Copyright
©The Author(s) 2025.
World J Gastroenterol. Jul 14, 2025; 31(26): 108375
Published online Jul 14, 2025. doi: 10.3748/wjg.v31.i26.108375
Published online Jul 14, 2025. doi: 10.3748/wjg.v31.i26.108375
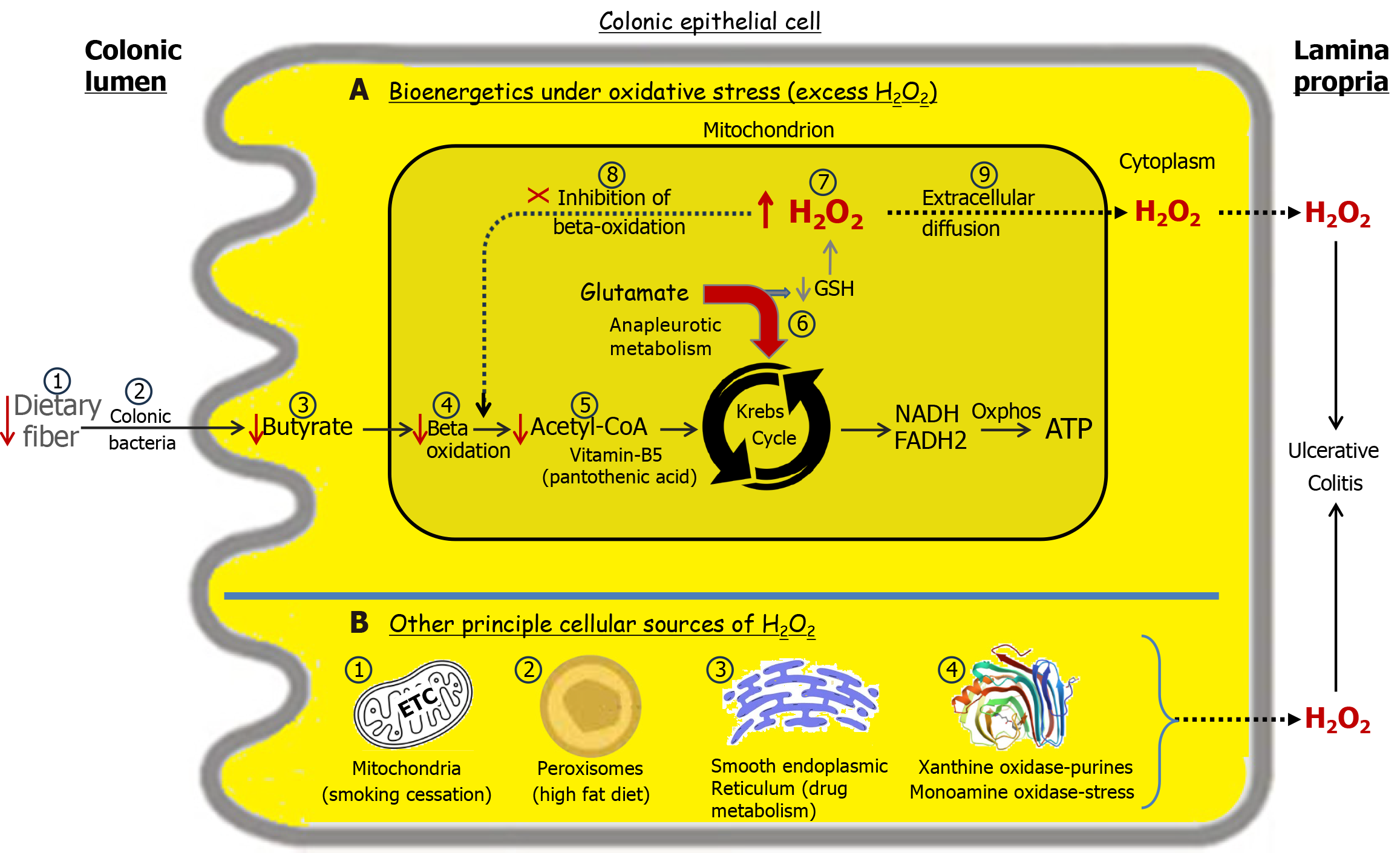
Figure 5 Transduction of environmental stimuli increases cellular hydrogen peroxide levels, elevating the risk of ulcerative colitis[27].
Normal energy flux in the colon begins with luminal soluble fiber, which is fermented by bacteria into n-butyrate, a key short-chain fatty acid. Butyrate is absorbed by colonic epithelial cells, where it undergoes beta-oxidation, generating acetyl-CoA. Subsequently, acetyl-CoA enters the Krebs cycle, producing the reducing equivalents NADH, nicotinamide adenine dinucleotide (reduced form) and FADH2, flavin adenine dinucleotide (reduced form) that drive oxidative phosphorylation to generate ATP, adenosine triphosphate. Panel A: Bioenergetics under oxidative stress. A deficiency in dietary soluble fiber (A1) results in inadequate butyrate production (A3) and reduced acetyl-CoA levels (A5). To offset the energy shortfall, the Krebs cycle engages in anaplerotic metabolism of glutamate (A6)[32,33]. This redirection of glutamate for energy production compromises glutathione synthesis required for hydrogen peroxide (H2O2) neutralization leading to elevated cellular H2O2 levels (A7). With sufficient accumulation, mitochondrial H2O2 can diffuse into the cytoplasm and across the cell membrane into the lamina propria, contributing to the development of ulcerative colitis (UC) (A9). A reduction in acetyl-CoA due to deficiencies in any factors involved in its synthesis, starting with luminal fiber, can lead to elevated cellular H2O2 levels, increasing the risk of UC. This is exemplified by a deficiency of vitamin B5, required for the synthesis of coenzyme A, which leads to a colitis in pigs analogous to human UC[34]. Initially, H2O2 accumulates within mitochondria, impairing beta oxidation, and is followed by relapse weeks later[21]. This delay reflects the time needed for mitochondrial H2O2 to establish a sufficient concentration gradient for cytoplasmic and extracellular diffusion. This explains why butyrate enemas are ineffective in resolving UC as H2O2 inhibits beta oxidation (A8)[26]. Sufficient damage to the colonic microbiota (A2) may increase the risk of developing or exacerbating UC. Panel B: The source of H2O2 production is influenced by the nature of the environmental stimuli[27]. B1: Disinhibition of the mitochondrial electron transport chain following smoking cessation can result in elevated H2O2 production, contributing to the development of ulcerative colitis[35]. B2: A diet rich in fats promotes increased peroxisomal beta-oxidation of long-chain fatty acids, leading to heightened H2O2 production, which predisposes individuals to UC. B3: The metabolism of certain drugs, such as nonsteroidal anti-inflammatory drugs, via cytochrome P450 enzymes in the smooth endoplasmic reticulum generates H2O2[36], which may contribute to the onset or relapse of UC. B4: Xanthine oxidase metabolizes purines found in red meat, producing significant amounts of H2O2[37]. Monoamine oxidase-A metabolizes serotonin released by enterochromaffin cells in the colon under stress, generating significant amounts of H2O2[38]. Oxidative stressors are stimuli that increase H2O2 in the body. More than one can be present simultaneously and contribute to UC development or relapse. H2O2: Hydrogen peroxide; NADH: Nicotinamide adenine dinucleotide (reduced); FADH2: Flavin adenine dinucleotide (reduced); ATP: Adenosine triphosphate; GSH: Glutathione.
- Citation: Pravda J. Ulcerative colitis: Timeline to a cure. World J Gastroenterol 2025; 31(26): 108375
- URL: https://www.wjgnet.com/1007-9327/full/v31/i26/108375.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i26.108375