INTRODUCTION
The number of patients with stress-induced gastric ulcers has increased dramatically[1], and stress is highly associated with several functional gastrointestinal disorders, such as functional dyspepsia and irritable bowel syndrome[2]. The nucleus tractus solitarius (NTS) is a relay nucleus for visceral primary afferent neural signaling. It receives sensory afferents from visceral organs and projects to the spinal cord to regulate respiratory and cardiovascular activity. The NTS is also closely connected with various brain nuclei[3]. Recent studies have demonstrated the role of the NTS in cardiovascular and respiratory regulation and the reflex regulation of intragastric pressure. Synapses mediate the vagal-dependent gastric reflex between vagal afferent fibers and NTS neurons, and through the vagal preganglionic parasympathetic neurons in the dorsal vagal complex[4]. Neuronal firing studies in the NTS have shown that H2S increases NTS-evoked postsynaptic currents by enhancing presynaptic glutamate release and affects the membrane potential of NTS neurons in a concentration-dependent manner[5].
Vagal afferent transmission primarily terminates in the NTS[6], which may act as a relay activator to inhibit spinal neuronal activity. Vagal-mediated glutamate release can regulate homeostasis by activating NTS neurons and metabotropic glutamate receptors, contributing to homeostatic regulation. Injection of oxytocin in the NTS has been observed to inhibit gastric smooth muscle diastole in rats, possibly through the cAMP-PKA signaling pathway[7]. The microinjection of monosodium glutamate in the NTS and the activation of NTS opioid receptors inhibit gastric motility and significant c-Fos expression in the dorsal motor nucleus of the vagus (DMV) neurons[8].
However, hydrogen sulfide (H2S) is a recently discovered gas transmitter endogenously produced in the human and animal brain and organ tissues. Cystathionine beta-synthase (CBS) mainly synthesizes H2S in the central nervous system and plays a significant physiological role[9] and has a protective role in neurodegenerative diseases such as Parkinson’s disease[10] and Alzheimer’s disease[11]. CBS is present in neurons and glial cells of the NTS, exerting excitatory effects and modulating synaptic neuronal activity[12]. Blocking CBS attenuates synaptic transmission in NTS neurons. Applying the H2S donor NaHS also enhances synaptic transmission in NTS neurons. H2S in the NTS plays an equally important role as a gaseous neuromodulator in maintaining or modulating autonomicand other systems[13]. In vitro experiments have shown that H2S relaxes gastrointestinal smooth muscles, inhibiting spontaneous movements and responses to chemical or electrical stimuli[14]. H2S also plays a role in other types of smooth muscle relaxation via K+-ATP channels[15], suggesting that endogenous H2S has a regulatory role in the gastrointestinal tract’s motor function.
The nuclear factor kappa-B (NF-κB) pathway is activated by various factors and plays a crucial role in the immune response and inflammation. A study found that NaHS administration in the rat intraperitoneal via sulfhydration caused NF-κB activation and lung inflammation, a significant increase in p65 protein levels, vascular congestion, and neutrophil infiltration. Also, slight neuronal degeneration was observed in the rat heart, liver, and brain, suggesting that H2S acts on NF-κB channels for messaging[16]. H2S also interacts with nitric oxide to cause vasodilation, down-regulates NF-kB pathway-induced inflammation, fibrosis and damage from prolonged or intense oxidative stress; protects tissues from ischemia- and reperfusion-induced injury; and reduces immune rejection by reducing oxygen free radicals produced in vivo[17]. Additionally, H2S demonstrates antioxidant and anti-apoptotic effects on neurons and glial cells[18].
Transient receptor potential vanilloid type 1 (TRPV1) is a non-selective calcium channel associated with nociceptive sensations in peripheral nerves. Its activation can lead to neurokinin 1 (NK1) receptor activation, pain, local neurogenic inflammation, and systemic anti-inflammatory/analgesic effects, and enhanced transmitter release in the NTS. TRPV1 involves various physiological and pathological processes[19,20]. Electrophysiological studies have shown that the activation of TRPV1 by capsaicin enhances glutamate release to visceral sensory neurons, affecting NTS preganglionic neurons[21]. H2S can cause peripheral inflammation and synaptic enhancement of glutamatergic signaling in the spinal cord by activating TRPV1 channels, thus stimulating other receptors at the terminals of capsaicin-sensitive neurons[22]. TRPV1 activation also leads to the release of substance P (SP), while NK1 receptors are responsible for neurally mediated digestive secretion and contributes to brain homeostasis and sensory neuronal transmission associated with depression, stress, anxiety and vomiting[23]. H2S causes gastric juice secretion by stimulating TRPV1 receptors on primary afferent nerve fibers and modulates cholinergic neurons by releasing SP to act on NK1, NK2, or NK3 receptors[24]. Therefore, we speculate that TRPV1 channels may be involved in the effect of H2S donors on gastric emptying.
SP is a neuroactive peptide involved in pain and inflammation[25]. It is widely present in the mammalian organism in the central, peripheral, and gastrointestinal nervous systems[26] and other tissues, participating in various physiopathological processes, including stress, emotional anxiety, and immune regulation. NK1 receptors are the primary receptors for SP and are widely expressed in the brain, contributing to stress and emotional anxiety[27]. SP is widely expressed in the NTS, mainly in the primary sensory neurons in the peripheral nervous system, and intrinsic neurons of the gastrointestinal tract. It has been shown to have neurotransmitter effects in the central and peripheral nervous systems and is associated with immune and inflammatory diseases of the respiratory and gastrointestinal systems[28].
Herein, we investigated the regulatory effects of H2S in the NTS on rat gastric function and explore whether these effects were mediated by SP release via NF-κB channel-dependent activation of the TRPV1 pathway.
MATERIALS AND METHODS
Animal
The animals used in this experiment were 240-280 g adult male Wistar rats purchased from Jinan Panyue Experimental Animal Breeding Co and housed in separate cages at a constant temperature (22 °C ± 2 °C) given appropriate food and water based on their body weight. To allow them to acclimate to their surroundings, the rats were exposed to natural rhythmic light for one week before the start of the experiment.
Before the experiment, the rats underwent a 24-h fasting period, during which they were allowed to freely drink. The other environmental conditions remained consistent throughout the experiment. The Experimental Animal Ethics Committee of Qilu Normal University approved the experiment. All experiments complied with internationally accepted ethical standards. The study also adhered to the guidelines set by the International Association for the Study of Pain[29].
Chemicals
NaHS, L703606, PDTC, Capsazepine, and protamine sky blue were purchased from Sigma-Aldrich (St. Louis, MO, United States). NaHS was dissolved in 0.9% saline, while the other chemicals were dissolved in dimethyl sulfoxide and reconstituted in saline. For the immunohistochemical fluorescence double labeling, the following reagents were purchased from Servicebio (Wuhan, China): Goat serum, anti-CBS rabbit pAb, FITC-conjugated goat anti-rabbit IgG, Cy3-conjugated goat anti-mouse IgG, and anti-c-Fos mouse pAb.
Immunohistochemical fluorescence double labeling
We used the restraint water immersion stress model to investigate acute stress-induced gastric mucosal injury in rats. This acute compound stress model causes changes in gastric function in rats under stress through enhanced parasympathetic activity in the innervated stomach[30]. Once anesthetized, the rats were swiftly removed from the bottle and secured to a wooden board using medical tape to immobilize their limbs and teeth. When awake, the rats were then immersed in cold water (21 °C ± 1 °C) with the sternal process aligned with the water level. To minimize experimental error, consistent time points were selected for each experiment.
The rats were randomly divided into three groups (n = 6) based on the duration of restraint water-immersion stress (RWIS) (0 h, 1 h, or 3 h). Cardiac perfusion was performed using 500 mL of prepared 0.01 mol/L phosphate-buffered saline (PBS) followed by 500 mL of 4% 0.1 mol/L paraformaldehyde (PFA). After administering an overdose of isoflurane to sacrifice the rat, the thoracic cavity was opened along the sternal process, and the heart was exposed. The infuser needle was inserted into the heart’s left ventricle, securing the heart, while the right auricle was incised to allow blood to drain. The rat’s liver was flushed with 0.01 mol/L PBS buffer until it turned white, followed by perfusion with 4% PFA solution using a “fast and then slow” principle, gradually reducing the flow rate when the rat’s limbs twitched.
Upon completion of perfusion, the rat’s head was severed, and the brain was extracted. The brain was placed in a small wide-mouth flask containing 4% PFA and kept at 4 °C for 24 h. Subsequently, the fixed rat brain was transferred to a 0.1 mol/L 30% sucrose solution for dehydration. The frozen target nuclei region was then sectioned into 30 μm thick coronal sections using a sectioning machine and stored in 0.01 mol/L PBS.
Next, each well of a multi-well plate was filled with 500 μL of 0.01 mol/L PBS buffer to clean the brain slices and remove impurities. A methanolic solution of 3% H2O2 was added to block endogenous peroxidase activity. The wells were then incubated with a goat serum closure solution for 1 h to enhance cell membrane permeability. Subsequently, 500 μL of each primary antibody working solution was added, consisting of mouse anti-c-Fos (diluted at 1:500) and rabbit anti-CBS (diluted at 1:500), and incubated overnight at 4 °C.
Finally, 500 μL of each fluorescent secondary antibody working solution was added for 1 h. Any residual fluorescent secondary antibody was washed off with PBST. Previously treated with chromium-vanadium gelatin, the brain slides were placed on glass slides and allowed to dry naturally. The slides were sealed with an anti-fluorescence quencher, ensuring the removal of air bubbles using a vacuum. Finally, the sealed fluorescent glass slide was placed under an Olympus Fluorescence confocal microscopy to observe and compare the brain atlas to determine the position of the NTS, observe the CBS and c-Fos-positive neurons number, and take pictures. The expression of c-Fos and CBS in the NTS was counted using Image Pro-Plus 6.0 software (Number/0.01 mm2).
Experimental grouping
We evaluated the following subgroups to investigate the regulatory effects of NaHS in the NTS on gastric function and its underlying mechanisms. The chosen doses were based on pre-experiments and relevant literature[31]: (1) The effect of microinjection of NaHS (0.1 µL, 4 nmol; n = 6) in NTS on gastric motility; (2) the effect of microinjection of NaHS (0.1 µL, 8 nmol; n = 6) in NTS on gastric motility; (3) the effect of microinjection of saline (0.1 µL; n = 6) in NTS on gastric motility as a control group; (4) the effect of microinjection of NaHS (0.1 µL, 4 nmol) + PDTC (0.1 µL; n = 6) in NTS on gastric motility; (5) microinjection of NaHS (0.1 µL, 4 nmol) + Capsazepine (0.1 µL, 4 nmol; n = 6) in NTS on gastric motility; and (6) microinjection of NaHS (0.1 µL, 4 nmol) + L703606 (0.1 µL, 4 nmol; n = 6) in NTS on gastric motility.
Microinjection
Before conducting the experiments, the rats were anesthetized with 4% chloral hydrate at (400 mg/kg body weight) by intraperitoneal injection until the eyelids and corneal reflexes disappeared, the muscles were relaxed, and the breathing was uniform. The anesthetized rat head was fixed on a brain stereotaxic apparatus (Stoelting 68002, Shenzhen Ruiwode Company, China).
Next, the animal was fixed according to the rat brain stereotaxic atlas (Paxinos and Watson, 2007) using the three points of the animal’s bilateral inner ear holes and incisors. With the left and right ear rods reading the same, fontanelle and bregma were kept at the same level with an error of no more than 0.3 mm.
The head hair was removed with hair clippers to expose the scalp and disinfected with 75% alcohol. Then, the scalp was cut along the sagittal suture of the skull with ophthalmic clippers to expose the skull, the excess connective tissue around the skull was cut away, and the surface of the skull was gently wiped with saline until the fontanelle and herringbone suture were exposed. The three-dimensional coordinates of fontanelle were used as the zero point. A small hole of approximately 2 mm in diameter was drilled in the skull ipsilateral to the coordinates of the NTS center point (13.3 mm posterior to fontanelle, 0.8 mm paracentral opening, 7.9 mm subdural) in the atlas using an electric cranial drill. A glass microelectrode with a tip of approximately 30 μm was placed into the brain at the depth of the coordinates.
Recording gastric motility
The anesthetized rats were placed abdomen side up, and a small incision was made in the fundus of the stomach to clean the gastric residue. A 5 mm diameter balloon filled with warm water was inserted into the pylorus of the gastric sinus and kept at a constant baseline pressure. The balloon inserted into the rat’s stomach was connected to the pressure transducer and BL-420 (Biological Function Experimental System; Chengdu Taimeng Company, China) via a polyethylene plastic tube. The stimulation parameters of the transducer were adjusted to 25 mm/min speed, 0.5 mV/cm sensitivity, and 10 Hz filter. Once the gastric motility curve was stabilized, the drug’s microinjections and contaminate sky blue were administered. A heat lamp was used throughout the experiment to maintain a constant ambient temperature, and gastric motility was recorded.
Histological identification of the microinjection site
After gastric motility recording, 2% pontamine sky blue (0.1 µL) was injected into the NTS, the rats were executed with an excess of sodium pentobarbital, and the thorax was opened for cardiac perfusion. After perfusion, the heads of the rats were cut off, and the brain tissues were removed and placed in a 4% formaldehyde solution for fixation.
Subsequently, the brain tissues were frozen at -16 °C for 30 min and sectioned into successive coronal sections with a thickness of 16 μm. The brainstem sections were stained, allowing for the identification of injection sites. The brain slices on slides were treated with a neutral red stain and dehydrated to achieve transparency. The sections were observed and photographed using a microscope (Nikon Optiphot, Nikon, Shanghai, China) and photographed with a digital camera (Magnafire; Optronics, Goleta, CA, United States) connected to a computer. The blue dot marking the precise location of the NTS was identified for further statistical analysis.
Data analysis and statistics
The gastric motility of the rats was assessed by counting the number of contractions before and 5 min after injection were counted respectively. The total duration of contraction waves (T.D.C.W) within 5 min, the total amplitude of contraction waves (T.A.C.W) within 5 min and the gastric motility index (the product of amplitude and duration) before and after the 5-min microinjection were evaluated statistically. To calculate the inhibition rate of gastric motility, the following formula was used: Inhibition rate (%) = (pre-injection value-post-injection value) × 100%/pre-injection value. The height between the highest point of the contraction curve and the baseline is the amplitude of the contraction wave. The time duration between the starting point and the ending point of the contraction wave is the time duration of the contraction wave.
Statistical analysis was performed using SPSS v25.0 (IBM SPSS Inc., Chicago, IL, United States) using Student’s t-test or one-way ANOVA, followed by a posthoc test using the Student-Newman-Keuls test. All data are presented as mean ± SE. A P value less than 0.05 was considered statistically significant.
RESULTS
Observation of c-Fos and CBS expression in NTS at different times in RWIS
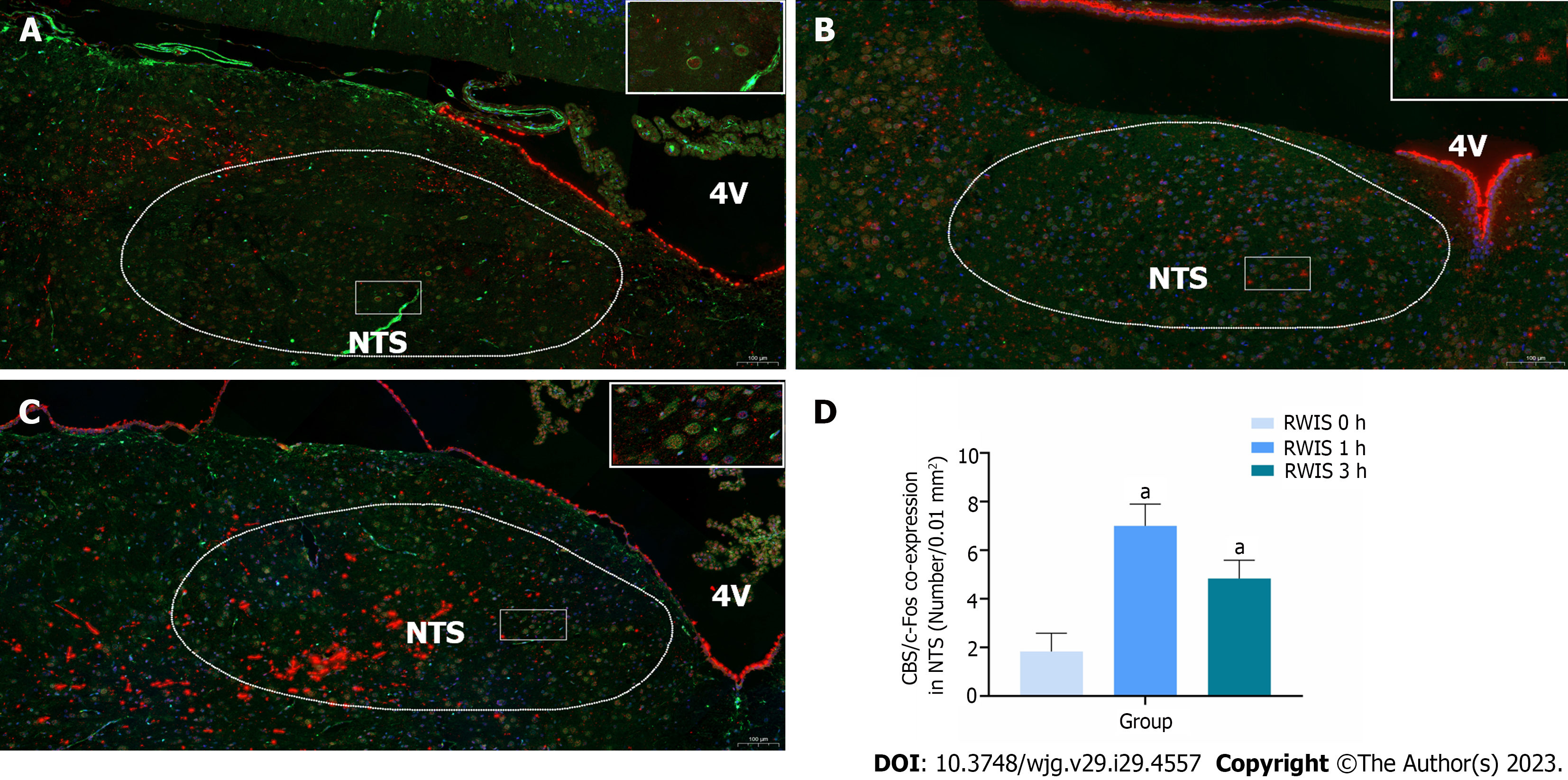
In this experiment, the number of CBS and c-Fos co-expressing neurons in the NTS (Figure 1, n = 6) was revealed by immunohistochemical fluorescence double-labeling. The expression of c-Fos protein in the NTS showed varying degrees of increase at 1 h (7.00 ± 0.37) and 3 h (4.83 ± 0.31) after RWIS compared to the control group at 0 h (1.83 ± 0.30) (P < 0.01). This finding indicates that CBS neurons in the NTS of rats were activated during the RWIS procedure.
Figure 1 Co-expression of cystathionine beta-synthase (green) and c-Fos (red) neurons in the nucleus tractus solitarius at different times of restraint water-immersion stress.
A: Expression of cystathionine beta-synthase (CBS) and c-Fos in restraint water-immersion stress (RWIS) at 0 h; B: Expressions of CBS and c-Fos in RWIS at 1 h; C: Expression of CBS and c-Fos in RWIS at 3 h; D: Quantification of neurons co-expressing CBS and c-Fos in the nucleus tractus solitarius (n = 6). aP < 0.01 vs RWIS 0 h group. NTS: Nucleus tractus solitarius; RWIS: Restraint water-immersion stress; CBS: Cystathionine beta-synthase.
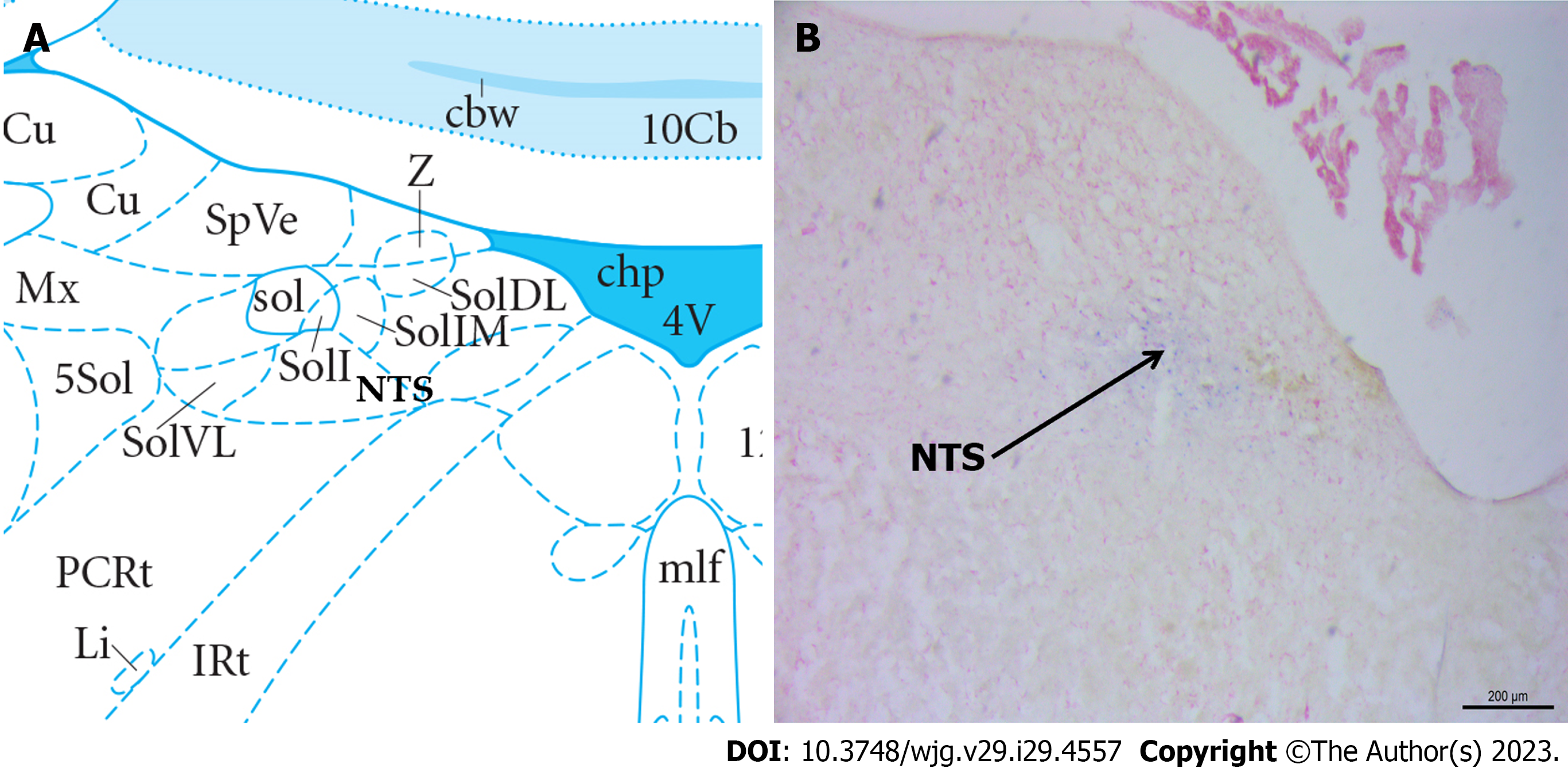
NTS injection site identification
Following neutral red staining, the brain slices were examined under a light microscope to determine the localization of the injected blue spots and drugs within the NTS. The observed data regarding the gastric motility of rats in the correct position were analyzed. Figure 2 presents the diagram for identifying the degree of tissue localization.
Figure 2 Histological identification of microinjections located at the nucleus tractus solitarius in the brain.
A: Location of the nucleus tractus solitarius (NTS) in the brain atlas; B: Neutral red stained brain section with blue dot sites representing injection into the NTS. NTS: Nucleus tractus solitarius.
NaHS inhibits gastrointestinal motility within NTS
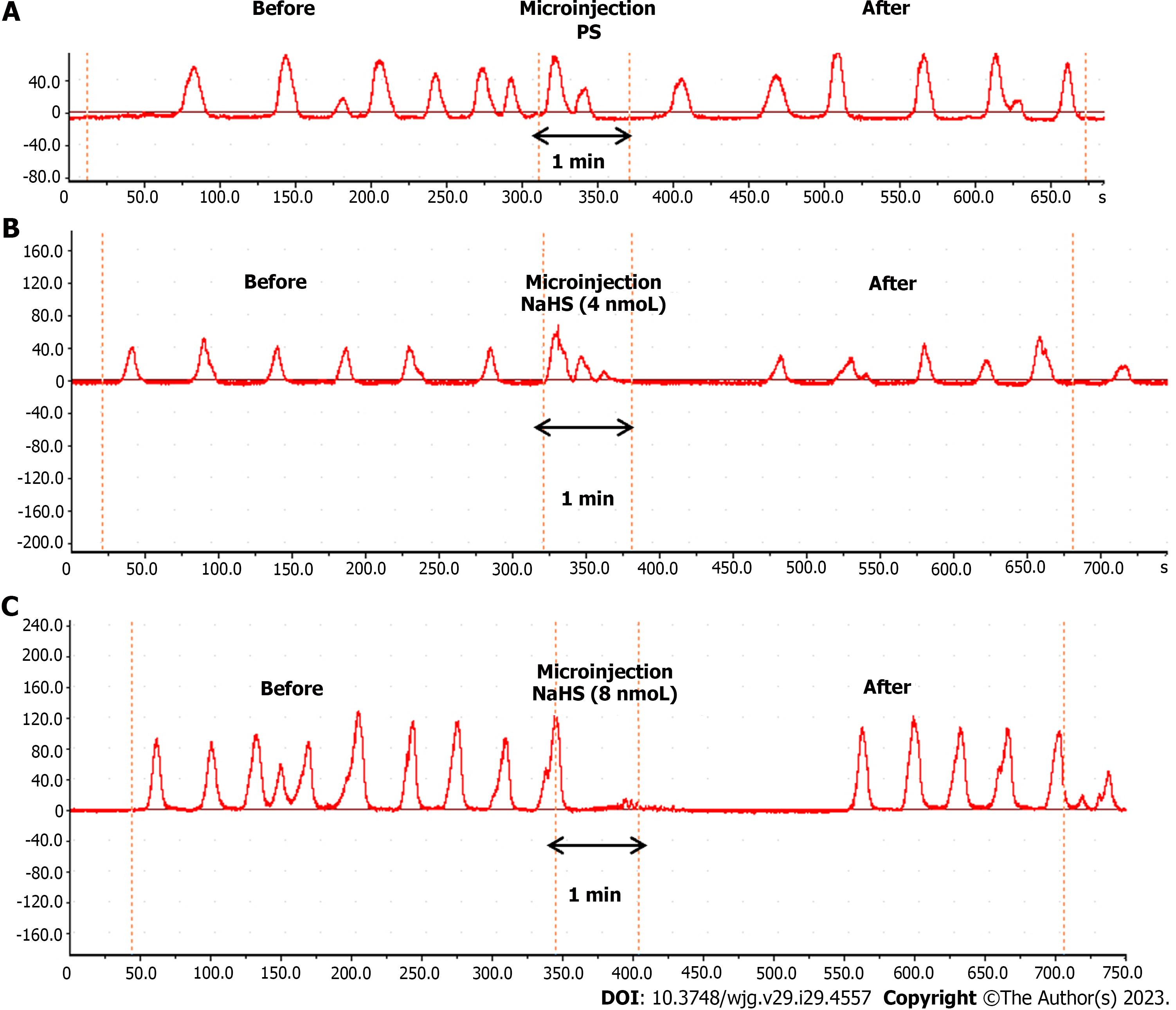
The microinjection of physiological saline (PS) (0.1 µL, n = 6) under the same conditions did not produce a significant change in gastric motility (Figure 3A). In contrast, the microinjection of NaHS at different concentrations (4 nmol and 8 nmol, 0.1 µL, n = 6) into the rat NTS resulted in significant inhibition of gastric motility (Figure 3B and C).
Figure 3 Effect of microinjection of drugs in the nucleus tractus solitarius on gastric motility in rats.
A: Gastric motility curves were recorded in rats microinjected with saline at the nucleus tractus solitarius (NTS); B: Gastric motility curves were recorded in rats microinjected with 4 nmol NaHS in the NTS; C: Gastric motility curves were recorded in rats microinjected with 8 nmol NaHS in the NTS. PS: Physiological saline.
We compared gastric motility curves measured before and 5 min after the drug injection and after 4 nmol NaHS injection in the NTS. The T.A.C.W. decreased from 553.08 mm 5 min-1 ± 9.59 mm 5 min-1 to 421.30 mm 5 min-1 ± 10.58 mm 5 min-1 (P < 0.01). The T.D.C.W. decreased from 179.79 s 5 min-1 ± 13.33 s 5 min-1 to 132.56 s 5 min-1 ± 6.67 s 5 min-1 (P < 0.01), and the gastric motility index (G.M.I.) decreased from 5219.88 ± 182.11 to 4250.28 ± 159.03 (P < 0.01). At a NTS microinjection dose of 8 nmol NaHS, the T.A.C.W. decreased from 587.62 mm 5 min-1 ± 9.58 mm 5 min-1 to 407.44 mm 5 min-1 ± 10.61 mm 5 min-1 (P < 0.01) and the T.D.C.W. decreased from 234.11 s 5 min-1 ± 11.74 s 5 min-1 to 145.13 s 5 min-1 ± 3.93 s 5 min-1 (P < 0.01) and the G.M.I. decreased from 5906.07 ± 181.71 to 4105.60 ± 49.35. After PS injection in the NTS, the T.A.C.W. decreased from 468.72 mm 5 min-1 ± 6.42 mm 5 min-1 to 467.34 mm 5 min-1 ± 5.04 mm 5 min-1 (P > 0.05), the T.D.C.W. from 236.96 s 5 min-1 ± 8.51 s 5 min-1 to 232.38 s 5 min-1 ± 16.31 s 5 min-1 (P > 0.05), and the G.M.I. from 5797.17 ± 141.87 to 5778.08 ± 125.32 (P > 0.05) (Figure 4A-C).
Figure 4 Effects of the microinjection of NaHS (4 nmol and 8 nmol) and physiological saline into the nucleus tractus solitarius before and after the gastric motility data.
A: Total amplitude of the contraction wave (T.A.C.W.); B: Total duration of the contraction wave (T.D.C.W.); C: Gastric motility index (G.M.I.); D: Inhibition rates of T.A.C.W., T.D.C.W., and G.M.I. aP < 0.01 vs before injection. PS: Physiological saline; T.A.C.W.: Total amplitude of the contraction wave; T.D.C.W.: Total duration of the contraction wave; G.M.I.: Gastric motility index.
The inhibition rates of the T.A.C.W. in the 4 nmol NaHS, 8 nmol NaHS, and saline groups were 23.83%, 30.69%, and 0.27%, respectively. The inhibition rates of T.D.C.W. in the 4 nmol NaHS, 8 nmol NaHS, and saline groups were 26.21%, 37.43%, and 2.06%, respectively. The inhibition rates of G.M.I. in the 4 nmol NaHS, 8 nmol NaHS, and saline groups were 18.55%, 30.17%, and 0.30%, respectively (Figure 4D). The data indicated that the inhibition rates of T.A.C.W., T.D.C.W., and G.M.I. were significantly higher in the 8 nmol NaHS group compared to the 4 nmol NaHS group. These findings suggest a dose-dependent inhibitory effect of NTS injection of NaHS on gastric motility.
PDTC eliminates the inhibitory effect of NaHS on gastric motility
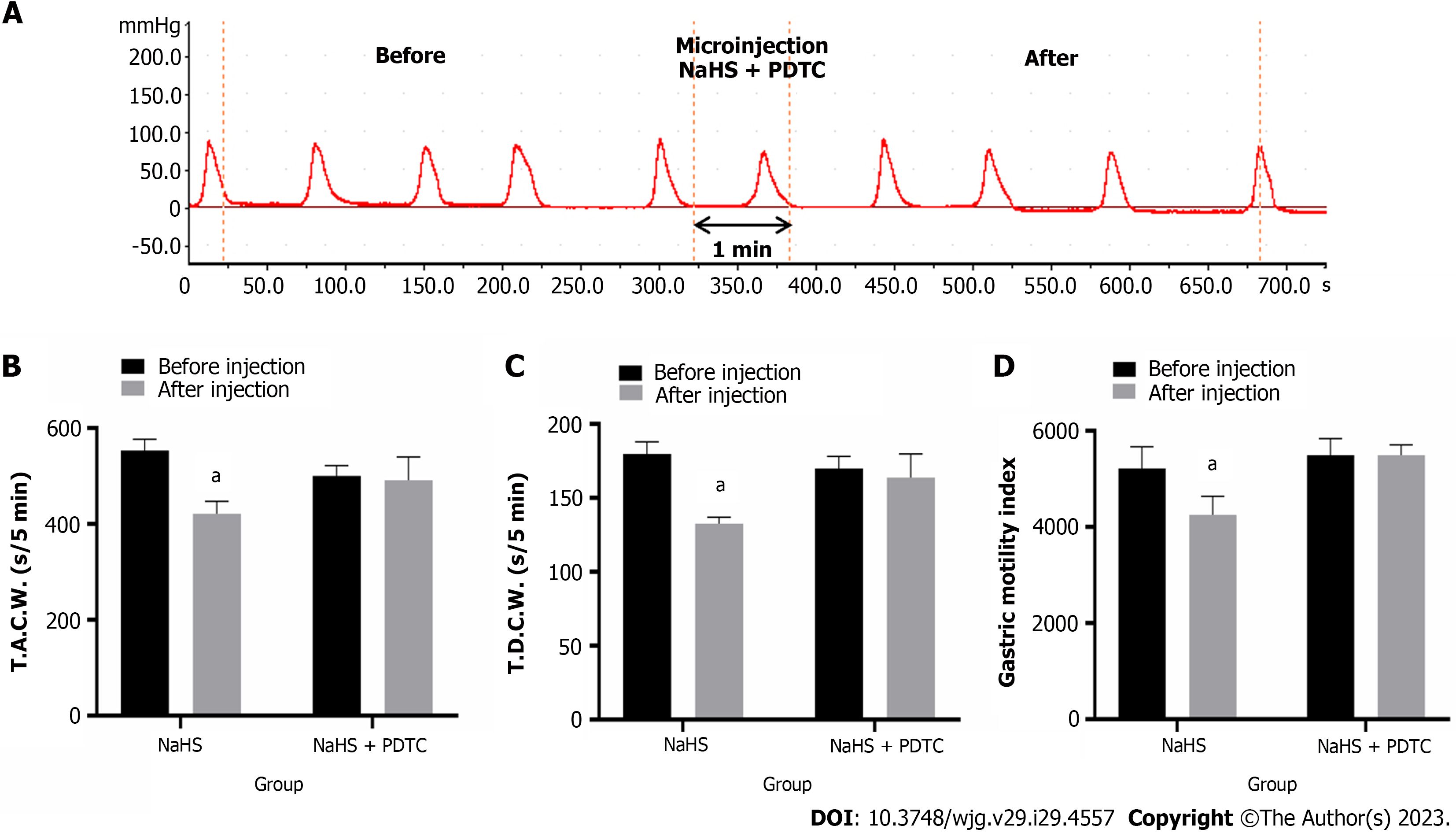
Injection of PDTC followed by NaHS into the NTS eliminated the inhibitory effect of NaHS on gastric motility (Figure 5A, n = 6). The T.A.C.W. changed from 500.15 mm 5 min-1 ± 7.56 mm 5 min-1 to 491.06 mm 5 min-1 ± 17.19 mm 5 min-1 (P > 0.05), the T.D.C.W. changed from 169.84 s 5 min-1 ± 3.40 s 5 min-1 (P > 0.05), and the G.M.I. changed from 5494.78 ± 140.32 to 5490.60 ± 88.80 after PDTC followed by NaHS injection (P > 0.05) (Figure 5B-D). These data suggest that NaHS can regulate gastric motility through the NF-κB signaling pathway.
Figure 5 Effect of microinjection of 4 nmol NaHS and 4 nmol NaHS + pyrrolidine dithiocarbamate into the nucleus tractus solitarius on gastric motility in rats.
A: Gastric motility curve recorded in rats with 4 nmol NaHS + pyrrolidine dithiocarbamate microinjection in nucleus tractus solitarius; B: Data of total amplitude of the contraction wave; C: Data of total duration of the contraction wave; D: Data of gastric motility index. aP < 0.01 vs before injection. PDTC: Pyrrolidine dithiocarbamate; T.A.C.W.: Total amplitude of the contraction wave; T.D.C.W.: Total duration of the contraction wave.
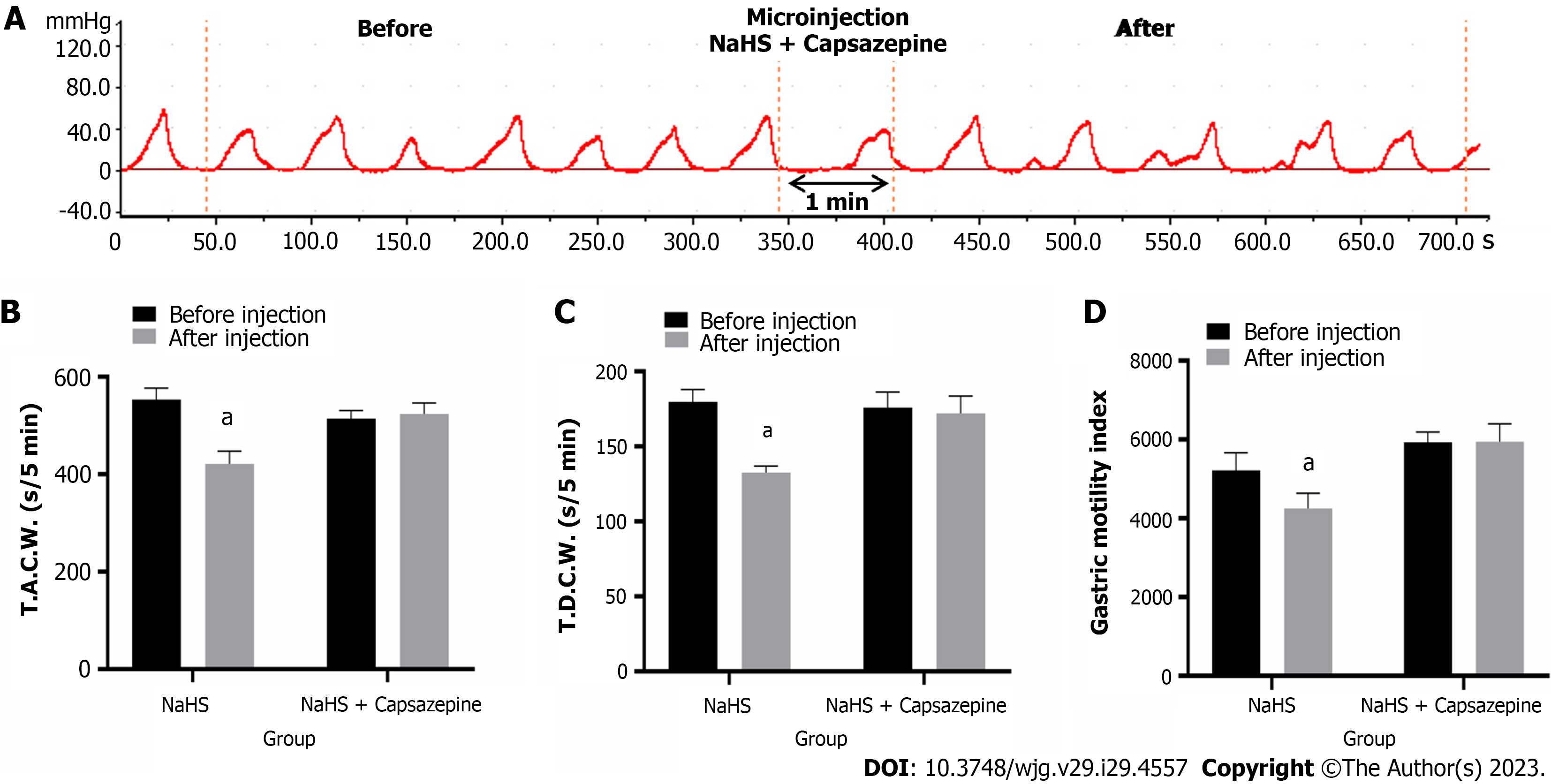
Capsazepine eliminates the inhibitory effect of NaHS on gastric motility
Injection of Capsazepine followed by NaHS into the NTS eliminated the inhibitory effect of NaHS on gastric motility (Figure 6A, n = 6). As a result, the T.A.C.W. changed from 514.46 mm 5 min-1 ± 6.56 mm 5 min-1 to 523.87 mm 5 min-1 ± 9.21 mm 5 min-1 (P > 0.05), the T.D.C.W. changed from 175.90 s 5 min-1 ± 4.22 s 5 min-1 to 172.13 s 5 min-1 ± 4.68 s 5 min-1 (P > 0.05), and the G.M.I. changed from 5932.97 ± 104.93 to 5946.45 ± 184.14 (P > 0.05) (Figure 6B-D). These data suggest that NaHS can regulate gastric motility through TRPV1 channels.
Figure 6 Effects of microinjection of 4 nmol NaHS and 4 nmol NaHS + Capsazepine into the nucleus tractus solitarius on gastric motility in rats.
A: Gastric motility curve recorded in rats with 4 nmol NaHS + Capsazepine microinjection in nucleus tractus solitarius; B: Data of total amplitude of the contraction wave; C: Data of total duration of the contraction wave; D: Data of gastric motility index. aP < 0.01 vs before injection. T.A.C.W.: Total amplitude of the contraction wave; T.D.C.W.: Total duration of the contraction wave.
L703606 eliminates the inhibitory effect of NaHS on gastric motility
Injection of L703606 followed by NaHS into the NTS eliminated the inhibitory effect of NaHS on gastric motility (Figure 7A, n = 6). The T.A.C.W. changed from 494.46 mm 5 min-1 ± 11.86 mm 5 min-1 to 490.53 mm 5 min-1 ± 14.00 mm 5 min-1 (P > 0.05), the T.D.C.W. changed from 164.10 s 5 min-1 ± 5.53 s 5 min-1 to 158.39 s 5 min-1 ± 10.64 s 5 min-1 (P > 0.05), and the G.M.I. changed from 5827.59 ± 133.74 to 5762.80 ± 114.34 (P > 0.05) (Figure 7B-D). These data suggest that NaHS can act on NK1 receptors to regulate gastric motility.
Figure 7 Effects of microinjection of 4 nmol NaHS and 4 nmol NaHS + L703606 into the nucleus tractus solitarius on gastric motility in rats.
A: Gastric motility curve recorded in rats with 4 nmol NaHS + L703606 microinjection in nucleus tractus solitarius; B: Data of total amplitude of the contraction wave; C: Data of total duration of the contraction wave; D: Data of gastric motility index. aP < 0.01 vs before injection. T.A.C.W.: Total amplitude of the contraction wave; T.D.C.W.: Total duration of the contraction wave.
DISCUSSION
Endogenous H2S concentrations in the brain range between 10 nM and 160 nM[32], and approximately 33% of H2S is produced by volatilization in NaHS solutions when measurements are made in a closed environment. H2S (10 mmol/L) in NTS neurons can maintain excitatory postsynaptic potential excitation for 10 minutes, which is equivalent to the time it takes for a microinjection NaHS to work[33]. Moreover, H2S can cross the cell membrane by free diffusion to modulate cellular properties[34]. We selected NaHS as an exogenous H2S rapid drug-delivery donor.
H2S, an emerging gaseous signaling molecule, plays an important role in regulating digestion and the nervous system[35]. Placing coronal NTS slices into NaHS solution was found to cause rapid concentration-dependent depolarization of neurons at NTS sites, with H2S increasing the postsynaptic currents in NTS neurons by promoting presynaptic glutamate release[36]. Herein, we observed a significant increase in the co-expression of neurons between CBS and c-Fos in rat NTS after RWIS, indicating that H2S in the NTS is involved in gastrointestinal regulation and stress.
In this experiment, we found that the amplitude and duration of gastric motility and the index of gastric motility were significantly lower in the NTS than in the control group, in a dose-dependent manner, after the injection of different concentrations of NaHS in rats. Sensory information from the upper gastrointestinal tract is transmitted to the NTS via vagal afferent fibers, and c-Fos-positive neurons are significantly increased in the NTS following stressful processes. Glutamate release from vagal afferent fibers activates NTS neurons, which can regulate gastrointestinal activity by inhibiting the vagal excitatory cholinergic efferent pathway via the inhibitory neurotransmitter GABA or by exciting the vagal inhibitory non-adrenergic non-cholinergic (NANC) efferent pathway[37]. In addition, vagal afferent nerves from the gastrointestinal tract can activate the NANC efferent pathway leading to gastric smooth muscle relaxation. Vagal efferent fibers activate the noradrenergic neurons in the NTS, which in turn activate the NANC pathway neurons in the DMV. These NANC-DMV neurons transmit to the gastrointestinal plexus to activate postganglionic cholinergic neurons, thus causing gastric relaxation[38].
In vivo studies found that the microinjection of D-glucose into the NTS resulted in decreased gastric motility and increased intragastric pressure in rats via K+-ATP channel relaxation of the smooth muscles and increased firing of GABAergic neurons[39]. Injection of cholecystokinin in the rat NTS modulates the gastrointestinal motility and secretory function in the upper gastrointestinal tract by activating postganglionic cholinergic excitatory or NANC inhibitory pathways[40]. Increased glutamate content within the NTS directly activates excitatory postsynaptic potentials in NTS neurons, which in turn stimulates local circuit GABAergic and glutamatergic neurons. GABAergic signals at the NTS determine the state of the gastric tone and contraction and mediate changes in gastric mechanical function at the onset of the vagal-vagal reflex[41,42]. The intra-NTS injection of GLP-1 reduces the gastric tone by activating NANC and delays gastric emptying in a dose-dependent manner[43]. Therefore, NaHS injection in the rat NTS may inhibit gastric motility by releasing inhibitory neurotransmitters such as GABA from preganglionic cholinergic neurons.
The activation of TRPV1 leads to the release of various pro-inflammatory cytokines, which can activate NF-kB translocation to the nucleus. Our experiments revealed that the injection of the NF-κB pathway blocker PDTC followed by NaHS eliminated the modulation of gastric function by NaHS. H2S is mainly synthesized in the brain by the CBS enzyme, andwe found that NaHS injection in both the ambiguous and paraventricular nuclei inhibited gastric motility in rats via the NF-κB pathway[44,45], that the injection of NaHS to the brain blocked inflammation-associated apoptosis, and that treatment with NaHS reduced the expression of inflammatory factors in astrocytes and microglia due to Alzheimer’s disease[46]. H2S reduces the levels of phosphorylated p38 MAPK and phosphorylated p65 NF-κB in vivo. NaHS also reduces LPS-induced inflammation by inhibiting p38 MAPK and p65 NF-κB in rat cells[47]. SP is the main presynaptically released excitatory transmitter from injured primary afferent fibers. It binds to NK1 receptors on the postsynaptic membrane to activate NF-kB-induced inflammatory factor synthesis. NF-κB is reportedly involved in the transcriptional regulation of various response-related genes, including gastrointestinal mucosal damage, and that the downregulation of NF-κB signaling can inhibit stress-induced local inflammatory responses in the gastric mucosa and can repair local lesions in the gastric mucosa[48,49]. Therefore, physiological concentrations of H2S can modulate the inflammatory process and regulate gastric dysfunction by down-regulating the inflammatory response.
To investigate the signaling pathways involved in H2S regulation, we focused on the TRPV1-SP-NF-kB pathway[50]. TRPV1 activation, enhanced pro-inflammatory cytokine expression, and oxidative stress via the NF-kB pathway led to reduced phosphorylation of the ERK signaling pathway, activation of PAG involved in the ERK-NF-kB pathway, and production of SP, which in turn regulated TRPV1-mediated neurogenic inflammation[51]. Our experiments showed that the administration of the TRPV1 blocker Capsazepine followed by NaHS eliminated the modulation of gastric function by NaHS. TRPV1 is widely expressed in spinal and vagal afferent neurons. It innervates the gastrointestinal tract, and its upregulated sensitivity may be associated with the pathophysiological functions of diseases such as visceral pain, irritable bowel syndrome, inflammatory bowel disease and pancreatitis[52]. TRPV1 presents two sides to inflammation. There are even studies showing the alternating effects of TRPV1 on inflammation[53]. Capsaicin induces inflammation in the stomach by activating TRPV1, damages the gastrointestinal mucosa, causes structural changes in the intestinal barrier and further leads to other gastrointestinal symptoms[54-56]. It also decreases the expression of anti-inflammatory cytokines in the stomach and intestine and promotes the release of gastrointestinal neuropeptide SP, which is closely associated with gastrointestinal visceral pain[57]. The findings suggest that the TRPV1 receptor antagonist caspofungin abrogates H2S donor-induced enhancement of gastric emptying and that TRPV1-dependent pathways have been shown to produce modulation of vagally mediated muscle contractions in the gastrointestinal tract[58]. Capsaicin also activates the enterocholinergic neurons in guinea pig’s small intestine and induces contractile effects and inhibition of gastric emptying, thus inducing the relaxation of smooth muscles of the fundus[59]. Capsazepine was also found to inhibit NaHS-induced pyloric smooth muscle relaxation, indicating that the effect of H2S donors on enhancing gastric emptying and inducing pyloric sphincter relaxation is mediated by the activation of TRPV1 receptors. Although there are many mechanisms by which this effect can be manifested, our data support the theory of smooth muscle relaxation induced by afferent neuronal TRPV1 receptor activation[45,60].
TRPV1 receptors may act as molecular sensors involved in processing cardiac injury information in spinal neurons. Harmful environmental stimuli can activate TRPV1 to produce pro-inflammatory mediators in the epithelial cells, thus triggering neurogenic inflammatory responses. TRPV1 antagonists reportedly inhibited H2S-induced neuropeptide release and bronchoconstriction in vitro, whereas H2S induced the release of an endogenous tachykinin, SP, by stimulating sensory nerve endings, and that NaHS-induced SP release is significantly reduced when the TRPV1 blocker Capsazepine is applied[61]. Capsaicin also slows down gastric motility through TRPV1-induced excitation of gastric sensory nerve fibers[62]. A possible role of capsaicin-sensitive vagal afferent nerves in gastric mucosal injury and prevention has been demonstrated and is associated with the release of SP[31].
We eliminated the inhibitory effect of NaHS on gastric motility in our experiments by injecting the NK1 receptor blocker L703606 followed by NaHS. SP is a brain-gut peptide abundant in mammals that acts as a neurotransmitter for specific receptors to mediate NANC expression in the autonomic nervous system. Numerous studies have shown its role in stress and nociceptive transmission[63]. Injection of exogenous SP into the NTS resulted in the reduction of gastric motility. In contrast, injection of NK1 blockers enhanced the gastric motility. Therefore, SP in the NTS may play a predominantly inhibitory role in gastrointestinal regulation. Administration of NK1 receptor antagonists also prevented H2S-induced contractile responses, indicating that SP released from sensory nerve endings is the ultimate mediator of H2S-induced excitation of smooth muscle in rats, possibly leading to altered gastric motility and the formation of gastric ulcers[64]. Injection of glutamate and SP in the NTS modulates gastric motility and emptying, and leads to a dose-dependent decrease in tonic gastric pressure and inhibition of gastric motility[65,66]. It has been shown that glutamate, acting through N-methyl-D-aspartic acid receptors with glutamate ion channels, and tachykinin, acting through NK1 and NK2 receptors, act synergistically in the transmission of acid stimuli from the gastric mucosa to the NTS[67]. SP regulates gastric smooth muscle contraction by both inhibition and enhancement mechanisms. Studies have found that SP microinjections in the brain have inhibitory effects on gastric motility. Furthermore, microinjections of SP in the rat DMV inhibited gastric EMG fast waves and gastric motility. These effects could be abolished by SP receptor antagonists and by severing the vagus nerve, respectively[68]. SP in the brain plays a role in stress-induced physiological and behavioral activity. The effects of NK1 can be blocked using injectable drugs or knockout methods[69-71]. Moreover, c-Fos expression can be downregulated, with the nucleus involved in stress in the brain and pain, causing pain through increased SP release in the hypothalamic and spinal cord tissue. This results in a sterile inflammatory response, with persistent pain leading to increased levels of SP receptors in the posterior horn of the spinal cord[72]. SP also plays a role in transmitting injurious information, with low doses given ventrally to produce analgesia, acting in a neuroendocrine manner on various immune cells involved in immune regulation and enhancing immune function[73]. We, therefore, consider that H2S enhances the inhibitory effect of SP on gastric motility by excitation of NTS neurons which are then transmitted to the DMV.
ARTICLE HIGHLIGHTS
Research background
Recent studies have revealed that hydrogen sulfide is the third class of gas signaling molecules after nitric oxide (NO) and carbon monoxide (CO). The high level of endogenous hydrogen sulfide found in the brain, which is mainly produced by cystathionine beta-synthase, suggests that it may have a physiological function, and the nucleus tractus solitarius is important nucleus that regulates the function of internal organs, so we want to elucidate the role of hydrogen sulfide in the NTS in regulating gastric function in rats.
Research motivation
To investigate whether hydrogen sulfide in the nucleus tractus solitarius is involved in the regulation of gastric dysfunction by restraint water-immersion stress, this study will examine the role of hydrogen sulfide in the nucleus tractus solitarius in the regulation of gastric motility.
Research objectives
It is the first time to propose that hydrogen sulfide in the nucleus tractus solitarius has a regulatory effect on the gastric motility caused by restraint water-immersion stress, and to investigate its mechanism of action, which can not only elucidate the mechanism of regulation of gastric dysfunction by hydrogen sulfide in the nucleus tractus solitarius, and also provide an important experimental basis for the prevention and treatment of stress gastric ulcer from the central aspect in clinical practice.
Research methods
We used immunohistochemical, fluorescent double-labeling technique and restraint water-immersion stress model to confirm the involvement of hydrogen sulfide-producing cystathionine beta-synthase neurons in the nucleus tractus solitarius in the regulation of gastric function, and physiological methods to record changes in gastric motility before and after their brain injection.
Research results
After restraint water-immersion stress, cystathionine beta-synthase neurons containing c-Fos were significantly increased and gastric motility was inhibited in rats after nucleus tractus solitarius injection of NaHS, and this inhibitory effect was eliminated after pre-injection of transient receptor potential vanilloid 1 channels, NF-κB channel blockers, and NK1 receptor antagonists followed by NaHS injection.
Research conclusions
Injection NaHS into the nucleus tractus solitarius can inhibit gastric motility in rats and this effect may be mediated by TRPV1 and NK1 receptors via NF-κB channel-dependent activation.
Research perspectives
Our next step will be to continue our work on the effects of endogenous hydrogen sulfide in the nucleus tractus solitarius on gastric function.