Gao K, He S, Kumar P, Farmer D, Zhou J, Wang A. Clonal isolation of endothelial colony-forming cells from early gestation chorionic villi of human placenta for fetal tissue regeneration. World J Stem Cells 2020; 12(2): 123-138
- URL: https://www.wjgnet.com/1948-0210/full/v12/i2/123.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i2.123
Over the past three decades, fetal medicine, especially fetal surgery, has been substantially developed for the treatment of congenital disorders. These include structural defects, such as spina bifida, congenital diaphragmatic hernia, sacrococcygeal teratoma, cardiac malformations[1-3], and genetic disorders, such as hemophilia[4,5], Duchenne muscular dystrophy[6] and cystic fibrosis[7,8]. For the past several years, our group has been exploring and establishing stem cell-based regenerative fetal treatments combined with tissue engineering for a variety of congenital disorders. For instance, we have successfully isolated placental mesenchymal stem/stromal cells (PMSCs) from the chorionic villus of early gestation placentas, and developed a PMSC-based fetal treatment for spina bifida (SB)[3,4,9-12]. Using the surgically-created fetal ovine SB model, we showed that augmenting in utero surgical repair of SB defects with PMSCs can rescue neurons and cure SB-associated motor function deficits at birth[3,9-11]. However, consistent with numerous other cases in which therapeutic effects were observed using MSCs, the transplanted PMSCs did not persist following transplantation, nor contribute to tissue regeneration by integration[3,13-17]. Rather, the PMSCs rescued neurons via paracrine mechanisms. In the aforementioned studies, small intestinal submucosa extracellular matrix (SIS-ECM) was the biomaterial scaffold used to deliver the stem cells in vivo[9,18,19]. Porcine small intestinal submucosa (SIS) scaffold is a Food and Drug Administration-approved natural ECM, which serves as a suitable provisional matrix for tissue regeneration. Based on our previous application of SIS-ECM, we believe it can be useful as a scaffold for delivering various types of stem cells to different affected tissues.
In another recent study of hemophilia A, we showed that when co-transplanting PMSCs with cord blood-derived endothelial colony-forming cells (CB-ECFCs), PMSCs integrated into the host environment and formed stable, long-term engraftment[20]. This observation suggests that ECFCs play a critical role in facilitating the long-term survival and engraftment of PMSCs[20].
The potential of ECFCs is also noted in vascularization, including vasculogenesis and angiogenesis. Vasculogenesis is the de novo formation of blood vessels, and is an essential physiological process that occurs during embryonic development and tissue regeneration. Angiogenesis is the growth of new capillaries from pre-existing blood vessels, which is observed both prenatally and postnatally[21]. ECFCs are highly proliferative endothelial progenitor cells that can differentiate into mature endothelial cells[22], and facilitate the functional formation of angiogenesis and thus vascularization. Therefore, cell therapies using ECFCs isolated from various tissue sources, such as bone marrow[23], adipose tissue[24], peripheral blood[25] and cord blood[20,26], have been sought as a therapeutic method to improve vascularization for various disorders[27]. Vascularization is vital to the development, maintenance, and regeneration of tissues. Angiogenesis, one vascularization process in which new blood vessels are formed from preexisting ones, plays a crucial role in embryonic and fetal development[21,28]. A defect in angiogenesis can lead to a variety of diseases, such as heart and brain ischemia, neurodegeneration, hypertension, osteoporosis, respiratory distress, and preeclampsia, to name a few[29]. Therefore, improving angiogenesis can ameliorate these aforementioned disorders by substantially increasing the supply of nutrients and oxygen to the affected tissues, and thus subsequently promoting tissue regeneration and functional repair[30-32]. Furthermore, the proliferative capacity of ECFCs, as well as their ability to integrate into the circulatory system, has allowed them to also be used as a delivery method of mutant genes to treat genetic vascular diseases[20,33]. Overall, the potential of ECFCs is greatly noted, and they may be ideal for treating the various disorders listed above, both adult and congenital. For example, an ideal long-term treatment strategy for congenital genetic diseases, such as hemophilia, is to apply appropriate stem cells during the first trimester of gestation, and treat the fetus prior to the development of a fetal immune system[4,34].
The placenta is a highly vascularized organ that plays a pivotal role in supporting and regulating fetal development with active vascularization beginning at an early gestational age[35]. During the first trimester of gestation, the placenta rapidly develops from the trophectoderm. The developmental process includes the formation of the villus tree and the extensive vasculature necessary to support the developing fetus. Hence, the early gestation placenta may pose a source from which we can reliably obtain a variety of progenitor cells such as ECFCs, in addition to the PMSCs that we have already established[35-37].
Several methods have been established to isolate ECFCs from term placentas. Patel et al[38] isolated large numbers of ECFCs by flow cytometry enrichment of CD45-CD34+-CD31Lo cells from term placentas, and found that placenta-derived ECFCs possessed angiogenic qualities similar to CB-ECFCs. Solomon and colleagues obtained ECFCs from the micro- and macrovascular tissues of human term placentas using CD31 magnetic bead sorting and colony isolation[39]. Thus far, a protocol for isolating ECFCs from early gestational preterm placentas has not been well-established, and the feasibility of using these ECFCs as an autologous source for fetal treatment has not been explored. In this study, we established a method for isolating ECFCs from early gestation placentas, and characterized their phenotype and angiogenic functions.
Discarded human early gestation (GA 12-16 wk) placentas were collected from the University of California, Davis, Medical Center. The study was submitted to the University of California, Davis, Institutional Review Board, and was determined to be exempt from review. The chorionic villi of the placenta were dissected into small pieces and washed three times with 1X phosphate-buffered saline (PBS) solution containing 100 IU/mL of penicillin and 100 μg/mL of streptomycin. Chorionic villus tissue (100 mg) was placed in a 100 mm dish and digested with 10 mL of enzyme solution containing 1 mg/mL collagenase Type I (Gibco), 0.1% trypsin (Invitrogen), and 0.2 mg/mL DNase (Invitrogen) by incubating at 37 °C, 5% CO2 for 20 min. The cell suspension was collected, neutralized with a medium containing 10% fetal bovine serum (FBS), and placed on ice. Fresh enzyme solution was added to the remaining tissue in the petri dish and incubated for an additional 20 min. The collection of cells and enzyme digestion was repeated until the whole tissue was completely dissociated. The cell suspensions were pooled, filtered through 70 μm nylon mesh, and incubated with red blood cell lysate buffer for 5 min at room temperature.
Cells that were obtained by enzyme dissociation, as described above, were labeled with magnetic bead antibodies, and sorted according to the manufacturer’s protocol (MACS cell separation system; Miltenyi Biotec). Briefly, cells were first labeled with PE-mouse anti-human CD31 antibody and then bound to anti-PE microbeads (MiltenyiBiotec). Labeled cells were passed through a separation column under the magnetic field, and CD31-positive cells were collected and seeded on a rat-tail collagen Type I (BD Biosciences Discovery)-coated tissue culture treated dish at a low seeding density (2000-4000 cells/cm2). Cells were cultured in Endothelial Cell Growth Medium MV-2 media (ECGM-MV2, PromoCell) with the addition of 250 ng/mL TGF-β inhibitor SB431542 (Stemcell Technologies) and 10 ng/mL vascular endothelial growth factor (VEGF; R&D Systems). Cells were fed every day for the first 7 d and every 2 d until the appearance of cell colonies.
Once a cell colony with a cobblestone-like morphology appeared and grew to about 20 cells, it was hand-picked using a cloning cylinder. The rim of a sterile cloning cylinder (Millipore/Sigma) was coated with sterile vacuum grease and placed onto the location of the target clone marked under the microscope. Trypsin-EDTA was used to detach the cells within the cloning cylinder. All cells obtained from each of the selected colonies was seeded into one well of a 24-well plate (Corning). After about 2-3 wks of culture and upon reaching about 90% confluency, the cells were passaged into a 6-well tissue culture treated dish. In addition, 5000 cells from each colony were seeded into one well of a 96-well plate for CD31 and VE-Cadherin staining to confirm the ECFC phenotype. The monoclonal cells that displayed cobblestone-like morphology and co-expressed CD31 and VE-Cadherin underwent further expansion. CV-ECFCs were frozen at passage five and subsequently used for all experiments.
Cord blood was collected from discarded term placentas obtained from the University of California Medical Center. CB-ECFCs were isolated as previously described[20]. They were cultured in ECGM-MV2 media (PromeCell). CB-ECFCs between passages 4-6 were used in this study.
Flow cytometry was used to characterize the cellular composition of the chorionic villus of human early gestation placental tissue, and the surface markers of isolated and expanded CV-ECFCs. The Attune NxT Flow Cytometer (ThermoFisher Scientific) was used for performing flow cytometry, and FlowJo software (FlowJo LLC) was used for data analyses. All antibodies were obtained from BD Biosciences. For characterization of early gestation placenta cellular composition, single cells obtained from enzymatic dissociation of placental villi were fractionated into tubes containing approximately 5 x 105 cells per sample and stained with PE-CD31 (555446), APC-CD13 (561698), APC-CD90 (561971), APC-CD34 (560940), APC-CD45 (560973), APC-CD105 (562408), APC-CD117 (561118), and Alexa Fluor 647-CD146 (563619). For phenotypic characterization of CV-ECFCs, cells were stained with PE-CD31 (555446), PE-CD34 (555446), PE-CD144 (560410), PE-CD90 (561972), PE-CD45 (555483), APC-CD105 (562408), Alexa Fluor 647-CD146 (563619), and Alexa Fluor 647-CD309 (560495). PE-Ms IgG1 κ (555749), APC-Ms IgG1 κ (550854), and Alexa Fluor 647-Ms IgG1 κ (557783) were used as isotype controls, and anti-mouse Igκ CompBeads were used to generate compensation controls. The LIVE/DEAD™ Fixable Near-IR Dead Cell Stain Kit (ThermoFisher Scientific) was used to exclude interference from the non-specific staining of dead cells. CV-ECFCs were transduced using an established protocol[20]. Transduction efficiency was assessed by tdTomato expression quantified by flow cytometry.
Chorionic villus tissue was dissected from human early gestation placentas, fixed with 10% formalin for 24 h, protected by 30% sucrose dehydration until the tissue specimen settled to the bottom of the tube, and then embedded in O.C.T compound (Sakura Finetek). Serial sections were made at 6 µm thickness using a Cryostat (Leica CM3050S) and mounted onto microscope slides (Matsunami Glass). O.C.T compound was washed off by water. Tissue sections were permeabilized by incubating the tissues with PBS containing 0.5% Triton X-100 for 10 min. Tissue sections were then incubated with PBS containing 5% bovine serum albumin (BSA) for 1 h at room temperature to block non-specific binding sites. Samples were incubated with primary antibody diluted in PBS containing 1% BSA at 4 °C overnight. The dilutions of primary antibodies were: Mouse anti-human CD146 (BD Biosciences) 1:50, mouse anti-human NG2 (BD Biosciences) 1:50, mouse anti-human CD34 (Dako) 1:25, rabbit anti-human von Willebrand factor (vWF) (Dako) 1:200. Tissue sections were then stained with the respective secondary antibodies: donkey anti-rabbit, conjugated with Alexa647 (ThermoFisher Scientific) and donkey anti-mouse, conjugated with Alexa647 (ThermoFisher Scientific), diluted 1:500 with 1% BSA in PBS, and incubated for 1 h at room temperature. The slides were then counterstained with 1:5000 dilution of DAPI for 5 min, mounted with Prolong Diamond Antifade Mountant (Invitrogen), and imaged with a Zeiss Observer Z1 microscope. For CV-ECFC immunocytochemical characterization, cells were seeded in 96-well plates and fixed with 10% formalin. Cells were stained using rabbit anti-VE-Cadherin (Cell Signaling) at 1:400 dilution or mouse anti-CD31 antibodies (Dako) at 1:40 dilution and imaged, as described above.
The CV-ECFCs were cultured in 0.5% BSA for 24 h and then incubated with 10 μg/mL Dil-Ac-LDL (Alfa Aesar) in serum-free culture medium at 37 °C for 5 h. Cells were then washed three times with PBS, fixed with 10% formalin for 15 min, and stained with DAPI (1:5000 in water) to label nuclei. The cells were imaged using a Zeiss Observer Z1 microscope.
Twenty-four-well culture dishes were coated with 300 µL Matrigel (BD Biosciences) per well and allowed to gel for 60 min at 37 °C. CV-ECFCs (100,000) were seeded onto the Matrigel-coated wells and incubated at 37 °C, 5% CO2. Phase contrast images were acquired 12 h after seeding using a Zeiss Observer Z1 microscope. Tube formation was quantified using the angiogenesis analyzer in ImageJ software for total junction numbers and total branch lengths.
CV-ECFCs could serve as a promising autologous cell source for the treatment of genetic diseases. To test if CV-ECFCs can be genetically modified, we transduced CV-ECFCs using lentiviral vectors for proof-of-concept evaluations. All lentiviral constructs were generated at the Institute for Regenerative Cures (IRC) Vector Core, University of California, Davis. CV-ECFCs were transduced in a transduction medium composed of DMEM high glucose, 10% FBS and 8 μg/mL protamine sulfate (MP Biomedicals) with pCCLc-MNDU3-LUC-PGK-Tomato-WPRE for quantitative analysis at a multiplicity of infection (MOI) of 10 for 6 h. After 6 h, the transduction medium was discarded, and cells were cultured in ECGM-MV2 medium for 72 h. A fluorescence microscope (Zeiss Observer Z1) was used to observe tdTomato expression, and flow cytometry was used to detect transduction efficiency.
CV-ECFCs represent a promising new cell source for various fetal or adult tissue engineering applications. To test the cytocompatibility of CV-ECFCs with biomaterial scaffolds, we used a clinical grade SIS-ECM scaffold as a representative scaffold to be tested in vitro. Punch-outs (6 mm) of the SIS-ECM scaffold (Biodesign® Dural graft, Cook Biotech, West Lafayette, IN) were incubated in the culture medium overnight. A quantity of 2 × 105 transduced CV-ECFCs were suspended in 10 μL culture medium, seeded onto the pre-equilibrated SIS-ECM scaffold, and cultured at 37 °C, 5% CO2 for 4 h to allow cells to attach. Then, the culture medium was subsequently added to cover the CV-ECFC/SIS scaffold composite, and incubated at 37 °C, 5% CO2 for 24 h. A fluorescence microscope (Zeiss Observer Z1) was then used to observe cell morphology on the scaffold.
All data analysis was performed using PRISM 7 software (GraphPad Software Inc.). Descriptive statistical data are reported as mean ± SD.
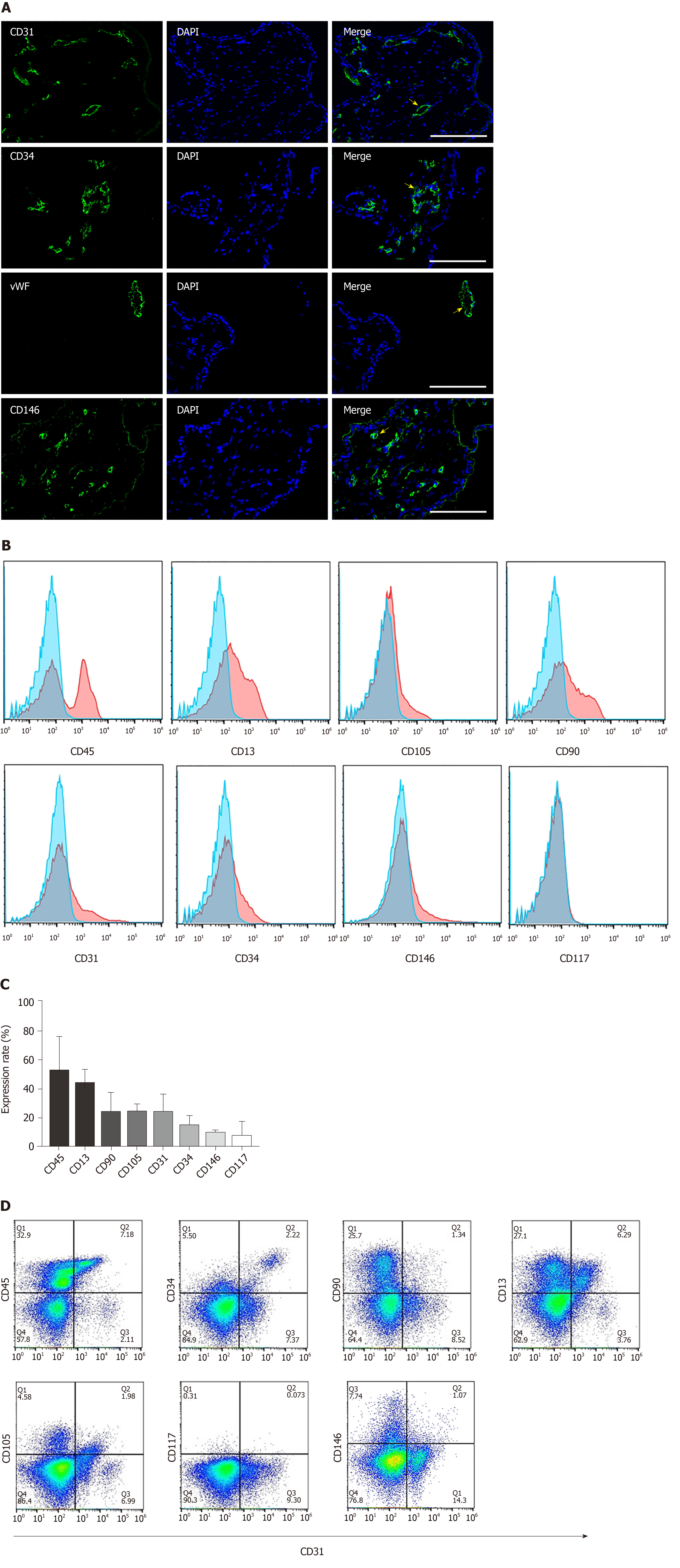
The placenta is composed of a variety of progenitor cells, and undergoes rapid development during the early gestational period of pregnancy. Hence, it serves as a unique source for obtaining progenitor cells that could be utilized for tissue engineering purposes. We first analyzed the cellular composition of early gestation placenta villi. The typical endothelial cell markers CD31, CD34 and vWF were used to characterize the endothelial cells present in the chorionic villus tissue (gestation age of 14 wk 5 d). Results confirmed that these endothelial cell markers were broadly distributed throughout the chorionic villus tissues (Figure 1A, the first three panels). CD146 is a marker expressed on the endothelium in capillaries and perivascular cells around the venules. We found that CD146-positive cells were widely present in placental villi tissues (Figure 1A, the 4th panel). Flow cytometry quantitative analysis was used to characterize the cell phenotypes derived from the enzymatic dissociation of placental tissue. We found that during early gestation (12-16 wk), placental chorionic villus tissues had 52.3% ± 24.1% CD45-positive cells , 43.9% ± 10.39% CD13-positive cells, 23.98% ± 14.28% CD90-positive cells, 24.5% ± 5.47% CD105-positive cells, 23.83% ± 13.66% CD31-positive cells, 14.79% ± 7.65% CD34-positive cells, 9.57% ± 2.5% CD146-positive cells, and 7.35% ± 10.79% CD117-positive cells (Figure 1B and C). Since CD31 is an established, specific endothelial cell marker, it was selected for the magnetic sorting strategy of CV-ECFCs from chorionic villi. We also analyzed the co-expression of CD31 and other markers on the isolated cells derived from early gestation placental chorionic villus tissues. We found that 12.56% ± 4.24% of CD31-positive cells were also CD45 positive, and most likely of the non-adherent hematopoietic lineage. A subpopulation of CD31-positive cells (4.22% ± 1.53%) were also positive for CD34, a marker of immature endothelial progenitor cells. This subpopulation will most likely be able to grow into colonies with high proliferative potential. A subpopulation of CD31-positive cells (1.34% ± 0.67%) were also positive for CD90, and CD90 is generally considered as an MSC marker (Figure 1D). Although the cells that simultaneously expressed CD31 and CD90 account for only a small fraction of all cells, their higher proliferative capacity overtook the expansion of CD31-sorted CV-ECFCs. Hence, manual cloning was necessary to obtain pure populations of CV-ECFCs.
By enzymatically dissociating tissues, we obtained 1.22 × 106 ± 0.32 × 106 single cells from 100 mg of chorionic villus tissue (n = 5). Single cells were then subjected to magnetic bead sorting and manual cloning. After 4-6 passages and confirmation of surface markers, an ECFC cell line with proliferative potential was obtained. A brief illustration of this process is shown in Figure 2A. The CD31-positive cells were added to ECGM-MV2 containing TGF-β inhibitor and VEGF. Cell colonies grown from single cells appeared 5-10 d after being cultured, and they presented with multiple morphologies (Figure 2B). CV-ECFC colonies exhibited a cobblestone-like morphology, whereas MSC clones exhibited a spindle-shaped morphology. There were also colonies that exhibited non-uniform morphologies. Cell colonies that displayed a cobblestone-like morphology were chosen using a cloning cylinder, seeded, and cultured in a 24-well plate. In order to get a uniform population and increased cell number for subsequent characterization by immunocytochemistry, cells underwent two additional passages. Those cell lines that co-expressed CD31 and CD144 were expanded for subsequent experiments (Figure 2C). After a total of four passages within 6-8 wk of culture, a total number of 1.8 × 107 CV-ECFCs per 100 mg of chorionic villus tissue was obtained.
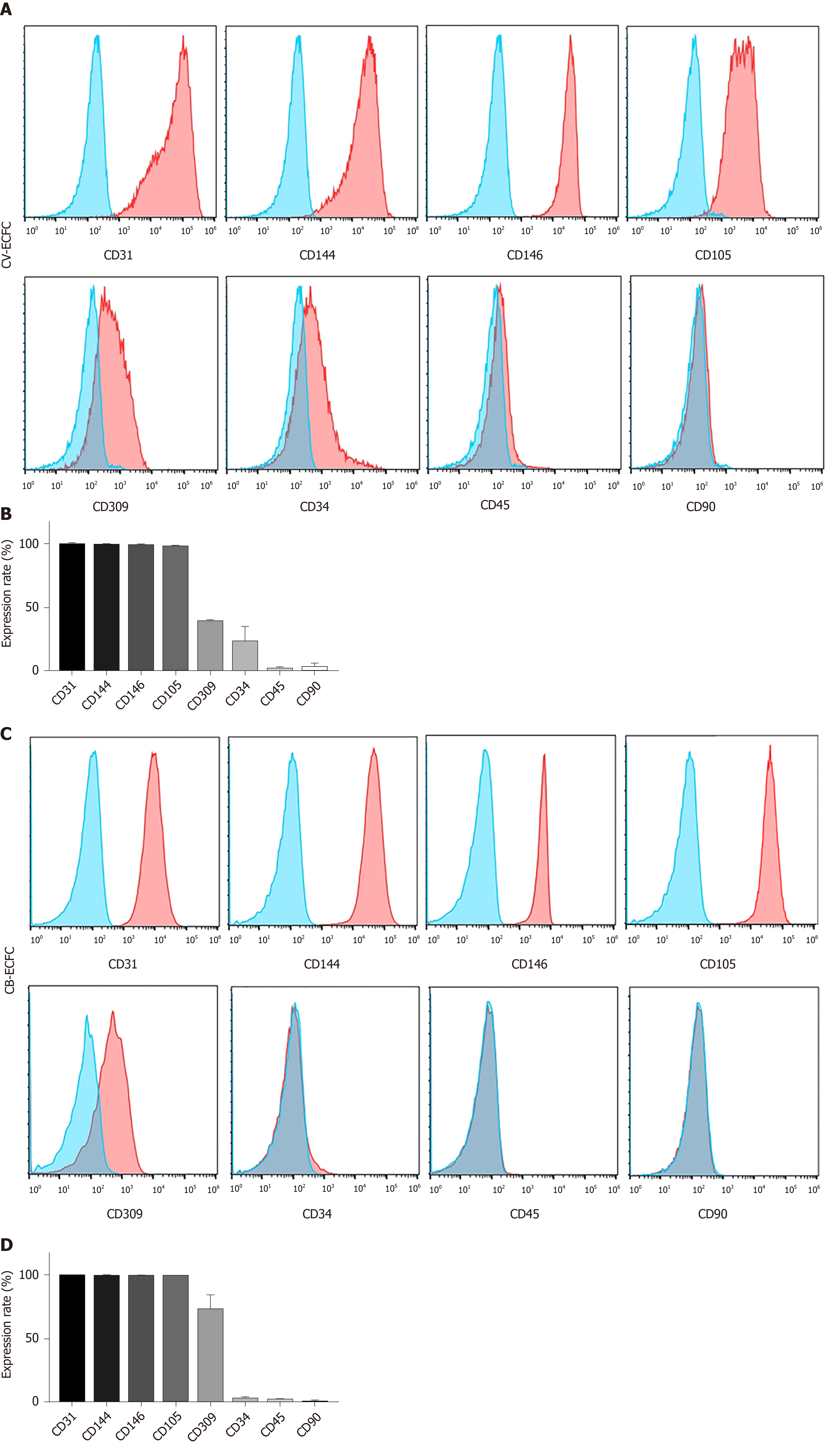
The immunophenotypic profile of CV-ECFCs was analyzed by flow cytometry. These cells were positive for well-established ECFC surface markers, including CD31 (99.55% ± 0.49%), CD144 (98.95% ± 0.64%), CD146 (98.3% ± 1.98%), CD105 (97.7% ± 1.56%), CD309 (39.6% ± 0.99%), and low expression of CD34 (23.2% ± 12.87%). They were negative for hematopoietic and MSC surface markers CD45 (1.87% ± 2.18%) and CD90 (2.95% ± 4.02%) (n = 3) (Figure 3). The expression levels of various surface markers were similar to CB-ECFCs, as previously published[20], except for the expression level of CD34 of CV-ECFCs, which was higher than that of CB-ECFCs (2.16% ± 1.06%).
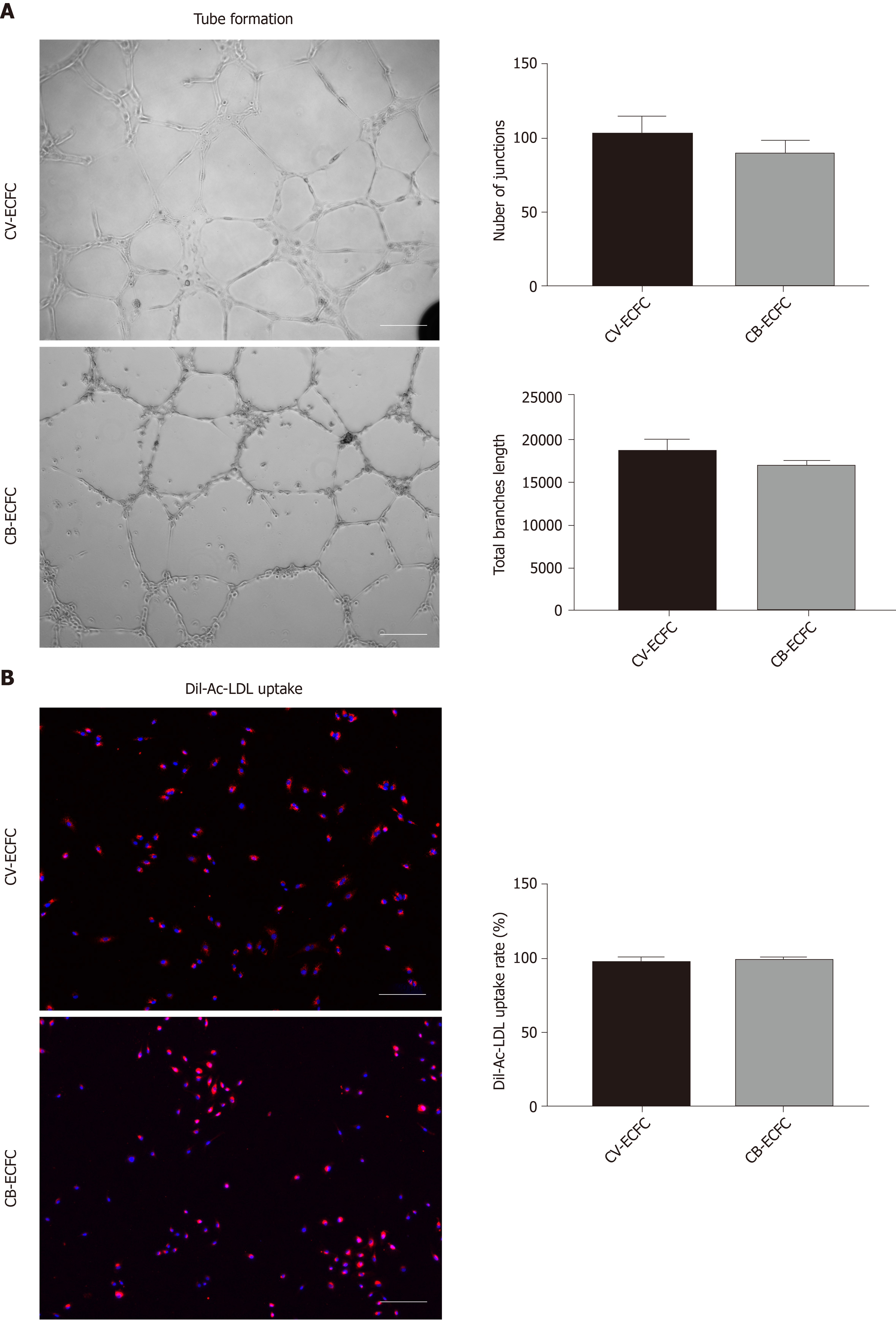
Tube formation and Dil-Ac-LDL uptake experiments are commonly used to identify endothelial cells from a functional perspective. Like CB-ECFCs, CV-ECFCs formed a similar tubular structure on the surface of Matrigel, which can persist for more than 48 h and then disintegrate. The capacity of in vitro angiogenesis of CV-ECFCs and CB-ECFCs showed no significant difference in quantity by the angiogenesis analyzer in ImageJ (Figure 4A). These CV-ECFCs showed a comparable ability of Dil-Ac-LDL uptake to CB-ECFCs (Figure 4B).
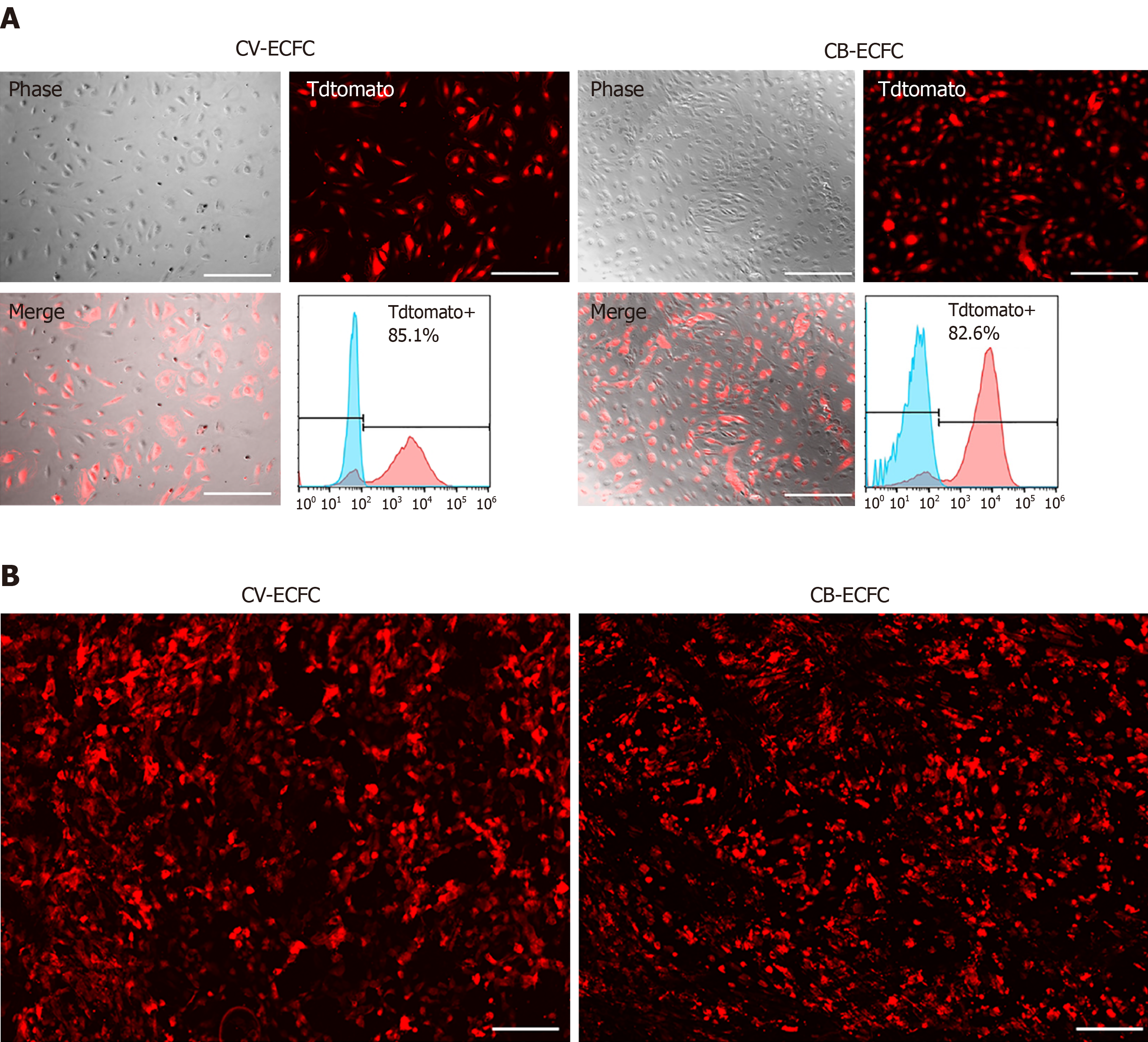
To explore the possibility of CV-ECFCs expressing exogenous proteins, and their potential for treating genetic defects, the transduction rate of these cells was explored. CV-ECFCs were transduced with a tdTomato-expressing lentiviral vector, and the transduction rate reached 85.1% at an MOI of 10 by flow cytometry analysis of transduced and non-transduced cells (Figure 5A). We tested the compatibility of CV-ECFCs with SIS biomaterial scaffold for future potential tissue engineering. CV-ECFCs adhered well to the surface of the SIS scaffold, and displayed normal morphology, similar to CB-ECFCs. These results show that CV-ECFCs can be delivered to a defective site via a SIS-ECM scaffold or other similar collagen-based scaffolds (Figure 5B).
The placenta is a highly vascularized organ, and the vascularization process begins at an early gestational age[35]. This study confirmed that endothelial cells account for about 20%-40% of total placental cells present during early gestation. We showed that endothelial cells are present in the chorionic villi and likely involved in the formation and development of blood vessels. Proliferative ECFCs are rare in placental cell populations, which makes the isolation of viable ECFCs technically challenging, especially since the number of available cells from clinical chorionic villus sampling (CVS) specimens is limited[40,41]. Flow cytometry sorting is one method that has been used to isolate subpopulations from mixed cells and tissues. However, the viability of flow cytometry-sorted cells is predominantly low, making it unfeasible to obtain large numbers of cells from limited amounts of tissue[41] for any in utero autologous fetal therapy. Hence, to isolate a viable and expandable purified population of endothelial progenitor cells (CV-ECFCs), we first utilized CD31 magnetic bead sorting, which resulted in two distinct populations of cells with high proliferative potential. One population had an endothelial progenitor cobble-stone like morphology and co-expressed CD31 and CD34, and are therefore most likely CV-ECFCs. The second population had a mesenchymal spindle shape morphology, and unlike PMSCs that are CD31-negative, these cells co-expressed CD31 and CD90. These cells had a higher proliferative capacity compared to CV-ECFCs, and when co-existing with CV-ECFCs, they outnumber CV-ECFCs. This observation was consistent with the findings of Rapp et al[42], where they isolated and cultured ECFCs from term placentas. The recent study showed the presence of bipotent progenitor cells in term placenta, where the CD31Lo population differentiated into both endothelial and mesenchymal colonies[40]. Hence, CD31/CD90-double positive cells, with the mesenchymal phenotype in our isolation method, could be these bipotential progenitor cells. In addition, according to our previous study, PMSCs could transform into the mesenchymal phenotype and undergo growth arrest when they were co-cultured[20]. Studies have reported that TGF-β can promote the endothelial-to-mesenchymal transition process to make endothelial cells gain a mesenchymal phenotype and induce growth arrest[43,44]. Therefore, we added TGF-β inhibitor to the ECGM-MV2 medium to maintain the endothelial cell phenotype. Due to the above reasoning, in order to separate these two cell populations, we picked colonies manually based on their morphology. The expanded cells were further characterized for surface expression of endothelial markers, leading to a pure ECFC cell line that was obtained within 8 wk of culture.
One key element of developing well-vascularized, viable regenerative therapeutics is the incorporation of autologous or allogeneic endothelial cells or endothelial progenitor cells derived from various tissue sources to the therapeutic modality or construct. This strategy has been widely used in treating vasculogenesis and/or angiogenesis-related diseases and conditions such as heart failure, acute kidney injury, stroke and wound healing[45-48]. CB-ECFCs are a good source of postnatal treatments, but cannot be used as an autologous cell source for fetal treatments. In this study, we developed a protocol to derive CV-ECFCs from early gestation placentas that could potentially be used as an autologous source of cells for fetal treatment. Our approach allows one to obtain CV-ECFCs from small amounts of tissue (about 100 mg), which is similar to the size of clinical CVS specimens. This method can also be applicable to large-scale expansion and banking of CV-ECFCs from large or whole placental tissue. CV-ECFCs are similar to CB-ECFCs with respect to cell phenotype, in vitro tube-forming capability, transducibility, and compatibility with biomaterial scaffolds. Compared with CB-ECFCs, the CV-ECFCs we isolated express higher levels of the stem cell marker CD34, which likely correlates with a more primitive function and therapeutic capacity. Finally, in combination with the current fetal surgery techniques, these cells hold promise as a novel autologous and/or allogeneic regenerative treatment for congenital anomalies or defects. Further in vivo functional evaluation of these cells is warranted.
In summary, we established a CD31 magnetic sorting-assisted approach to isolate clonal CV-ECFCs from human early gestation placentas that hold promise to be used as an autologous cell source for fetal treatment of congenital anomalies or defects.
Fetal medicine and fetal surgery have been substantially developed for the treatment of congenital defects. Stem cell transplantation is an important means of tissue reconstruction and repair of genetic defects. Endothelial colony-forming cells (ECFCs) represent a promising cell candidate for their unique role in facilitating the formation of angiogenesis and vascularization.
ECFCs isolated from the chorionic villus tissue of early gestation placentas can serve as a source of cells for prenatal autologous fetal cell transplantation, as well as provide a basis for studying the development, physiological function, congenital disease, and fetal treatment of the developing fetus and placenta.
The objective of this study is to establish an isolation protocol to obtain ECFCs from the chorionic villus of human early gestation placentas, as well as to investigate the characterization of these cells and their potential applications in gene delivery and tissue engineering.
Dissected chorionic villus tissues were enzymatically digested to obtain single cells. Then, magnetic bead sorting, monoclonal culture and colony isolation were performed to obtain chorionic villi-derived ECFCs (CV-ECFCs). Immunohistochemical identification, flow cytometry, Matrigel tube formation assays, LDL uptake assays and lentiviral transduction were carried out to characterize the morphology, phenotype and function of the purified and expanded CV-ECFCs.
Using the established isolation protocol, we were able to obtain 1.8 × 107 pure CV-ECFCs from a single cell colony culture within 6-8 wks. CV-ECFCs showed typical endothelial phenotypes and functions. CV-ECFCs have demonstrated the ability to be transduced with lentiviruses, and function as carriers for gene therapy. They also possess good biocompatibility with biomaterial delivery vehicles, such as small intestinal submucosa extracellular matrix for potential tissue engineering applications.
This study shows that ECFCs are present in early gestation placental chorionic villi, and can be isolated and expanded to a significant number in a short period of time. These CV-ECFCs possess typical endothelial cell phenotypes and functions, and hold the potential of being used in gene therapy and tissue engineering applications.
CV-ECFCs isolated from early gestation placentas provide a new source of ECFCs for the fetal treatment of congenital disorders. Combined with existing in utero treatment technologies, this cell therapy could be widely applied toward a variety of diseases and conditions. In future research, we will further explore the in vivo applications of these cells in various animal models. Investigating the phenotype and functions of CV-ECFCs will also facilitate our understanding of the development, cellular composition, and function of the developing placenta and its interaction with the developing fetus.
The authors thank Cook Biotech Inc. for generously providing us with the ECM material. We acknowledge Alexandra Maria Iavorovschi for the help with manuscript editing.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Huang YC S-Editor: Wang YQ L-Editor: Filipodia E-Editor: Liu MY
| 1. | Deprest JA, Flake AW, Gratacos E, Ville Y, Hecher K, Nicolaides K, Johnson MP, Luks FI, Adzick NS, Harrison MR. The making of fetal surgery. Prenat Diagn. 2010;30:653-667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 2. | Harrison MR. The University of California at San Francisco Fetal Treatment Center: a personal perspective. Fetal Diagn Ther. 2004;19:513-524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Wang A, Brown EG, Lankford L, Keller BA, Pivetti CD, Sitkin NA, Beattie MS, Bresnahan JC, Farmer DL. Placental mesenchymal stromal cells rescue ambulation in ovine myelomeningocele. Stem Cells Transl Med. 2015;4:659-669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 4. | Kumar P, Gao K, Wang C, Pivetti C, Lankford L, Farmer D, Wang A. In Utero Transplantation of Placenta-Derived Mesenchymal Stromal Cells for Potential Fetal Treatment of Hemophilia A. Cell Transplant. 2018;27:130-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Porada CD, Rodman C, Ignacio G, Atala A, Almeida-Porada G. Hemophilia A: an ideal disease to correct in utero. Front Pharmacol. 2014;5:276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Koppanati BM, Li J, Reay DP, Wang B, Daood M, Zheng H, Xiao X, Watchko JF, Clemens PR. Improvement of the mdx mouse dystrophic phenotype by systemic in utero AAV8 delivery of a minidystrophin gene. Gene Ther. 2010;17:1355-1362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Keswani SG, Balaji S, Le L, Leung A, Katz AB, Lim FY, Habli M, Jones HN, Wilson JM, Crombleholme TM. Pseudotyped AAV vector-mediated gene transfer in a human fetal trachea xenograft model: implications for in utero gene therapy for cystic fibrosis. PLoS One. 2012;7:e43633. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Conese M, Ascenzioni F, Boyd AC, Coutelle C, De Fino I, De Smedt S, Rejman J, Rosenecker J, Schindelhauer D, Scholte BJ. Gene and cell therapy for cystic fibrosis: from bench to bedside. J Cyst Fibros. 2011;10 Suppl 2:S114-S128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Kabagambe S, Keller B, Becker J, Goodman L, Pivetti C, Lankford L, Chung K, Lee C, Chen YJ, Kumar P, Vanover M, Wang A, Farmer D. Placental mesenchymal stromal cells seeded on clinical grade extracellular matrix improve ambulation in ovine myelomeningocele. J Pediatr Surg. 2017;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Vanover M, Pivetti C, Lankford L, Kumar P, Galganski L, Kabagambe S, Keller B, Becker J, Chen YJ, Chung K, Lee C, Paxton Z, Deal B, Goodman L, Anderson J, Jensen G, Wang A, Farmer D. High density placental mesenchymal stromal cells provide neuronal preservation and improve motor function following in utero treatment of ovine myelomeningocele. J Pediatr Surg. 2019;54:75-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Chen YJ, Chung K, Pivetti C, Lankford L, Kabagambe SK, Vanover M, Becker J, Lee C, Tsang J, Wang A, Farmer DL. Fetal surgical repair with placenta-derived mesenchymal stromal cell engineered patch in a rodent model of myelomeningocele. J Pediatr Surg. 2017;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 12. | Lankford L, Selby T, Becker J, Ryzhuk V, Long C, Farmer D, Wang A. Early gestation chorionic villi-derived stromal cells for fetal tissue engineering. World J Stem Cells. 2015;7:195-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Pittenger M. Sleuthing the source of regeneration by MSCs. Cell Stem Cell. 2009;5:8-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9:11-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1127] [Cited by in F6Publishing: 1144] [Article Influence: 95.3] [Reference Citation Analysis (0)] |
| 15. | Cheng ZJ, He XJ. Anti-inflammatory effect of stem cells against spinal cord injury via regulating macrophage polarization. J Neurorestoratology. 2017;5:31-38. [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Shi Zj, Huang HY, Feng SQ. Stem cell-based therapies to treat spinal cord injury: A review. J Neurorestoratology. 2017;5:125-131. [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Zhang ZR, Wang FY, Song MJ. The cell repair research of spinal cord injury: A review of cell transplantation to treat spinal cord injury. J Neurorestoratology. 2019;7:55-62. [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Denost Q, Adam JP, Pontallier A, Montembault A, Bareille R, Siadous R, Delmond S, Rullier E, David L, Bordenave L. Colorectal tissue engineering: A comparative study between porcine small intestinal submucosa (SIS) and chitosan hydrogel patches. Surgery. 2015;158:1714-1723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Lankford L, Chen YJ, Saenz Z, Kumar P, Long C, Farmer D, Wang A. Manufacture and preparation of human placenta-derived mesenchymal stromal cells for local tissue delivery. Cytotherapy. 2017;19:680-688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Gao K, Kumar P, Cortez-Toledo E, Hao D, Reynaga L, Rose M, Wang C, Farmer D, Nolta J, Zhou J, Zhou P, Wang A. Potential long-term treatment of hemophilia A by neonatal co-transplantation of cord blood-derived endothelial colony-forming cells and placental mesenchymal stromal cells. Stem Cell Res Ther. 2019;10:34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Zygmunt M, Herr F, Münstedt K, Lang U, Liang OD. Angiogenesis and vasculogenesis in pregnancy. Eur J Obstet Gynecol Reprod Biol. 2003;110 Suppl 1:S10-S18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 302] [Cited by in F6Publishing: 313] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 22. | Medina RJ, Barber CL, Sabatier F, Dignat-George F, Melero-Martin JM, Khosrotehrani K, Ohneda O, Randi AM, Chan JKY, Yamaguchi T, Van Hinsbergh VWM, Yoder MC, Stitt AW. Endothelial Progenitors: A Consensus Statement on Nomenclature. Stem Cells Transl Med. 2017;6:1316-1320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 274] [Cited by in F6Publishing: 269] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 23. | Yu S, Li Z, Zhang W, Du Z, Liu K, Yang D, Gong S. Isolation and characterization of endothelial colony-forming cells from mononuclear cells of rat bone marrow. Exp Cell Res. 2018;370:116-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Szöke K, Reinisch A, Østrup E, Reinholt FP, Brinchmann JE. Autologous cell sources in therapeutic vasculogenesis: In vitro and in vivo comparison of endothelial colony-forming cells from peripheral blood and endothelial cells isolated from adipose tissue. Cytotherapy. 2016;18:242-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Kolbe M, Dohle E, Katerla D, Kirkpatrick CJ, Fuchs S. Enrichment of outgrowth endothelial cells in high and low colony-forming cultures from peripheral blood progenitors. Tissue Eng Part C Methods. 2010;16:877-886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Zhang H, Tao Y, Ren S, Liu H, Zhou H, Hu J, Tang Y, Zhang B, Chen H. Isolation and characterization of human umbilical cord-derived endothelial colony-forming cells. Exp Ther Med. 2017;14:4160-4166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Shafiee A, Patel J, Lee JS, Hutmacher DW, Fisk NM, Khosrotehrani K. Mesenchymal stem/stromal cells enhance engraftment, vasculogenic and pro-angiogenic activities of endothelial colony forming cells in immunocompetent hosts. Sci Rep. 2017;7:13558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | Drake CJ. Embryonic and adult vasculogenesis. Birth Defects Res C Embryo Today. 2003;69:73-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 118] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 29. | Tahergorabi Z, Khazaei M. A review on angiogenesis and its assays. Iran J Basic Med Sci. 2012;15:1110-1126. [PubMed] [Cited in This Article: ] |
| 30. | Hao D, Xiao W, Liu R, Kumar P, Li Y, Zhou P, Guo F, Farmer DL, Lam KS, Wang F, Wang A. Discovery and Characterization of a Potent and Specific Peptide Ligand Targeting Endothelial Progenitor Cells and Endothelial Cells for Tissue Regeneration. ACS Chem Biol. 2017;12:1075-1086. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 31. | Novosel EC, Kleinhans C, Kluger PJ. Vascularization is the key challenge in tissue engineering. Adv Drug Deliv Rev. 2011;63:300-311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 743] [Cited by in F6Publishing: 666] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 32. | Böttcher-Haberzeth S, Biedermann T, Reichmann E. Tissue engineering of skin. Burns. 2010;36:450-460. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 197] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 33. | Lin Y, Chang L, Solovey A, Healey JF, Lollar P, Hebbel RP. Use of blood outgrowth endothelial cells for gene therapy for hemophilia A. Blood. 2002;99:457-462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 138] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 34. | Almeida-Porada G, Atala A, Porada CD. In utero stem cell transplantation and gene therapy: rationale, history, and recent advances toward clinical application. Mol Ther Methods Clin Dev. 2016;5:16020. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 35. | Robin C, Bollerot K, Mendes S, Haak E, Crisan M, Cerisoli F, Lauw I, Kaimakis P, Jorna R, Vermeulen M, Kayser M, van der Linden R, Imanirad P, Verstegen M, Nawaz-Yousaf H, Papazian N, Steegers E, Cupedo T, Dzierzak E. Human placenta is a potent hematopoietic niche containing hematopoietic stem and progenitor cells throughout development. Cell Stem Cell. 2009;5:385-395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 36. | Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Péault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2900] [Cited by in F6Publishing: 2768] [Article Influence: 173.0] [Reference Citation Analysis (0)] |
| 37. | Parolini O, Alviano F, Bagnara GP, Bilic G, Bühring HJ, Evangelista M, Hennerbichler S, Liu B, Magatti M, Mao N, Miki T, Marongiu F, Nakajima H, Nikaido T, Portmann-Lanz CB, Sankar V, Soncini M, Stadler G, Surbek D, Takahashi TA, Redl H, Sakuragawa N, Wolbank S, Zeisberger S, Zisch A, Strom SC. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells. 2008;26:300-311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 721] [Cited by in F6Publishing: 721] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 38. | Patel J, Seppanen E, Chong MS, Yeo JS, Teo EY, Chan JK, Fisk NM, Khosrotehrani K. Prospective surface marker-based isolation and expansion of fetal endothelial colony-forming cells from human term placenta. Stem Cells Transl Med. 2013;2:839-847. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 39. | Solomon I, O'Reilly M, Ionescu L, Alphonse RS, Rajabali S, Zhong S, Vadivel A, Shelley WC, Yoder MC, Thébaud B. Functional Differences Between Placental Micro- and Macrovascular Endothelial Colony-Forming Cells. Stem Cells Transl Med. 2016;5:291-300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 40. | Shafiee A, Patel J, Hutmacher DW, Fisk NM, Khosrotehrani K. Meso-Endothelial Bipotent Progenitors from Human Placenta Display Distinct Molecular and Cellular Identity. Stem Cell Reports. 2018;10:890-904. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 41. | Gumina DL, Su EJ. Endothelial Progenitor Cells of the Human Placenta and Fetoplacental Circulation: A Potential Link to Fetal, Neonatal, and Long-term Health. Front Pediatr. 2017;5:41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 42. | Rapp BM, Saadatzedeh MR, Ofstein RH, Bhavsar JR, Tempel ZS, Moreno O, Morone P, Booth DA, Traktuev DO, Dalsing MC, Ingram DA, Yoder MC, March KL, Murphy MP. Resident Endothelial Progenitor Cells From Human Placenta Have Greater Vasculogenic Potential Than Circulating Endothelial Progenitor Cells From Umbilical Cord Blood. Cell Med. 2012;2:85-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Cooley BC, Nevado J, Mellad J, Yang D, St Hilaire C, Negro A, Fang F, Chen G, San H, Walts AD, Schwartzbeck RL, Taylor B, Lanzer JD, Wragg A, Elagha A, Beltran LE, Berry C, Feil R, Virmani R, Ladich E, Kovacic JC, Boehm M. TGF-β signaling mediates endothelial-to-mesenchymal transition (EndMT) during vein graft remodeling. Sci Transl Med. 2014;6:227ra34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 286] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 44. | Heldin CH, Landström M, Moustakas A. Mechanism of TGF-beta signaling to growth arrest, apoptosis, and epithelial-mesenchymal transition. Curr Opin Cell Biol. 2009;21:166-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 476] [Cited by in F6Publishing: 506] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 45. | Kim JY, Song SH, Kim KL, Ko JJ, Im JE, Yie SW, Ahn YK, Kim DK, Suh W. Human cord blood-derived endothelial progenitor cells and their conditioned media exhibit therapeutic equivalence for diabetic wound healing. Cell Transplant. 2010;19:1635-1644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 46. | Yerebakan C, Sandica E, Prietz S, Klopsch C, Ugurlucan M, Kaminski A, Abdija S, Lorenzen B, Boltze J, Nitzsche B, Egger D, Barten M, Furlani D, Ma N, Vollmar B, Liebold A, Steinhoff G. Autologous umbilical cord blood mononuclear cell transplantation preserves right ventricular function in a novel model of chronic right ventricular volume overload. Cell Transplant. 2009;18:855-868. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 47. | Burger D, Viñas JL, Akbari S, Dehak H, Knoll W, Gutsol A, Carter A, Touyz RM, Allan DS, Burns KD. Human endothelial colony-forming cells protect against acute kidney injury: role of exosomes. Am J Pathol. 2015;185:2309-2323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 160] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 48. | Li YF, Ren LN, Guo G, Cannella LA, Chernaya V, Samuel S, Liu SX, Wang H, Yang XF. Endothelial progenitor cells in ischemic stroke: an exploration from hypothesis to therapy. J Hematol Oncol. 2015;8:33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |