Published online May 14, 2023. doi: 10.3748/wjg.v29.i18.2818
Peer-review started: February 28, 2023
First decision: March 14, 2023
Revised: March 16, 2023
Accepted: April 13, 2023
Article in press: April 13, 2023
Published online: May 14, 2023
Helicobacter pylori (H. pylori) is the main pathogen that causes a variety of upper digestive diseases. The drug resistance rate of H. pylori is increasingly higher, and the eradication rate is increasingly lower. The antimicrobial resistance of H. pylori is an urgent global problem. It has been confirmed that Banxia Xiexin decoction (BXXXT) demonstrates the effects of treating gastrointestinal diseases, inhibiting H. pylori and protecting gastric mucosa. The purpose of the present study is to further explore the therapeutic effects of BXXXT on drug-resistant H. pylori.
To confirm that BXXXT demonstrates therapeutical effects in vivo and in vitro on gastritis mice with drug-resistant H. pylori and explain its mechanism to provide an experimental basis for promoting the application of BXXXT.
The aqueous extract of BXXXT was gained by water decocting method. The inhibitory effect of the aqueous extract on H. pylori was detected by dilution in vitro; drug-resistant H. pylori cells were used to build an acute gastritis model in vivo. Thereafter, the model mice were treated with the aqueous extract of BXXXT. The amount of H. pylori colonization, the repair of gastric mucosal damage, changes of inflammatory factors, apoptosis, etc., were assessed. In terms of mechanism exploration, the main medicinal compositions of BXXXT aqueous extract and the synergistic bacteriostatic effects they had demonstrated were analyzed using mass spectrometry; the immune function of peripheral blood cells such as CD3+ T and CD4+ T of mice with gastritis before and after treatment with BXXXT aqueous extract was detected using a flow cytometry; the H. pylori transcriptome and proteome after treatment with BXXXT aqueous extract were detected. Differently expressed genes were screened and verification was performed thereon with knockout expression.
The minimum inhibitory concentration of BXXXT aqueous extract against H. pylori was 256-512 μg/mL. A dose of 28 mg/kg BXXXT aqueous extract treatment produced better therapeutical effects than the standard triple therapy did; the BXXXT aqueous extract have at least 11 ingre
BXXXT aqueous extract could demonstrate good therapeutic effects on drug-resistance H. pylori in vitro and in vivo and its mechanism comes down to the synergistic or additional antibacterial effects of berberine, emodin and luteolin, the main components of the extract; the extract could activate the immune function and enhance bactericidal effects; BXXXT aqueous extract, with main targets of BXXXT aqueous extract related to urease, virulence factors, etc., could reduce the urease and virulence of H. pylori, weaken its colonization, and reduce its inflammatory damage to the gastric mucosa.
Core Tip: The failure rate of treating Helicobacter pylori (H. pylori) infectious diseases is increasing, leading to an urgent need to study and develop anti-H. pylori drugs. Banxia Xiexin decoction (BXXX) has a good effect on Hp infection and Hp-infection-related diseases. However, its pharmacological mechanism remains unclear, and whether it has an effect on drug-resistant H. pylori infection has not been confirmed by animal experiments. Our study confirms that BXXX decoction (BXXXT) has good therapeutic effects on drug-resistant H. pylori infection through in vivo and in vitro experiments in mice, then the composition of BXXXT and effective components, the immunomodulatory effect, the main target were verified. We preliminarily explain why BXXXT has a good effects.
- Citation: Li XH, Xu JY, Wang X, Liao LJ, Huang L, Huang YQ, Zhang ZF. BanXiaXieXin decoction treating gastritis mice with drug-resistant Helicobacter pylori and its mechanism. World J Gastroenterol 2023; 29(18): 2818-2835
- URL: https://www.wjgnet.com/1007-9327/full/v29/i18/2818.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i18.2818
Helicobacter pylori (H. pylori) is the main pathogen that causes a variety of upper digestive diseases[1], such as chronic gastritis, peptic ulcer, and gastric cancer[2]. At present, the treatment options for H. pylori infections include standard triple therapy, bismuth-containing quadruple therapy, and sequential therapy. The drug resistance rate of H. pylori is increasingly higher, and the eradication rate is increasingly lower. Clarithromycin-resistant H. pylori has been listed as a focus for the research and development of antibiotics by the World Health Organization in 2017. The antimicrobial resistance of H. pylori is an urgent global problem. Multiple antibiotic resistance existed in 16847 H. pylori strains isolated in Wenzhou from 2013-2020. Separate statistics on the resistance rates of H. pylori to the six commonly used antibiotics revealed that the resistance rates to levofloxacin, clarithromycin and metronidazole were high in the region, respectively 32.81%, 26.02%, and 95.67%. Therefore, how to avoid, overcome and eradicate H. pylori and H. pylori’ s drug resistance brings challenges to the current clinical work. In China, traditional Chinese medicine could be used to treat a variety of refractory diseases, reducing drug resistance and improving the eradication rate of H. pylori[3]. “BXXXT” comes from Zhang Zhongjing’ s Treatise on Febrile Diseases, which consists of 15 g Pinellia ternate, 9 g Radix scutellariae, 9 g Dried ginger, 9 g Ginseng, 9 g Roasted licorice, 3 g Coptis chinensis, and 4 Jujubes. At present, experimental studies and clinical efficacy of this prescription have been reported for gastrointestinal dysfunction, peptic ulcer, chronic gastritis, atrophic gastritis and other digestive system diseases[4-6]. There are numerous studies at home and abroad on the efficacy of this prescription[7-9], and it has been confirmed that BXXXT demonstrates the effects of treating gastrointestinal diseases, inhibiting H. pylori and protecting gastric mucosa[10-12]. However, whether it has the same effect on the refractory gastritis caused by drug-resistant H. pylori remains unreported.
Therefore, the purpose of the present study is to further explore the therapeutic effects of BXXXT on drug-resistant H. pylori through establishing mouse models, and to study the molecular mechanism of BXXXT applied in the treatment of the refractory gastritis caused by drug-resistant H. pylori through ingredient analysis, immune regulation, identification of therapeutical targets, etc., so as to provide an experimental basis for improving the application efficiency and of this prescription worldwide.
H. pylori strains (standard 26695, G27, NSH57, and multi-drug-resistant BHKS159 all provided by Professor Bi Hongkai of Nanjing Medical University) containing the preservation solution and stored at -80 °C, clinical strains HPBS001-HPBS016 that had been isolated by Huang Yanqiang Laboratory, Youjiang Medical College for Nationalities were used. The H. pylori strains were cultured in a Columbia (OXOID, United Kingdom) medium with 10% serum or a BHI (OXOID, United Kingdom) medium in a microaerobic environment (85% nitrogen, 5% oxygen, and 10% carbon dioxide) at 37 °C.
SPF C57BL/6 mice aged six to eight weeks were purchased from Changsha Tianqin Biological Co., Ltd.; the number of SPF animal license: SYXK Gui 2017-0004; animal experiment ethics number: No. 2019112501.
The prescription consists of 15 g Pinellia ternate, 9 g Radix scutellariae, 9 g Dried ginger, 9 g Ginseng, 9 g Roasted licorice, 3 g Coptis chinensis, and 4 g Jujubes. In the first round, the medical materials used to prepare BXXXT were crashed to crude powder that was placed in a beaker with sterile distilled water at a ratio of 1:10, soaked for 4 h, treated with diluted and boiled for 0.5 h with the liquid filtered out. In the second round, the filtered powder was treated with distilled water in a ratio of 1:5 and boiled for 20 min with the filtrate filtered out; in the third round, the filtered powder was treated following the same steps described in the second round but boiled for 5 min with the liquid filtered out. The liquids prepared during the three rounds were combined, concentrated to crude drug (1 g/mL), evaporated under atmospheric pressure, sterilized, and stored for later use.
A medium of BXXXT aqueous extract that had been diluted two-fold was prepared as the treatment group: BXXXT aqueous extract was mixed with BHI and diluted two-fold with berberine set as the positive drug control group. H. pylori cells were adjusted at OD600 = 0.03 (equivalent to 1.0 × 107 CFU/mL). Bacterial solution (100 μL, equivalent to 1.0 × 106 CFU/mL) was inoculated into a 96-well plate that contained medicated BHI and incubated at 37 °C for 48 h to determine the results with the lowest concentration of drugs that could inhibit H. pylori growth being minimum inhibitory concentration (MIC).
BXXXT aqueous extract, amoxicillin (Sigma-Aldrich, Germany), clarithromycin (Sigma-Aldrich, Germany) and omeprazole (Sigma-Aldrich, Germany) were all dissolved and diluted to 10 mg/mL. C57BL/6 model mice (BHKS159) were divided into four groups: The omeprazole + amoxicillin + clarithromycin group (the dose was 138.2 mg/kg of omeprazole, 28.5 mg/kg of amoxicillin, and 14.3 mg/kg of clarithromycin), the omeprazole + BXXXT aqueous extract (28 mg/kg), the omeprazole + BXXXT aqueous extract (7 mg/kg) and the phosphate-buffered saline (PBS) group, with six mice in each group. Mice were given administration once a day for three consecutive times. Two days after drug withdrawal, the blood was collected from the eyeballs of the mice. The mice were then sacrificed through cervical dislocation with tissues taken from their stomach and broken to acquire H. pylori that was then isolated, cultured, and identified with the amount of colonization calculated. Part of the stomach tissues was made into paraffin sections with HE staining, TUNEL immunohistochemistry and fluorescence immunoassay performed thereon.
This analysis was performed by Beijing Bio-Tech Pack Technology Company Ltd using a TripleTOF5600 + and an AB SCIEX™ with the ion source being ESI. The chromatographic column was SHIMADZU InerSustain C18 (100.0 mm × 2.1 mm, 2 µm) with the column temperature being 35 °C and flow rate of 0.300 (mL/min). The mobile phase: (1) Equate = “acetonitrile”; and (2) equate = “0.1% CH3COOH-H2O”. The chromatographic conditions were shown in Table 1. The scanning range of mass spectrometry conditions was m/z 100-1500. The scanning mode: DIA. Capillary voltage: 5000 V (positive) and 4500 V (negative). Capillary Temp: 500 °C, DP 60 V, CE 35 V, and CES 15 V.
| Time (min) | Parameter |
| 0 | A: 0%, B: 100% |
| 10 | A: 50%, B: 50% |
| 13 | A: 95%, B: 5% |
| 14 | A: 0%, B: 100% |
| 15 | A: 0%, B: 100% |
According to the protocols of the checkerboard method, drug A in the first row and drug B in the first column were diluted two-fold respectively. Then the transverse and longitudinal drugs were also cross-diluted two-fold and treated with 100 μL bacterial suspension, with the optimal combination effect selected to calculate the antimicrobial concentration index (FICI) after culture for 48 h. FICI= MIC of A drugs used in combination/MIC of A drugs used alone + MIC of B drugs used in combination/MIC of B drugs used alone. Criteria: synergistic effects (FICI ≤ 0.5); additive effect (0.5 < FICI ≤ 1.0); no effect (1.0 < FICI ≤ 2.0); antagonistic effects (FICI > 2.0).
During the process of testing the efficacy of BXXXT aqueous extract described previously in section 1.5, the peripheral blood of mice before and after administration was collected and cells thereof were treated with anticoagulant and then with 50 µL antibody mixtures: CD3, CD4, and CD8, etc. After the membranes were fixed and broken, the cells were treated with 100 µL antibody mixtures: IFN and IL-4. After filtration, the expressions of immune cells and cytokines were detected using a flow cytometry.
BHKS159 bacteria were cultured on a Columbia plate overnight. Thereafter, the single colony was selected and diluted to 0.5 Mcfarland standard (MCF) with 2 μL taken and added to 5 mL BXXXT aqueous extract (1/2 MIC, prepared by being treated with BHI). The negative control group was induced with PBS, shaken at 37 °C and centrifuged to collect the bacteria solutions after it had been treated with the aqueous extract for 4 h and 8 h. The bacteria solutions were delivered to Nanjing Medical University where they were observed under a transmissive electron microscope. H. pylori cells were treated with BXXXT aqueous extract at the half inhibitory concentration that had been detected before for 8 h, after which the samples were collected and frozen in liquid nitrogen for 10 min, frozen with dry ice and delivered to Beijing Allwegene Technology Co., Ltd. for detection and analysis of transcriptome and proteome. The transcriptome analysis was performed using the Illumina PE150 sequencing strategy; the length of RNA fragments was detected using Agilent 2100; the alignment and transcript assembly analysis were performed using Boetie2 and the Rockhhoper software; quantitative protein analysis was performed using the ITRAQ labeling quantitative strategy, ITRAQ/TMT labeling performed using isoheavy isotope labeling, and the quantitative detection of target genes performed using a fluorescent polymerase chain reaction (PCR) instrument and Western blotting. Strains with low expressions of related target genes were used to verify MIC changes and mutant strains were constructed with reference to the protocols described in the previous studies[13]. The relevant mRNA amplification primers are shown in Table 2, and the relevant antibody information is displayed in Table 3.
| No. | Primer | Sequence (5’ to 3’) | Company |
| 1 | UREA F | GCCAATGGTAAATTAGTT | Shanghai Invitrogen Biotech Co., Lt |
| 2 | UREA R | CTCCTTAATTGTTTTTAC | |
| 3 | UREB F | TCTATCCCTACCCCACAACC | |
| 4 | UREB R | CCATCCACGAACACATGGTA | |
| 5 | CagA F | ACCCCTAGTCGGTAATG | |
| 6 | CagA R | GCTTTAGCTTCTGATACTGC | |
| 7 | VacA F | GTCAGCATCACACCGCAAC | |
| 8 | VacA R | CTGCTTGAATGCGCCAAAC | |
| 9 | 16sRNA F | CTGGAGAGACTAAGCCCTCC | |
| 10 | 16sRNA R | AGGATCAAGGTTTAAGGATT |
| Name | Art. No. | Company |
| CagA (A-10) | sc-32746 | Santa Cruz |
| VacA | sc-28368 | Santa Cruz |
| m-IgGk BP-HRP | sc-516102 | Santa Cruz |
| GAPDH Ab | AF7021 | Affinity Biosciences |
| Goat anti-rabbit IgG (H+L) HPR | S0001 | Affinity Biosciences |
Statistical analysis and mapping were performed using the Graphpad Prism software, version 8.0. Continuous data were expressed as mean ± SD. Differences between groups were analyzed using the one-way ANOVA. P < 0.05 was considered statistically significant.
The MICs of BXXXT aqueous extract on three sensitive H. pylori strains and 11 drug-resistant H. pylori strains were detected by applying the solid plate method. BXXXT aqueous extract was found to have antibacterial effects on strains, both resistant and sensitive (the MIC was 256-512 μg/mL). The antibacterial effect of BXXXT aqueous extract was compared with that of berberine (the MIC was 512-2048 μg/mL), as shown in Table 4. The results suggested that there was a two-to-four-fold difference between them and that BXXXT aqueous extract produced better antibacterial effects than 98% pure berberine. The reason might be that although berberine accounts for a small portion of the prescription, there might be other antibacterial components, or there might be synergistic or additive effects among these components.
| Strain | Drug-resistanct strain | BXXXT aqueous extract | Berberine |
| 26695 | Sensitive | 512 | 1024 |
| G27 | Sensitive | 512 | 1024 |
| NSH57 | Sensitive | 256 | 512 |
| BHKS159 | Resistant to levofloxacin, clarithromycin and metronidazole | 512 | 1024 |
| HPBS001 | Resistant to levofloxacin, clarithromycin and metronidazole | 512 | 1024 |
| HPBS002 | Resistant to metronidazole | 512 | 1024 |
| HPBS003 | Resistant to clarithromycin | 512 | 1024 |
| HPBS004 | Resistant to levofloxacin | 512 | 1024 |
| HPBS005 | Resistant to levofloxacin and metronidazole | 256 | 1024 |
| HPBS006 | Resistant to clarithromycin and metronidazole | 256 | 1024 |
| HPBS007 | Resistant to clarithromycin | 512 | 1024 |
| HPBS010 | Resistant to metronidazole, clarithromycin and levofloxacin | 512 | 2048 |
| HPBS011 | Resistant to metronidazole and clarithromycin | 512 | 1024 |
| HPBS013 | Resistant to metronidazole, clarithromycin and levofloxacin | 512 | 1024 |
| HPBS014 | Resistant to metronidazole, clarithromycin, amoxicillin and levofloxacin | 512 | 1024 |
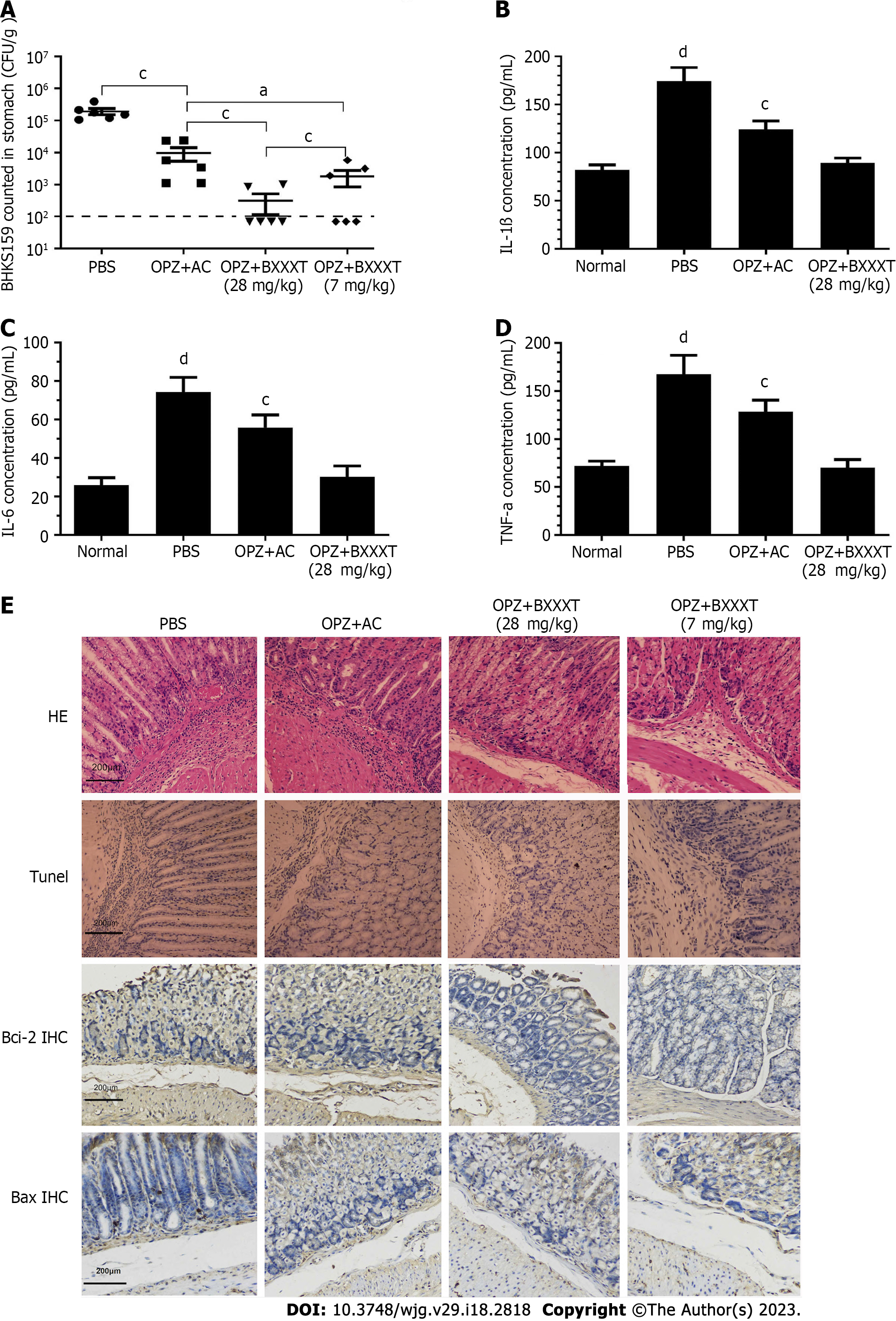
Model mice with acute gastritis caused by the drug-resistant strain BHKS159 were constructed and treated with PBS, omeprazole (OPZ) + amoxicillin clarithromycin (AC), OPZ + BXXXT (28 mg/kg) and OPZ + BXXXT (7 mg/kg), respectively. Although H. pylori colonization, inflammatory factors IL-1β, IL-6 and tumor necrosis factor-alpha (TNF-α), inflammatory damage, and apoptosis factors Bcl-2 and Bax were improved in OPZ + AC treatment group, there were still significant differences compared with OPZ + BXXXT (28 mg/kg) treatment group. After OPZ + BXXXT (28 mg/kg) treatment, the mice could basically recover to the normal level, implying therapeutical effects significantly better than that of the triple group (Figure 1). However, amoxicillin which does not develop drug resistance, produced therapeutical effects that contributed to the improvement in the OPZ + AC treatment group when combined with omeprazole. BXXXT, which did not demonstrate good effects in vitro, produced obvious therapeutical effects in vivo, which might be related to the synergistic and immunomodulatory effects of BXXXT aqueous extract.
MS-DIAL 3.70 (MS-DIAL: Data independent MS/MS deconvolution for comprehensive metabolome analysis) (Nature Methods, 12, 523-526, 2015).The original LC-MS data of BXXXT aqueous extract were imported into MS-DIAL, version 3.70 for preprocessing (MS-DIAL: Data independent MS/MS deconvolution for comprehensive metabolome analysis) (Nature Methods, 12, 523-526, 2015), including peak value extraction, noise-removal, deconvolution and peak alignment, and thereafter the three-dimensional data matrix in comma-separated values format was derived (original data matrix). The peak information extracted was compared with the database, with the full database search of MassBank, Respect and GNPS (14951 records in total). About 428 monomer components were identified, among which, as the related literature suggests, there were a total of 78 major components related to H. pylori resistance. Eleven species including berberine, emodin, baicalin, quercetin have been widely reported and demonstrate good antibacterial effects. Their ion additions and molecular structure are displayed in Table 5. It could be suggested that among the components of BXXXT aqueous extract, in addition to berberine, other components such as emodin also have inhibitory effects, which provides experimental basis for the better antibacterial effects in vitro BXXXT aqueous extract could produce compared with berberine.
| Name | Molecular structural formula |
| Berberine | COC1=C(OC)C2=C[N+]3=C(C=C2C=C1)C1=CC2=C(OCO2)C=C1CC3 |
| Baicalin | C1=CC=C(C=C1)C2=CC(=O)C3=C(C(=C(C=C3O2)O[C@H]4[C@@H]([C@H]([C@@H]([C@H](O4)C(=O)O)O)O)O)O)O |
| Luteolin | OC1=CC(O)=C2C(=O)C=C(OC2=C1)C1=CC(O)=C(O)C=C1 |
| Gallic acid | OC(=O)C1=CC(O)=C(O)C(O)=C1 |
| Gingerol | CCCCCC(O)CC(=O)CCC1=CC(OC)=C(O)C=C1 |
| Wogonoside | COC1=C(O)C=C(O)C2=C1OC(=CC2=O)C1=CC=CC=C1 |
| Rosmarinic acid | OC(=O)[C@H](CC1=CC(O)=C(O)C=C1)OC(=O)\C=C\C1=CC(O)=C(O)C=C1 |
| Aloe-emodin | OCC1=CC2=C(C(O)=C1)C(=O)C1=C(C=CC=C1O)C2=O |
| Catechin | CCCCCC(CC(=O)CCC1=CC(=C(C=C1)O)OC)O |
| Naringenin | OC1=CC=C(C=C1)[C@@H]1CC(=O)C2=C(O)C=C(O)C=C2O1 |
| Quercetin | OC1=CC(O)=C2C(OC(=C(O)C2=O)C2=CC(O)=C(O)C=C2)=C1 |
Six groups of berberine and emodin, berberine and luteolin, luteolin and gallic acid, luteolin and rosmarinic acid, catechuic acid and quercetin, catechuic acid and emodin were selected from 12 main anti-HP components of water extract of BXXXT aqueous extract for combined drug sensitivity detection. The results suggested that the six groups demonstrated additive or synergistic effects on H. pylori (Table 6), berberine and emodin, luteolin and gallic acid in particular producing better synergistic effects. Similar effects might also be found in other component combinations that had not been verified, which provides further experimental evidence that BXXXT aqueous extract could produce better antibacterial effects in vitro than berberine.
| Strain | MIC of drugs used alone | MIC of drugs used in combinations | FIC | Effect | ||
| Berberine | Emodin | Berberine | Emodin | |||
| G27 | 1024 | 512 | 256 | 256 | 0.75 | Addictive |
| 26695 | 1024 | 512 | 256 | 128 | 0.50 | Synergistic |
| BHKS159 | 1024 | 512 | 256 | 128 | 0.50 | Synergistic |
| Berberine | Luteolin | Berberine | Berberine | |||
| G27 | 1024 | 1024 | 512 | 512 | 1.00 | Addictive |
| 26695 | 1024 | 1024 | 512 | 256 | 0.75 | Addictive |
| BHKS159 | 1024 | 1024 | 512 | 512 | 1.00 | Addictive |
| Luteolin | Gallic acid | Luteolin | Gallic acid | |||
| G27 | 1024 | 1024 | 256 | 256 | 0.50 | Synergistic |
| 26695 | 1024 | 1024 | 256 | 256 | 0.50 | Synergistic |
| BHKS159 | 1024 | 1024 | 256 | 256 | 0.50 | Synergistic |
| Luteolin | Rosmarinic acid | Luteolin | Rosmarinic acid | |||
| G27 | 1024 | 1024 | 512 | 256 | 0.75 | Addictive |
| 26695 | 1024 | 1024 | 512 | 512 | 1.00 | Addictive |
| BHKS159 | 1024 | 1024 | 512 | 256 | 0.75 | Addictive |
| Catechuic acid | Quercetin | Catechuic acid | Quercetin | |||
| G27 | 1024 | 1024 | 512 | 256 | 0.75 | Addictive |
| 26695 | 1024 | 1024 | 512 | 256 | 0.75 | Addictive |
| BHKS159 | 1024 | 1024 | 512 | 256 | 0.75 | Addictive |
| Catechuic acid | Emodin | Catechuic acid | Emodin | |||
| G27 | 1024 | 512 | 512 | 256 | 1.00 | Addictive |
| 26695 | 1024 | 512 | 512 | 256 | 1.00 | Addictive |
| BHKS159 | 1024 | 512 | 256 | 256 | 0.75 | Addictive |
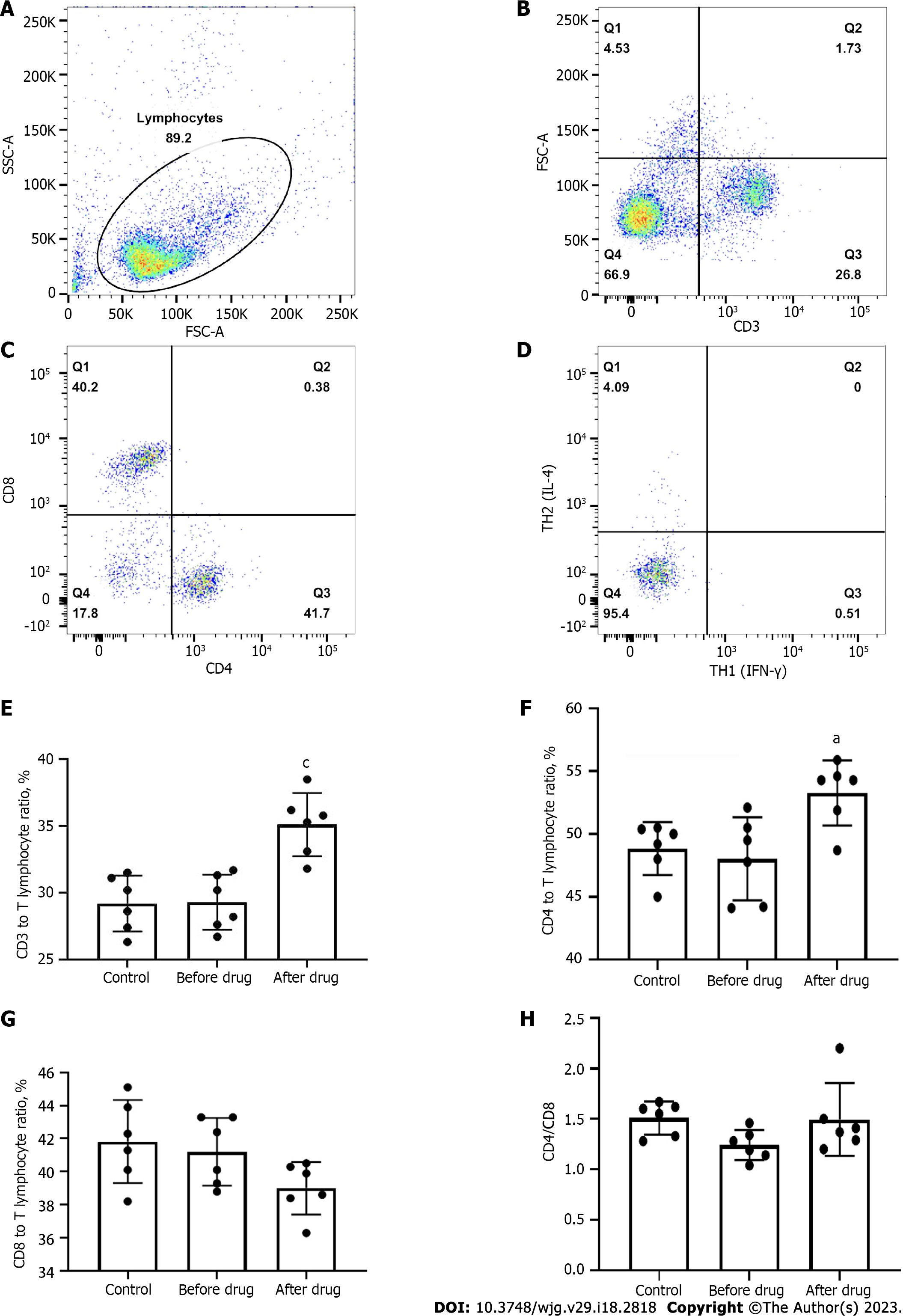
The t-test was used to analyze the proportion of CD3+ T, CD4+ T, and CD8+ T cells in total lymphocytes before and after administration. After administration, the proportion of CD3+ T and CD4+ T in total lymphocytes increased (P1 = 0.0009, P2 = 0.0115), as shown in Figure 2A-F; no significant difference was found between CD8+ T cells and TH1 cells (P3 = 0.1937, P4 = 0.8061), Figure 2A, C, D, and G, and the ratio of CD4+ T/CD8+ T cells was increased (P5 = 0.0280) as displayed in Figure 2H. These results indicated that BXXXT aqueous extract could improve the ratio of lymphocytes CD3+ T and CD4+ T to the total number of lymphocytes and the ratio of CD4+ T/CD8+ T cells. However, BXXXT aqueous extract could enhance immune functions, thus helping improve the immunity and antibacterial ability of the body, which might explain why BXXXT could produce better treatment effects in vivo.
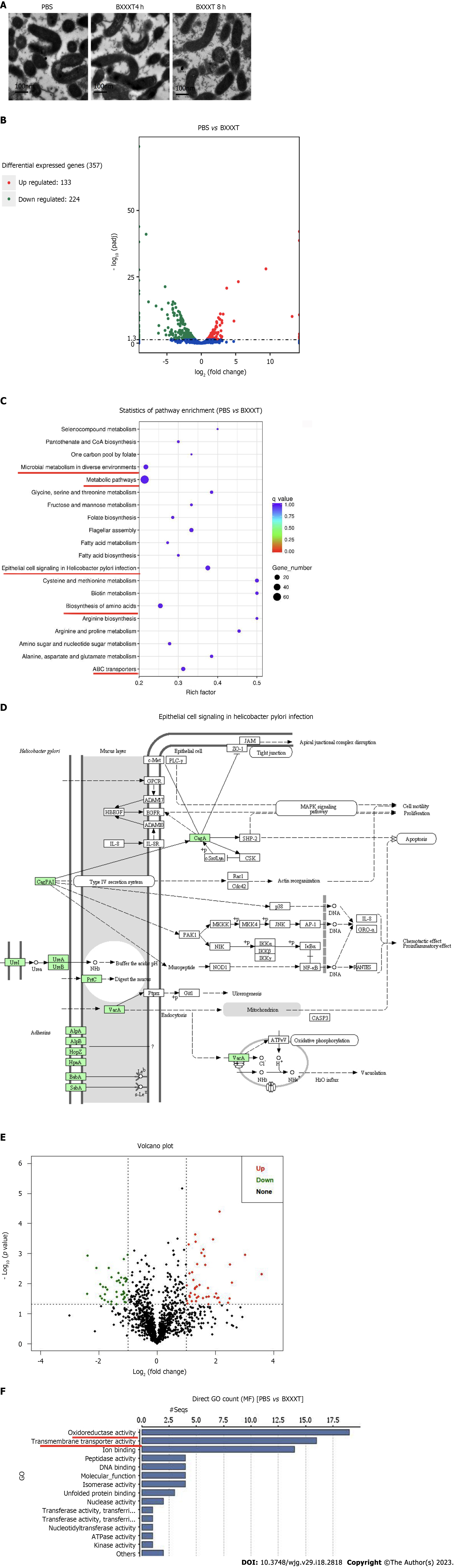
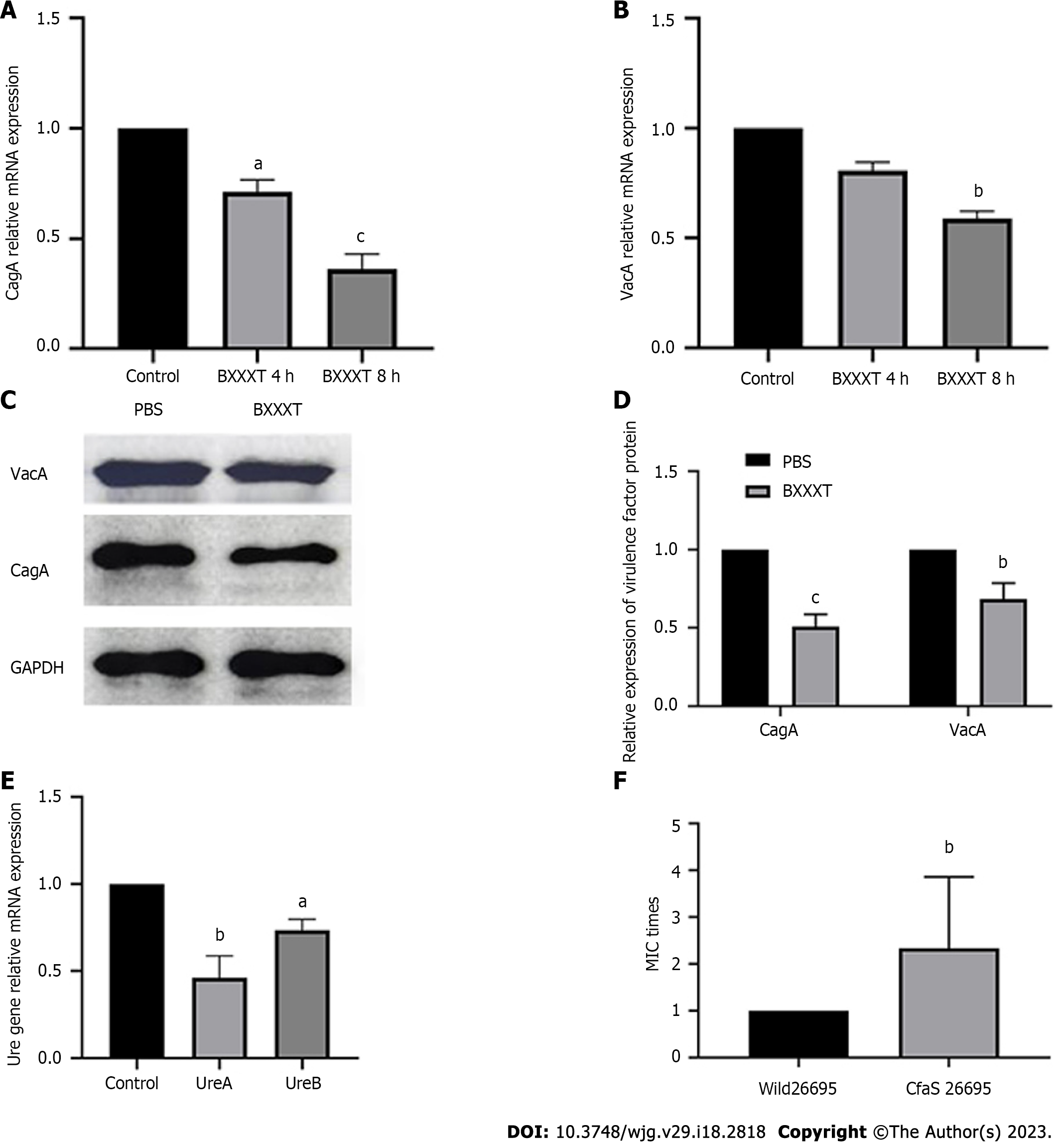
No significant changes were found at × 10000 magnification on the morphological structures of H. pylori after 4 h and 8 h of BXXXT treatment (Figure 3A), indicating that BXXXT did not produce antibacterial effects by directly destroying morphological structures. In the samples treated with BXXXT for 8 h, a total of 357 differentially expressed genes were detected after transcriptome analysis, among which 133 genes were up-regulated and 224 genes down-regulated (Figure 3B), mainly concentrating in five metabolic pathways including metabolic pathways, the epithelial cell signaling in H. pylori infection and the microbial metabolism in diverse environments (Figure 3C). The epithelial cell signaling in H. pylori infection pathway suggested that it is closely related to urease genes and virulence genes, as shown in Figure 3D. The proteome detection found 86 differentially expressed genes, among which 44 were up-regulated and 42 down-regulated (Figure 3E), mainly concentrating in oxidoreductases and transferases pathways (Figure 3F). Among the related genes and proteins found in transcriptome and proteome, respectively, and concentrating in the main pathways, five possible proteins of BXXXT were screened, among which four were urease-related and one was related to the virulence gene CagA (Table 7). The quantitative PCR (Q-PCR) and Western blot detection were performed to confirm the correlation between BXXXT action and virulence genes and urease genes. The results suggested that the mRNAs and protein expressions of CagA and VacA after BXXXT treatment were significantly decreased (Figure 4A-D), providing additional evidence for the obvious effects of BXXXT in vivo from the point that BXXXT could reduce virulence of H. pylori. The gene CFAs related to environmental regulation, urease and drug resistance was mutated (Figure 4E), after which the MIC of the mutant strains increased 2-4 times (Figure 4F), further proving that the urease-related gene CFAs might be one of the main targets of BXXXT. However, the decrease of urease could affect the adaptive regulation of stomach acid for which H. pylori’ s ability to colonize would be significantly reduced.
| Uniprot Accession Number | Protein name | Protein amino acid sequence | Pvalue | Reliability |
| E6NPU2 | Urease subunit alpha OS = Helicobacter pylori (strain F57), OX = 866346, GN = urea, PE = 3, SV = 1 | MKLTPKELDKLMLHYAGELARKRKEKGIKLNYVEAVALISAHIMEEARAGKKSAAEL | 0.001211988 | High |
| A0A2A6SFH9 | Urease (fragment) OS = Helicobacter pylori, OX = 210, GN = BB479_08100, PE = 4, SV = 1 | MKLTPKELDKLMLHYAGELARKRKEKGIKLNYVEAVALIXAHIMEEARAGKKTAAEL | 0.033815784 | High |
| A0A0L0QH58 | Urease subunit alpha OS = Helicobacter pylori, OX = 210, GN = urea, PE = 3, SV = 1 | MKLTPKELDKLMLHYAGELAKKRKEKGIKLNYVEAVALISAHIMEEARAGKKSAAEL | 0.033744196 | High |
| N4TND2 | Urease accessory protein UreG OS = Helicobacter pylori Hp A-11, OX = 992035, GN = ureG, PE = 3, SV = 1 | MVKIGVCGPVGSGKTALIEALTRHMSKDYDMAVITNDIYTKEDAEFMCKNSVMPRER | 0.016839824 | High |
| Q8RRP6 | Cytotoxin associated protein CagA (Fragment), OS = Helicobacter pylori, OX = 210, GN = cagA, PE = 4, SV = 1 | ALADLKNFSKEQLAQQAQKNESFNAGKKFEFSQSVRNGVNGTLVGNGFSQAEATTL | 0.03652953 | High |
There are about 4.4 billion people with H. pylori infections worldwide, with an average infection rate of 62.8%. Southeast Asia could be considered as high-incidence areas, mainly China, Japan, and South Korea[14,15]. The eradication of H. pylori has proven to prevent gastric cancer. However, the overuse of antibiotics leads to serious drug resistance. The drug resistance rate varies in different countries and regions and will change over time, indicated by those of clarithromycin, metronidazole, and levloxacin, all increasing over time. For example, the drug resistance rate of clarithromycin rose to 21% between 2012 and 2016[16-18]. Therefore, the failure rate of treating H. pylori infectious diseases is increasing, leading to an urgent need to study and develop anti-H. pylori drugs. In 2018, Hu et al[19] from Peking University proposed that non-antibiotic drugs such as traditional Chinese medicine, mucosal protective agents and probiotics could be used to treat H. pylori infection. Traditional Chinese medicine, including BXXXT, has a good effect on H. pylori infection and H. pylori-infection-related diseases[20,21]. However, its pharmacological mechanism remains unclear, and whether it has an effect on drug-resistant H. pylori infection or not has not been confirmed by animal experiments.
This study confirms that BXXXT has good therapeutic effects on drug-resistant H. pylori infection through in vivo and in vitro experiments in mice, which provides an experimental basis for elaborating that BXXXT could treat refractory gastritis caused by drug-resistant bacteria. While the efficacy of BXXXT is well established, explaining its mechanism is difficult. Traditional Chinese medicine, especially compound prescriptions, has complex components and a very complex mechanism of action in the body, which might be affected by multiple factors, especially those in stomach. Besides, it produces effects that are multi-target. As the content of the main components of the prescription is not high, the effects on the target may not always appear[22-24], which makes it difficult to elaborate on the mechanism of action. In the present study, the composition of BXXXT was analyzed, the effective anti-HP components were screened out with reference to the related literature and reports, the material basis of the efficacy was identified, and the synergistic effects among some of the effective components was verified. It was found that most of the components had additive or synergistic effects, such as berberine and emodin, luteolin and gallic acid. This indicated that though only accounting for a small portion, the active components of the Chinese medicine prescription, which could produce synergistic or additive effects demonstrated better antibacterial effects. The MIC of BXXXT against H. pylori is 256-512 μg/mL, much worse than that of clinical antibiotics but producing better therapeutical effects in vivo, especially for drug-resistant H. pylori, why?
H. pylori can adhere to the gastrointestinal mucosa, produce virulence factors, damage gastric epithelial cells, and induce, control and regulate inflammatory responses. CagA encodes variety of proteins, including CagA and VacA, ect[25]. Karbalaei et al[26] found that CagA and VacA genes were potentially associated with resistance to clarithromycin, metronidazole, amoxicillin, tetracycline and levofloxacin. The VacA can not only destroy mitochondria[27,28], but also reduce the proliferation of T cells, B cells, and the other immune cells, and affect the immune response[29-31]. Among the components of BXXXT, Gingerol, a crude extract containing gingerol, is able to can inhibit the growth of H. pylori strains (MIC range is 0.78 μg/mL to 12.5 μg/mL) and has significant activity against CagA+ strains[32]. Kaempferol is able to reduce the transcription of subunit protein A by the type IV secretory system, reduce the expression of pro-inflammatory cytokines (TNF-α, IL-1β) and IL-8 production in cells[33]. Urease can increase the pH value of the stomach and provide a suitable environment for H. pylori colonization. Palmatine in Coptis coptitis can act on sulfhydryl at the active site of urease and inhibit the conformational change of the urease molecules, and reduce urease activity[34]. Hesperidin can reduce the expression of UreA and UreB[35].
In the present paper, the immunomodulatory effect of BXXXT was analyzed, and it was found that it could up-regulate the expressions of immune factors such as CD4+ T and enhance immunity and the ability of sterilization; the main target of BXXXT was urease-related gene CFAs, which was related to the virulence factors CagA and VacA. When urease was destroyed, H. pylori cells could not survive in the gastric acid environment, and its colonization ability would be significantly weakened. With low expressions of H. pylori virulence factors, the inflammatory damage caused by H. pylori to gastric mucosa would be reduced. The effects of these three aspects could preliminarily explain why BXXXT has good effects in vivo. However, clarifying the action mechanism of BXXXT is very complicated and difficult, for there are many components of BXXXT, among which many are anti-H. pylori, the transcriptome and proteome analyses in this study also suggested that many membrane transporter genes were involved, such as ABC transporter, etc. Besides, we also found that berberine and other components of BXXXT could inhibit HefA gene to reverse drug resistance[25] and CFAs in this study might also be related to drug resistance[36,37]. Therefore, the action mechanism of BXXXT will be further studied.
BXXXT aqueous extract could demonstrate good therapeutic effects on drug-resistance H. pylori in vitro and in vivo and its mechanism comes down to the synergistic or additional antibacterial effects of berberine, emodin and luteolin, the main components of the extract; the extract could activate the immune function and enhance bactericidal effects; BXXXT aqueous extract, with main targets of BXXXT aqueous extract related to urease, virulence factors, etc., could reduce the urease and virulence of H. pylori, weaken its colonization, and reduce its inflammatory damage to the gastric mucosa.
Helicobacter pylori (H. pylori)’ s drug resistance brings challenges to the current clinical work. In China, traditional Chinese medicine could be used to treat a variety of refractory diseases, reducing drug-resistance, and improving the eradication rate of H. pylori. The study is to explore the therapeutic effects of Banxia Xiexin Decoction (BXXXT) on drug-resistant H. pylori.
H. pylori’ s drug resistance brings challenges to the current clinical work. The study is to explore the therapeutic effects of BXXXT on drug-resistant H. pylori, avoid, overcome H. pylori’ s drug resistance.
To confirm that BXXXT demonstrates therapeutical effects in vivo and in vitro on gastritis mice with drug-resistant H. pylori and explain its mechanism.
The aqueous extract of BXXXT was gained by water decocting method. The inhibitory effect of the aqueous extract on H. pylori was detected by dilution in vitro. In terms of mechanism exploration, the main medicinal compositions of BXXXT aqueous extract and the synergistic bacteriostatic effects they had demonstrated were analyzed using mass spectrometry; the immune function of peripheral blood cells such as CD3+ T and CD4+ T of mice were detected using a flow cytometry; the H. pylori transcriptome and proteome were detected. Differently expressed genes were screened and verification was performed thereon with knockout expression.
BXXXT aqueous extract against H. pylori was better therapeutical effects in vivo and in vitro; BXXXT aqueous extract was found to stimulate the expressions of CD3+ T and CD4+ T and increase the number of CD4+ T/CD8+ T in gastritis mice; the detection of transcriptome and proteome, quantitative polymerase chain reaction, Western blot and knockout verification revealed that the main targets of BXXXT aqueous extract are CFAs related to urea enzymes, and CagA, VacA, etc.
BXXXT aqueous extract could demonstrate good therapeutic effects on drug-resistance H. pylori in vitro and in vivo and its mechanism is related to reduce the urease and virulence of H. pylori, weaken its colonization, and reduce its inflammatory damage to the gastric mucosa, etc.
BXXXT aqueous extract has good therapeutic effects on drug-resistance H. pylori, can overcome H. pylori’s drug resistance.
Thank Professor Bi Hongkai of Nanjing Medical University for the gift of Helicobacter pylori strain.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Biochemistry and molecular biology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lee SC, Korea; Razek AAKA, Japan; Sakamoto S, Egypt S-Editor: Chen YL L-Editor: A P-Editor: Zhang XD
| 1. | Sultan S, Ahmed SI, Murad S, Irfan SM. Primary versus secondary immune thrombocytopenia in adults; a comparative analysis of clinical and laboratory attributes in newly diagnosed patients in Southern Pakistan. Med J Malaysia. 2016;71:269-274. [PubMed] [Cited in This Article: ] |
| 2. | Nagy P, Johansson S, Molloy-Bland M. Systematic review of time trends in the prevalence of Helicobacter pylori infection in China and the USA. Gut Pathog. 2016;8:8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 140] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 3. | Du JY, Pan J, Zhou QJ, Tang QQ, Yang NM, Zhang JZ. The Helicobacter Pylori Antibiotic Resistance in Wenzhou, Zhejiang from 2013 to 2020. Zhongguo Quanke Yixue. 2023;26:825-829. [DOI] [Cited in This Article: ] |
| 4. | Li RJ, Dai YY, Qin C, Huang GR, Qin YC, Huang YY, Huang ZS, Luo XK, Huang YQ. Application of traditional Chinese medicine in treatment of Helicobacter pylori infection. World J Clin Cases. 2021;9:10781-10791. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 9] [Cited by in F6Publishing: 9] [Article Influence: 3.0] [Reference Citation Analysis (2)] |
| 5. | Yang M, Chen J, Xu L, Shi X, Zhou X, An R, Wang X. A Network Pharmacology Approach to Uncover the Molecular Mechanisms of Herbal Formula Ban-Xia-Xie-Xin-Tang. Evid Based Complement Alternat Med. 2018;2018:4050714. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Li YT, You J, Song Y, Wang YJ, Wang HB. Meta-analysis of Banxia Xiexin Decoction in the treatment of chronic atrophic gastritis associated with Helicobacter pylori. Beijing Zhongyiyao. 2021;40:1143-1148. [DOI] [Cited in This Article: ] |
| 7. | Jong MS, Hwang SJ, Chen YC, Chen TJ, Chen FJ, Chen FP. Prescriptions of Chinese herbal medicine for constipation under the national health insurance in Taiwan. J Chin Med Assoc. 2010;73:375-383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Jhang JS, Livneh H, Yang SY, Huang HJ, Chan MWY, Lu MC, Yeh CC, Tsai TY. Decreased risk of colorectal cancer among patients with type 2 diabetes receiving Chinese herbal medicine: a population-based cohort study. BMJ Open Diabetes Res Care. 2020;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Murai T, Matsuo M, Tanaka H, Manabe Y, Takaoka T, Hachiya K, Yamaguchi T, Otsuka S, Shibamoto Y. Efficacy of herbal medicine TJ-14 for acute radiation-induced enteritis: a multi-institutional prospective Phase II trial. J Radiat Res. 2020;61:140-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Huang CY, Lai WY, Sun MF, Lin CC, Chen BC, Lin HJ, Chang CM, Yang CH, Huang KC, Yen HR. Prescription patterns of traditional Chinese medicine for peptic ulcer disease in Taiwan: A nationwide population-based study. J Ethnopharmacol. 2015;176:311-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Huang YP, Zhan DF, Huang H, Chen SF. The effect of Banxia Xiexin Decoction human serum containing drugs on TGF of HP-infected GES-1 cells- β/ Influence of Smad signal pathway. Zhongguo Shiyan Fangjixue Zazhi. 2018;14:91-96. [DOI] [Cited in This Article: ] |
| 12. | Ye ZM, Zhang H. Clinical effect observation of modified Banxia Xiexin Decoction on Hp-related chronic gastritis. Linchuang Yixue Gongcheng. 2022;29:1515-1516. [DOI] [Cited in This Article: ] |
| 13. | Liu XD. Clinical efficacy observation of Banxia Xiexin Decoction on patients with gastric ulcer. Zhongguo Yiyao Zhinan. 2021;19:97-99. [DOI] [Cited in This Article: ] |
| 14. | Jiang X, Duan Y, Zhou B, Guo Q, Wang H, Hang X, Zeng L, Jia J, Bi H. The Cyclopropane Fatty Acid Synthase Mediates Antibiotic Resistance and Gastric Colonization of Helicobacter pylori. J Bacteriol. 2019;201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Camorlinga-Ponce M, Gómez-Delgado A, Aguilar-Zamora E, Torres RC, Giono-Cerezo S, Escobar-Ogaz A, Torres J. Phenotypic and Genotypic Antibiotic Resistance Patterns in Helicobacter pylori Strains From Ethnically Diverse Population in México. Front Cell Infect Microbiol. 2020;10:539115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E. Prevalence of Antibiotic Resistance in Helicobacter pylori: A Systematic Review and Meta-analysis in World Health Organization Regions. Gastroenterology. 2018;155:1372-1382.e17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 617] [Cited by in F6Publishing: 657] [Article Influence: 109.5] [Reference Citation Analysis (0)] |
| 17. | Loffeld RJ, Werdmuller BF. Changes in Antibiotic Susceptibility of Helicobacter pylori in the Course of Eight Years in the Zaanstreek Region in The Netherlands. Gastroenterol Res Pract. 2013;2013:625937. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Liu DS, Wang YH, Zeng ZR, Zhang ZY, Lu H, Xu JM, Du YQ, Li Y, Wang JB, Xu SP, Chen Y, Lan CH, Cheng H, Jiang MD, Zhang LX, Huo LJ, Chen SY, Zhang GX, Wu KC, Zhu X, Chen YX, Zhu Y, Shu X, Xie Y, Lu NH. Primary antibiotic resistance of Helicobacter pylori in Chinese patients: a multiregion prospective 7-year study. Clin Microbiol Infect. 2018;24:780.e5-780.e8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 19. | Hu FL, Zhang SS. National consensus on integrated treatment of "disease syndrome" related to Helicobacter pylori. Linchuang Gandanbing Zazhi. 2018;27:1008-1016. [DOI] [Cited in This Article: ] |
| 20. | Wei P. Clinical effect of modified Banxia Xiexin Decoction on Helicobacter pylori positive peptic ulcer and its influence on inflammatory factors and gastrointestinal hormones. Linchuang Heliyongyao Zazhi. 2022;15:73-76. [DOI] [Cited in This Article: ] |
| 21. | Liu Yan, Ji XQ, Kong LJ, Gao ZH, Xiong X, Zhao YT, Wu JY. Study on the influence of different processing methods on the chemical composition and product quality of Scutellaria baicalensis. Shi Zhen Guoyi Guoyao. 2019;3:605-608. [DOI] [Cited in This Article: ] |
| 22. | Zhong JB, Wang J, Wang YY. The complexity of prescriptions and the development of new drugs of traditional Chinese medicine. Zhongguo Zhongyiyao Zazhi. 2002;5:321-323. [DOI] [Cited in This Article: ] |
| 23. | Zhu SN. The complexity of the material basis of efficacy. Shandong Zhongyiyao Daxue Xuebao. 2008;4:267-269. [DOI] [Cited in This Article: ] |
| 24. | Jin Y, Lu H, Ge JH, Wang YY, Yu S, Bian HS, Huang LL. Progress in the research on the material basis of Viola yedoensis. Zhonghua Zhongyiyao Xuekan. 2022;40:21-26. [DOI] [Cited in This Article: ] |
| 25. | Huang YQ, Huang GR, Wu MH, Tang HY, Huang ZS, Zhou XH, Yu WQ, Su JW, Mo XQ, Chen BP, Zhao LJ, Huang XF, Wei HY, Wei LD. Inhibitory effects of emodin, baicalin, schizandrin and berberine on hefA gene: treatment of Helicobacter pylori-induced multidrug resistance. World J Gastroenterol. 2015;21:4225-4231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 58] [Cited by in F6Publishing: 50] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 26. | Karbalaei M, Talebi Bezmin Abadi A, Keikha M. Clinical relevance of the cagA and vacA s1m1 status and antibiotic resistance in Helicobacter pylori: a systematic review and meta-analysis. BMC Infect Dis. 2022;22:573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 27. | Sharndama HC, Mba IE. Helicobacter pylori: an up-to-date overview on the virulence and pathogenesis mechanisms. Braz J Microbiol. 2022;53:33-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 57] [Article Influence: 28.5] [Reference Citation Analysis (1)] |
| 28. | McClain MS, Beckett AC, Cover TL. Helicobacter pylori Vacuolating Toxin and Gastric Cancer. Toxins (Basel). 2017;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 29. | Djekic A, Müller A. The Immunomodulator VacA Promotes Immune Tolerance and Persistent Helicobacter pylori Infection through Its Activities on T-Cells and Antigen-Presenting Cells. Toxins (Basel). 2016;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | Reshetnyak VI, Burmistrov AI, Maev IV. Helicobacter pylori: Commensal, symbiont or pathogen? World J Gastroenterol. 2021;27:545-560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 45] [Cited by in F6Publishing: 34] [Article Influence: 11.3] [Reference Citation Analysis (2)] |
| 31. | Altobelli A, Bauer M, Velez K, Cover TL, Müller A. Helicobacter pylori VacA Targets Myeloid Cells in the Gastric Lamina Propria To Promote Peripherally Induced Regulatory T-Cell Differentiation and Persistent Infection. mBio. 2019;10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 32. | Mahady GB, Pendland SL, Yun GS, Lu ZZ, Stoia A. Ginger (Zingiber officinale Roscoe) and the gingerols inhibit the growth of Cag A+ strains of Helicobacter pylori. Anticancer Res. 2003;23:3699-3702. [PubMed] [Cited in This Article: ] |
| 33. | Yeon MJ, Lee MH, Kim DH, Yang JY, Woo HJ, Kwon HJ, Moon C, Kim SH, Kim JB. Anti-inflammatory effects of Kaempferol on Helicobacter pylori-induced inflammation. Biosci Biotechnol Biochem. 2019;83:166-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 34. | Lee JW, Mase N, Yonezawa T, Seo HJ, Jeon WB, Cha BY, Nagai K, Woo JT. Palmatine attenuates osteoclast differentiation and function through inhibition of receptor activator of nuclear factor-κb ligand expression in osteoblast cells. Biol Pharm Bull. 2010;33:1733-1739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Kim HW, Woo HJ, Yang JY, Kim JB, Kim SH. Hesperetin Inhibits Expression of Virulence Factors and Growth of Helicobacter pylori. Int J Mol Sci. 2021;22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 36. | Barkan D, Liu Z, Sacchettini JC, Glickman MS. Mycolic acid cyclopropanation is essential for viability, drug resistance, and cell wall integrity of Mycobacterium tuberculosis. Chem Biol. 2009;16:499-509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 37. | Chen YY, Gänzle MG. Influence of cyclopropane fatty acids on heat, high pressure, acid and oxidative resistance in Escherichia coli. Int J Food Microbiol. 2016;222:16-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |