Published online May 28, 2022. doi: 10.3748/wjg.v28.i20.2214
Peer-review started: September 30, 2021
First decision: March 11, 2022
Revised: March 25, 2022
Accepted: April 21, 2022
Article in press: April 21, 2022
Published online: May 28, 2022
Processing time: 239 Days and 3.3 Hours
Direct acting antiviral (DAA) therapy has enabled hepatitis C virus infection to become curable, while histological changes remain uncontained. Few valid non-invasive methods can be confirmed for use in surveillance. Gadolinium-ethoxybenzyl-diethylenetriamine penta-acetic acid (Gd-EOB-DTPA) is a liver-specific magnetic resonance imaging (MRI) contrast, related to liver function in the hepatobiliary phase (HBP). Whether Gd-EOB-DTPA-enhanced MRI can be used in the diagnosis and follow up of hepatic fibrosis in patients with chronic hepatitis C (CHC) has not been investigated.
To investigate the diagnostic and follow-up values of Gd-EOB-DTPA-enhanced MRI for hepatic histology in patients with CHC.
Patients with CHC were invited to undergo Gd-EOB-DTPA-enhanced MRI and liver biopsy before treatment, and those with paired qualified MRI and liver biopsy specimens were included. Transient elastography (TE) and blood tests were also arranged. Patients treated with DAAs who achieved 24-wk sustained virological response (SVR) underwent Gd-EOB-DTPA-enhanced MRI and liver biopsy again. The signal intensity (SI) of the liver and muscle were measured in the unenhanced phase (UEP) (SIUEP-liver, SIUEP-muscle) and HBP (SIHBP-liver, SIHBP-muscle) via MRI. The contrast enhancement index (CEI) was calculated as [(SIHBP-liver/SIHBP-muscle)]/[(SIUEP-liver/SIUEP-muscle)]. Liver stiffness measurement (LSM) was confirmed with TE. Serologic markers, aspartate aminotransferase-to-platelet ratio index (APRI) and Fibrosis-4 (FIB-4), were also calculated according to blood tests. The grade of inflammation and stage of fibrosis were evaluated with the modified histology activity index (mHAI) and Ishak fibrosis score, respectively. Fibrosis regression was defined as a ≥ 1-point decrease in the Ishak fibrosis score. The correlation between the CEI and liver pathology was evaluated. The diagnostic and follow-up values of the CEI, LSM, and serologic markers were compared.
Thirty-nine patients with CHC were enrolled [average age, 42.3 ± 14.4 years; 20/39 (51.3%) male]. Twenty-one enrolled patients had eligible paired Gd-EOB-DTPA-enhanced MRI and liver tissues after achieving SVR. The mHAI median significantly decreased after SVR [baseline 6.0 (4.5-13.5) vs SVR 2.0 (1.5-5.5), Z = 3.322, P = 0.017], but the median stage of fibrosis did not notably change (P > 0.05). Sixty pairs of qualified MRI and liver tissue samples were available for use to analyze the relationship between the CEI and hepatic pathology. The CEI was negatively correlated with the mHAI (r = -0.56, P < 0.001) and Ishak score (r = -0.69, P < 0.001). Further stratified analysis showed that the value of the CEI decreased with the progression of the stage of fibrosis rather than with the grade of necroinflammation. For patients with Ishak score ≥ 5, the areas under receiver operating characteristics curve of the CEI, LSM, APRI, and FIB-4 were approximately at baseline, 0.87–0.93, and after achieving SVR, 0.83–0.91. The CEI cut-off value was stable (baseline 1.58 and SVR 1.59), but those of the APRI (from 1.05 to 0.24), FIB-4 (from 1.78 to 1.28), and LSM (from 10.8 kpa to 7.1 kpa) decreased dramatically. The APRI and FIB-4 cannot be used as diagnostic means for SVR in patients with Ishak score ≥ 3 (P > 0.05). Seven patients achieved fibrosis regression after achieving SVR. In these patients, the CEI median increased (from 1.71 to 1.83, Z = -1.981, P = 0.048) and those of the APRI (from 1.71 to 1.83, Z = -2.878, P = 0.004) and LSM (from 6.6 to 4.8, Z = -2.366, P = 0.018) decreased. However, in patients without fibrosis regression, the medians of the APRI, FIB-4, and LSM also changed significantly (P < 0.05).
Gd-EOB-DTPA-enhanced MRI has good diagnostic value for staging fibrosis in patients with CHC. It can be used for fibrotic-change monitoring post SVR in patients with CHC treated with DAAs.
Core Tip: In this prospective, comparative study, the correlation between the contrast enhancement index (CEI) in the hepatobiliary phase of gadolinium-ethoxybenzyl-diethylenetriamine penta-acetic acid enhanced magnetic resonance imaging and liver pathology measures was analyzed in patients with chronic hepatitis C. It was determined that the CEI has good diagnostic performance and is more useful than serological markers and transient elastography for hepatic-fibrosis monitoring in patients achieving sustained virological response.
- Citation: Li XH, Huang R, Yang M, Wang J, Gao YH, Jin Q, Ma DL, Wei L, Rao HY. Gadolinium-ethoxybenzyl-diethylenetriamine penta-acetic acid-enhanced magnetic resonance imaging for evaluating fibrosis regression in chronic hepatitis C patients after direct-acting antiviral. World J Gastroenterol 2022; 28(20): 2214-2226
- URL: https://www.wjgnet.com/1007-9327/full/v28/i20/2214.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i20.2214
Chronic hepatitis C (CHC) remains one of the major etiologies of chronic liver disease, causing substantial morbidity and mortality globally[1,2]. Remarkably well-tolerated, effective, and short-time direct acting antiviral (DAA) regimens have revolutionized therapy for hepatitis C virus (HCV) infection, achieving a high rate of sustained virological response (SVR). However, it has also been reported that despite achieving SVR, patients may still experience disease progression[3,4]. It is critical to follow up pathological changes in the liver after DAA therapy. A growing number of studies have focused on the use of non-invasive tests to substitute liver biopsy[5-7]. However, there are few validated thresholds for longitudinal assessment that correspond to histologic changes in fibrosis, and there is no definite non-invasive method for diagnosing the stage changes in fibrosis after SVR.
Gadolinium-ethoxybenzyl-diethylenetriamine penta-acetic acid (Gd-EOB-DTPA; Promovist® in Europe and Eovist® in the United States, Bayer Healthcare, Berlin, Germany) is a liver-specific magnetic resonance imaging (MRI) contrast agent[8]. The hepatocyte uptake and biliary excretion of Gd-EOB-DTPA mainly take place via organic anion transporting polypeptides OATP1B1/3 on the sinusoidal membrane and multidrug resistance-associated proteins MRP2 on the canalicular membrane. Therefore, after intravenous injection, Gd-EOB-DTPA shows extracellular distribution in the dynamic phase and hepatocyte-specific transportation in the hepatobiliary phase (HBP). Decrease in the number of normal hepatocytes and impaired hepatocyte function may reduce the hepatic enhancement of the HBP[9]. It has been reported using Gd-EOB-DTPA-enhanced MRI to identify hepatocellular carcinoma[10], evaluate liver function[11,12], stage liver fibrosis[13,14], and predict liver failure after hepatectomy[15].
To the best of our knowledge, no studies have used Gd-EOB-DTPA-enhanced MRI to evaluate changes in fibrosis after SVR with paired liver biopsy. The primary aim of our study was to evaluate the diagnostic performance of Gd-EOB-DTPA-enhanced MRI in staging liver fibrosis. The secondary aim was to confirm whether this diagnostic test can be used for longitudinal assessment of liver fibrosis in patients with CHC after achieving SVR treated by DAA regimens.
Patients with chronic HCV infection treated with DAAs in our hepatology center between January 2014 and December 2016 were prospectively invited to undergo Gd-EOB-DTPA-enhanced MRI and liver biopsy. Patients with qualified Gd-EOB-DTPA-enhanced MRI data and liver biopsy samples were enrolled. The selection of DAA regimens was based on the genotype and state of liver disease. The patients who achieved SVR after DAA therapy underwent the examinations a second time. Exclusion criterias were: (1) Non-HCV etiology-related chronic liver disease (such as chronic hepatitis B, drug-or alcohol-related liver disease, non-alcoholic steatohepatitis, etc.); (2) Clinical hepatic decompensation; (3) Solid organ transplantation; (4) Malignancy; (5) Combined with other systemic disease (immune system disease, blood system disease, etc.) and (6) Contraindications for MRI and liver biopsy. SVR was defined as undetectable HCV RNA 24 wk after the end of treatment.
Liver biopsy was performed within 1 mo before treatment and 3 mo after SVR. MRI was performed prior to liver biopsy within 1 mo. MRI with marked motion artifacts and specimens of liver tissue with lengths < 10 mm or < six portal areas under the microscope were regarded as inappropriate[16]. Demographic information, virologic data, and laboratory findings were collected.
All liver MRI examinations were performed with a Discover 750 3.0 T MR scanner (GE Healthcare, Milwaukee, WI, United States) using a 32-channel torso array coil. Patients were in the supine position during horizontal axis scanning and had received prior training on how to breathe. Gd-EOB-DTPA (Promovist®, Bayer Healthcare) was injected intravenously as a contrast agent at a dose of 0.1 mL/kg body weight with a flow rate of 2 mL/s, followed by a 20-mL 0.09% NaCl flush. Three-dimensional T1-weighted contrast-enhanced MRI was conducted in the unenhanced phase (UEP), arterial phase (AP, 25 s), portal phase (60 s), and HBP (20 min).
The signal intensities (SIs) of the liver parenchyma and paraspinal muscle in the UEP (SIUEP-liver and SIUEP-muscle) and HBP (SIHBP-liver and SIHBP-muscle) were independently measured by two professional radiologists with nearly 4 and 6 years of experience in interpreting abdominal MRIs, using regions of interests (ROIs). The radiologists were blinded to the patients’ clinical data and pathological changes.
Four ROIs were manually circled (size: 100–150 mm2) in the posterior and anterior segments of the right hepatic lobe and inner and lateral segments of the left hepatic lobe at the hepatic hilar level. The location of the ROIs had to be at the center of each segment, far from the abdominal wall, and avoiding visible blood vessels, bile ducts, and lesions. The ROI of the paraspinal muscle was mainly circled on the left side.
The contrast enhancement index (CEI) was calculated using the following formula[17]:
CEI = (SIHBP-liver/SIHBP-muscle)/( SIUEP-liver/SIUEP-muscle)
The change rate in the CEI (ΔCEI%) between pre-treatment (CEIpre) and post-SVR (CEIpost) was calculated as (CEIpost/CEIpre × 100%).
Specimens were fixed in formalin immediately after liver biopsy and embedded in paraffin; 4-μm-thick sections were cut and stained with hematoxylin and eosin and Masson’s trichrome. Two professional hepato-pathologists, both with nearly 30 years of working experience, assessed the degree and stage of necroinflammation and fibrosis of the specimens using the Ishak scoring system [modified histological activity index (mHAI) and Ishak score][18]. The pathologists were blinded to the imaging results and patient clinical data. The stages of liver fibrosis were divided into three groups according to the Ishak score, 0-2, 3-4, and 5-6, respectively. Necro-inflammatory severity was graded according to the mHAI score as 0-4, 5-8, 9-12, and 13-18[19]. Fibrosis regression was defined as a decrease of at least one point in the Ishak score after SVR[20].
Experienced technicians conducted liver stiffness measurements (LSMs) with FibroScan (Echosens, Paris, France). An effective result should have a successful rate > 60% and interquartile range/median ratio ≤ 30%.
With respect to serologic biomarkers of fibrosis, the aspartate aminotransferase (AST)-to-platelet ratio (APRI) and Fibrosis-4 (FIB-4) were calculated as following:
APRI = [(AST/ULN)/platelet count (× 109/L)] × 100[21]; FIB-4 = (age × AST)/[(platelet count) (× 109/L) × ALT1/2][22]
The change rates in APRI, FIB-4, and LSM between pre-SVR (valuepre) and post-SVR (valuepost) were calculated similarly with the CEI calculation.
Descriptive analyses were performed for sociodemographic characteristics (age, sex), clinical data [alanine aminotransferase (ALT), AST, total bilirubin (TB), albumin (Alb), platelet (PLT) count, HCV RNA, genotype, FIB-4, APRI, LSM, and CEI], histological grading, and staging.
Descriptive analyses were performed for sociodemographic characteristics, clinical data, histological characteristics, and the CEI. The value of HCV RNA was logarithmically transformed with 10 as the base. Continuous variables are expressed as median (interquartile range) (ALT, AST, TB, PLT, APRI, FIB-4, and LSM) or mean ± SD [age, Alb, international normalized ratio (INR), HCV RNA, mHAI, and CEI]. Categorical variables (sex, numbers of patients in the different mHAI and Ishak score groups) are presented as counts and percentages. The absolute values of the interclass correlation coefficients (ICC) were measured for SIs to confirm the interobserver reliability between reviewers. Student’s t-test (age, Alb, INR, HCV RNA, mHAI, and CEI) and Mann-Whitney U test (ALT, AST, TB, and PLT) were used to compare continuous variables, and the chi-square test was used for classified variables (sex, HCV genotype). The comparison between pre-and post-SVR was performed with the Wilcoxon sign rank test (ALT, AST, TB, PLT, FIB-4, APRI, and LSM) and paired sample t-test (Alb, INR, and CEI). Spearman’s correlation coefficient was calculated for the CEI, mHAI, and Ishak score. The predictive value of the CEI, APRI, FIB-4, and LSM for liver fibrosis was assessed using the area under the receiver operating characteristic curve (AUROC). Sensitivity, specificity, positive predictive value, and negative predictive value were also calculated. An optimal cut-off value was chosen to maximize the Youden index, which is defined as (sensitivity + specificity-1). P values < 0.05 were considered statistically significant. Statistical analyses were performed using IBM SPSS version 26.0 (IBM Corp., Armonk, NY, United States).
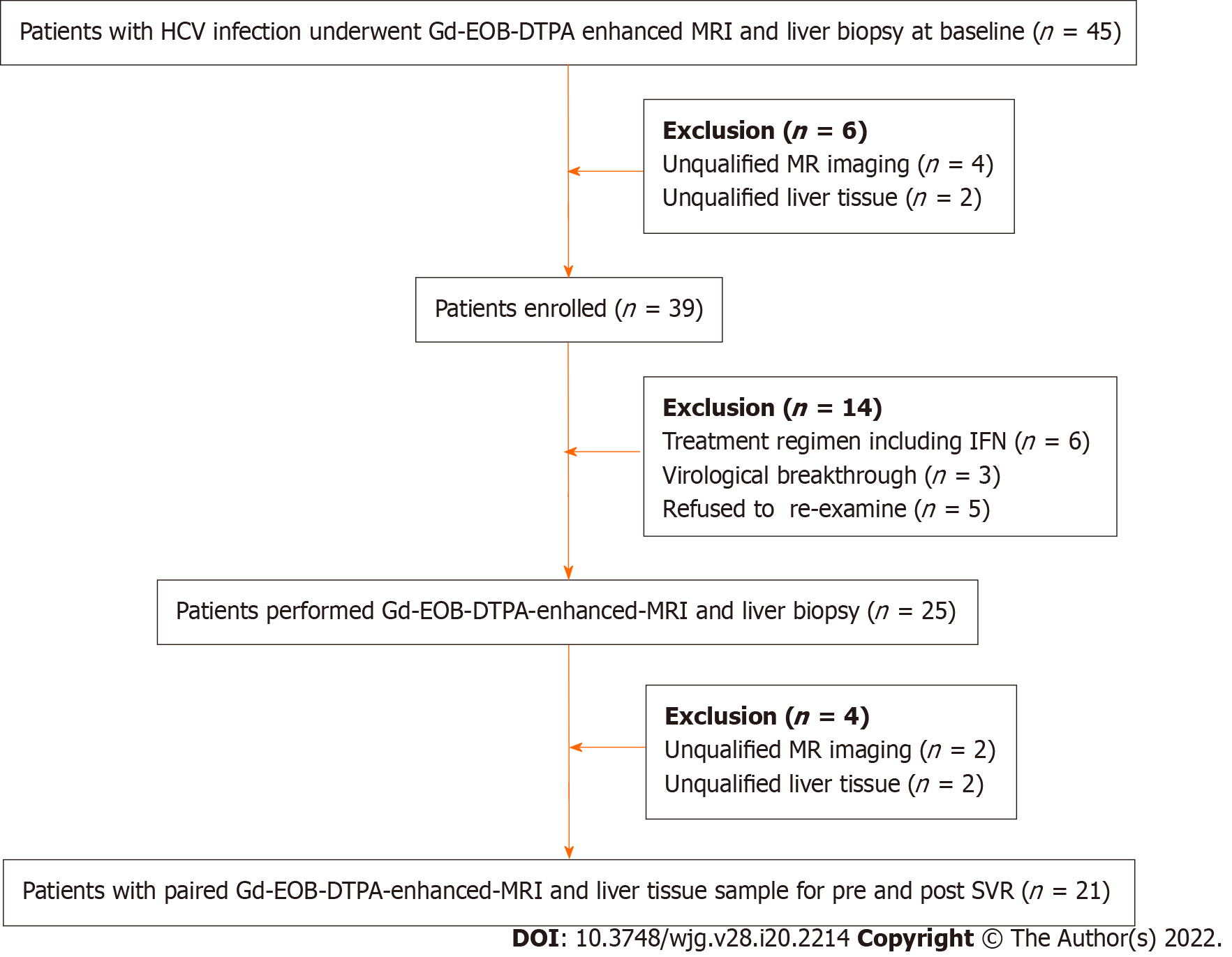
Forty-five patients underwent Gd-EOB-DTPA-enhanced MRI and liver biopsy at baseline and six with unqualified samples were excluded. Among the enrolled patients (n = 39), six were recommended to receive treatment with DAA plus Peg-IFN, and three had virological breakthrough. Twenty-five patients underwent MRI and liver biopsy again after achieving SVR, and 21 pairs of data were eligible. Therefore, there were 60 qualified and pairable MRI images and liver tissue samples available for analyzing correlation between CEI and liver pathology. The study flow chart is shown in Figure 1.
Two radiologists interpreted the Gd-EOB-DTPA-enhanced MRI images. The ICC for the measured SI values were excellent (greater than 0.9) for before and after achieving SVR. The ICC of the CEI was 0.729 (0.481, 0.858) and 0.886 (0.744, 0.952) pre and post SVR, respectively. Details of the inter-observer agreements of the ROI measurements and CEI are presented in Supplementary Table 1.
The 39 patients enrolled had a mean age of (42.3 ± 14.4) years, mean HCV RNA of (6.4 ± 0.7) log10 IU/mL, and 20 (51.3%) were male. The distribution of HCV genotypes (GT) was 1, 2, 3, and 6 in 19 (48.7%), 4 (10.3%), 13 (33.3%), and 3 (7.7%) patients, respectively. Patients with Ishak score of 5-6 were elder, had higher ALT and lower PLT levels than those with Ishak scores of 0–2 (P < 0.05) (Table 1).
| Parameters | Ishak 0-2 (n = 9) | Ishak 3-4 (n = 18) | Ishak 5-6 (n = 12) |
| Age (yr) | 36.3 ± 15.5 | 37.9 ± 12.4 | 53.1 ± 11.0b |
| Male, n (%) | 2 (22.2) | 11 (61.1) | 7 (58.3) |
| ALT (U/L) | 44 (22.5) | 52.5 (35.5) | 75.5 (98.7)a |
| TB (μmol/L) | 11 (7.5) | 14 (3.5) | 12.5 (7.5) |
| Alb (g/L) | 46.78 ± 3.77 | 45.72 ± 3.75 | 44.17 ± 3.35 |
| INR | 0.99 ± 0.07 | 1.00 ± 0.09 | 1.09 ± 0.16 |
| PLT (× 109/L) | 171 (62.5) | 177.5 (64.7) | 114 (67.5)b |
| HCV RNA (log10 IU/mL) | 6.43 ± 0.96 | 6.50 ± 0.71 | 6.33 ± 0.64 |
| mHAI score | 3.4 ± 1.7 | 5.3 ± 1.6a | 11.3 ± 4.0c |
Of the 21 patients who achieved SVR and had paired MRI and liver tissue samples, 13 (62%) received sofosbuvir/ribavirin therapy, and the other eight (38%) received daclatasvir/asunaprevir regimens. As expected, the values of ALT, AST (P < 0.001), and necroinflammation grade (P = 0.023) significantly decreased post SVR (Table 2). No significant change in fibrosis stage was observed. Among the noninvasive measurements, the median of LSM, FIB-4, and APRI decreased significantly, the mean of the CEI increased slightly without statistically significant (P = 0.29) (Table 2). After achieving SVR, 7 (33%) patients achieved fibrosis regression. No patient with Ishak 5-6 (n = 7) achieved fibrosis regression.
| Pre SVR | Post SVR | Z/t value | P value | |
| ALT (U/L) | 55 (29.5) | 17 (11) | -4.015 | < 0.001 |
| AST (U/L) | 40 (29.5) | 20 (10.5) | -4.016 | < 0.001 |
| TB (μmol/L) | 14 (6.5) | 15.5 (11.3) | -0.541 | 0.588 |
| Alb (g/L) | 44.90 ± 3.40 | 47.43 ± 3.06 | -3.919 | 0.001 |
| Platelet (× 109/L) | 165 (87) | 199 (130) | -2.576 | 0.01 |
| INR | 1.04 ± 0.14 | 1.02 ± 0.07 | 0.425 | 0.675 |
| mHAI score, n (%) | -2.362 | 0.023 | ||
| 0-4 | 5 (24) | 15 (71) | ||
| 5-8 | 10 (48) | 4 (9) | ||
| 13-18 | 6 (28) | 2 (10) | ||
| Ishak score, n (%) | -0.370 | 0.713 | ||
| 0-2 | 5 (24) | 7 (33) | ||
| 3-4 | 9 (43) | 7 (33) | ||
| 5-6 | 7 (33) | 7 (33) | ||
| APRI | 0.58 (1.32) | 0.25 (0.40) | -4.015 | < 0.001 |
| FIB-4 | 1.34 (3.61) | 0.99 (1.81) | -3.007 | 0.003 |
| LSM (kpa) | 6.6 (7.5) | 5.8 (4.0) | -2.746 | 0.006 |
| CEI | 1.65 ± 0.11 | 1.68 ± 0.16 | -1.087 | 0.29 |
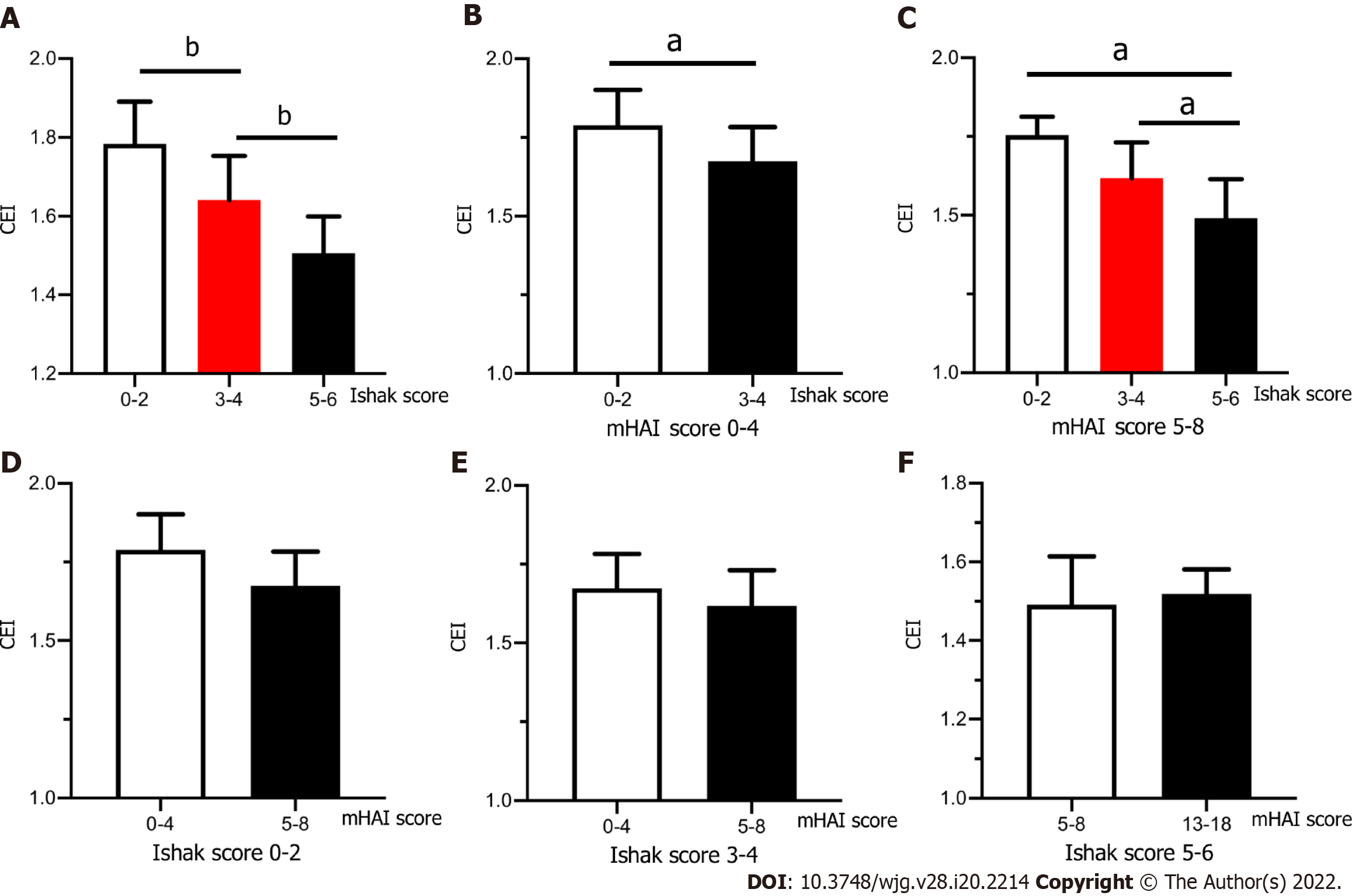
In patients with CHC, the CEI was negatively correlated with the grade of inflammation (r = -0.56, P < 0.001) and stage of fibrosis (r = -0.69, P < 0.001). The CEI decreased significantly among patients with Ishak scores 0–2, 3–4, and 5–6 (1.78 ± 0.11, 1.64 ± 0.11, and 1.50 ± 0.09, respectively, P < 0.001) (Figure 2A). To further analyze the relationship between the CEI and liver pathology, we stratified the patients according to the mHAI (0–4, 5–8, 9–12, and 13–18) and Ishak score (0–2, 3–4, and 5–6; the numbers of patients in each subgroup are shown in Table 3).
| Stratified analysis | Group 1 | Group 2 | Group 3 |
| mHAI score | Ishak score | ||
| 0-2 | 3-4 | 5-6 | |
| 0-4 | 14 | 11 | 1 |
| 5-8 | 3 | 13 | 9 |
| 13-18 | 0 | 0 | 9 |
| Ishak score | mHAI score | ||
| 0-4 | 5-8 | 13-18 | |
| 0-4 | 14 | 2 | 0 |
| 3-4 | 10 | 15 | 0 |
| 5-6 | 1 | 9 | 9 |
In patients with a mHAI of 0–4, the CEI in Group 2 (n = 11) was significantly lower than that in Group 1 (n = 14) [(1.67 ± 0.11) vs (1.79 ± 0.11), P = 0.021] and the CEI of the only patient in Group 3 was 1.52 (Figure 2B). When the mHAI was 5–8, the CEI decreased in the order of fibrosis Group 1 (n = 3), 2 (n = 13), and 3 (n = 9) at 1.75 ± 0.06, 1.62 ± 0.11, and 1.49 ± 0.12, respectively (P = 0.032) (Figure 2C). All patients with an mHAI of 13–18 had liver cirrhosis (n = 9), and they were not grouped. In contrast, after subgrouping the patients based on the fibrosis grade, there were no significant differences in the CEI among the inflammation groups (all P > 0.05) (Figure 2D–F). Therefore, we believe that decrease in the CEI is mainly associated with the progression of fibrosis.
Table 4 shows a comparison of the predictive values between the CEI and other non-invasive methods. According to the AUROCs and cut-off values, we found that the diagnostic efficacy of the CEI, LSM, APRI, and FIB-4 before and after DAA treatment was similar for liver cirrhosis (Ishak score ≥ 5), while the cut-off values of serological markers such as the APRI and FIB-4 significantly decreased post SVR. The cut-off value of the LSM also showed a similar trend. For significant fibrosis (Ishak score ≥ 3), post SVR, the diagnostic efficacy of the LSM decreased, and the APRI and FIB-4 showed no diagnostic value.
| Ishak score ≥ 5 | Ishak score ≥ 3 | |||
| Pre SVR | Post SVR | Pre SVR | Post SVR | |
| CEI | ||||
| AUROC (95%CI) | 0.93 (0.74, 0.97) | 0.87 (0.65, 0.97) | 0.88 (0.79, 1.00) | 0.87 (0.71, 1.00) |
| Cut-off value | 1.58 | 1.59 | 1.71 | 1.68 |
| LSM | ||||
| AUROC (95%CI) | 0.87(0.71, 1.00) | 0.87(0.79, 1.00) | 0.91(0.78, 1.00) | 0.80(0.60,0.98) |
| Cut-off value | 10.8 | 7.1 | 6.2 | 5.95 |
| APRI | ||||
| AUROC (95%CI) | 0.89(0.72, 1.00) | 0.89(0.74, 1.00) | 0.83(0.64, 1.00) | N2 |
| Cut-off value | 1.05 | 0.241 | 0.39 | N2 |
| FIB-4 | ||||
| AUROC (95%CI) | 0.92(0.80, 1.00) | 0.92(0.79, 1.00) | 0.80(0.58, 1.00) | N2 |
| Cut-off value | 1.78 | 1.281 | 0.87 | N2 |
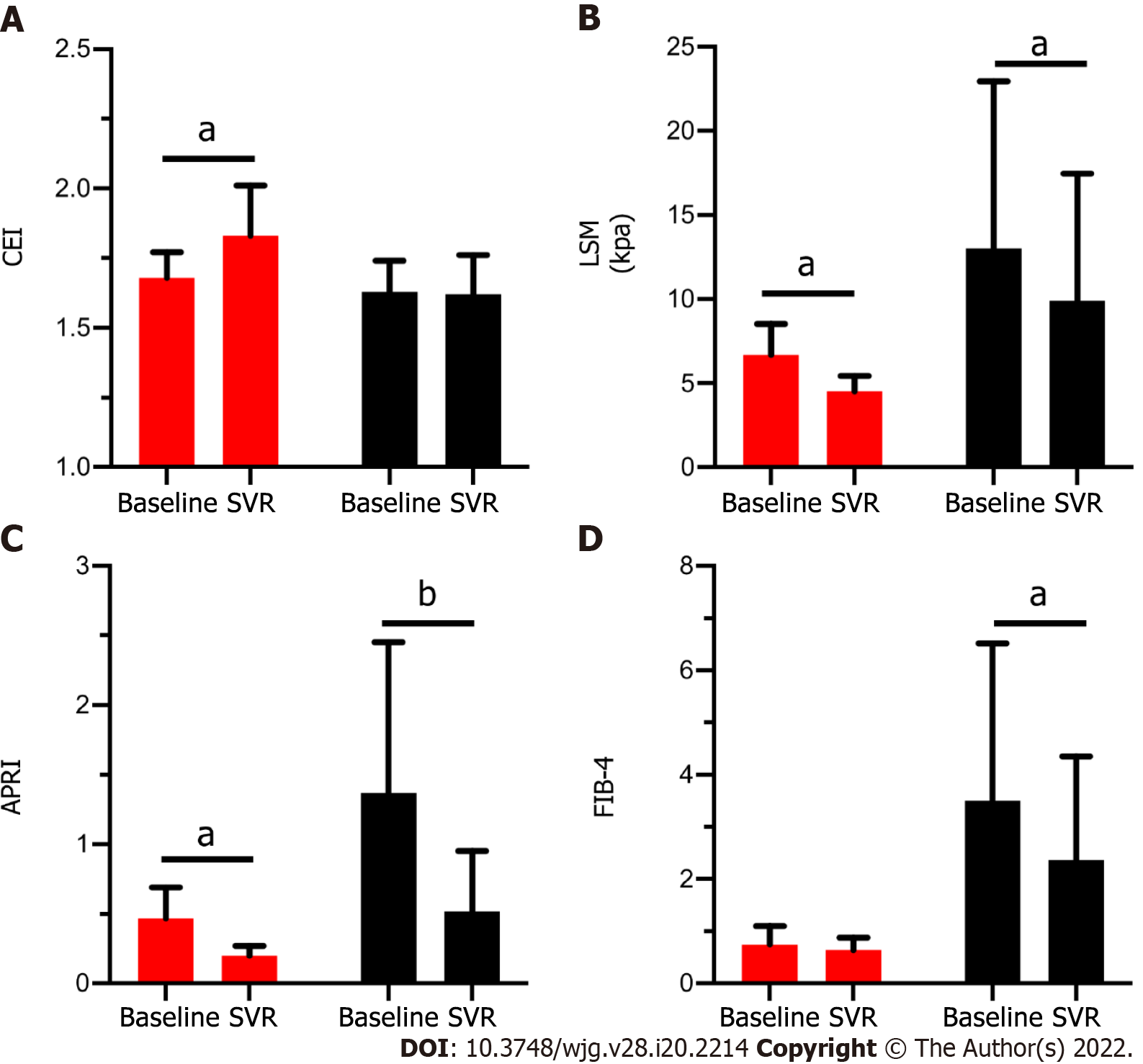
Figure 3 shows the change of the CEI and other non-invasive methods in patients with (red column) and without (black column) fibrosis regression. Among the patients with fibrosis regression (n = 7), the CEI increased significantly (from 1.68 ± 0.09 to 1.83 ± 0.18, P = 0.043) after DAA treatment; similarly, the LSM [from 6.6 (2.6) to 4.8 (1.2), P = 0.018] and APRI [from 0.37 (0.22) to 0.20 (0.08), P = 0.018] values decreased significantly. For patients who did not achieve fibrosis regression (n = 14), only the CEI remained stable (P > 0.05), while the LSM, APRI, and FIB-4 decreased significantly (P < 0.05). It is suggested that the decrease in the latter three noninvasive measurements after treatment may not be related to fibrosis regression. By comparing the change ratios of the four noninvasive indexes before and after treatment, only CEI% changed significantly, and CEI% was moderately positively correlated with fibrosis regression (r = 0.50, P = 0.021) (Table 5).
| Fibrosis regression | P value | ||
| Yes (n = 7) | No (n = 14) | ||
| CEI%1 | 107.36 ± 6.33 | 99.23 ± 7.14 | 0.020 |
| LSM% | 72.06 ± 20.32 | 81.31 ± 27.44 | 0.441 |
| APRI% | 45.40 ± 13.16 | 42.51 ± 16.41 | 0.702 |
| FIB-4% | 90.39 ± 24.09 | 75.69 ± 23.66 | 0.936 |
In this study, paired liver biopsy and Gd-EOB-DTPA-enhanced MRI data of patients with CHC before and after SVR were reported for the first time. This study concluded that the CEI of Gd-EOB-DTPA-enhanced MRI in the HBP decreased with the progression of liver fibrosis. For patients with CHC, the CEI can be used to distinguish among the different stages of liver fibrosis at baseline and after achieving SVR more effectively than the APRI, FIB-4, and LSM. The change in the CEI between pre and post SVR was related to fibrosis regression. This result increased the options for dynamic assessment of liver fibrosis after achieving SVR.
In our study, patients treated with DAA plus interferon were excluded. Although the combination therapy may have no effect on the evaluation of liver pathology, based on the current situation of CHC treatment, majority of patients can be cured by simple DAAs. So, we pay more attention on correlation between CEI and pathology changes in patients cured by simple DAAs. It is worth mentioning that, HCV RNA was both detected at 12- and 24-wk after the end of treatment. The value of HCV RNA at 12-wk were also undetectable in patients achieving SVR24. Since patients were enrolled between 2014-2016, SVR12 and SVR24 both could be used at that time, while the 24-wk SVR last longer, it was used in our article. We specially agree that the current definition of SVR as an undetectable HCV RNA at 12 wk after the end of treatment.
In the correlation analysis, we found that the CEI was mild negative related to both grade of inflammation and stage of fibrosis. Further hierarchical analysis showed that the CEI mainly decreased with the progression of liver fibrosis, which was consistent with the results of a previous multiple regression analysis[23]. As mentioned above, after achieving SVR, the overall liver fibrosis status was not notably improved, and the CEI also did not change significantly. Therefore, we combined 60 pairs of CEI and liver pathology data before and after treatment for analysis. It should be mentioned that the overall mHAI decreased after treatment, which may have affected the results. However, in our pre-analysis, we found that in the 21 paired MRI and liver biopsy samples after treatment, the correlation between the mHAI and Ishak score (r = 0.74, P < 0.001) remained significant. This may be because the patients with a mHAI > 13 at baseline had liver cirrhosis. After achieving SVR, there was no significant improvement in fibrosis or in the inflammation status. It was also shown that the correlation between the CEI and fibrosis stage remained relatively stable and was not related to the treatment state.
In patients with liver cirrhosis (Ishak score 5-6), the diagnostic values of the CEI and of the other non-invasive methods were similar, which was also partly previously reported by a cross-sectional comparative analysis[23]. As the inflammatory status improved with antiviral treatment, the cut-off values of the APRI (from 1.05 to 0.24) and FIB-4 (from 1.78 to 1.28) both substantially decreased for the same fibrosis status. The serological biomarkers had no diagnostic value for significant fibrosis after HCV eradication, mainly because with the rapid regression of liver necroinflammation, the ALT and AST levels returned to normal, reducing the diagnostic accuracy of serological biomarkers. Although accessible and common, the APRI and FIB-4 may not be suitable for the surveillance of patients with CHC post SVR[21,24].
Other than the serological biomarkers, the LSM obtained with TE is currently considered useful for fibrosis monitoring[25-27]. However, several studies have reported a rapid decrease in the LSM mainly related to inflammation regression, and its cut-off values are influenced by liver morphometry. Hence, the decrease in the LSM may be misinterpreted as change in the liver fibrosis stage[6,28]. A longitudinal study of 2 years showed that following SVR attainment, the improvements in the LSM were overstated compared to histologic staging[28]. Therefore, the follow-up value of liver fibrosis regression in patients with HCV SVR needs to be further verified. Our findings strengthened this notion in a relative short follow-up time (median of 6.2 mo). The cut-off value of the LSM decreased slightly in patients with liver cirrhosis (from 10.8 kPa to 7.1 kPa), wherein an LSM value of 7.1 kPa obtained with TE was defined as the threshold for absence of or minimal fibrosis in patients with CHC[29]. The comparative analysis also showed significant decrease in the LSM value in patients without fibrosis regression.
Apart from the CEI, several studies have reported multiple hepatobiliary liver enhancement indexes of Gd-EOB-DTPA-enhanced MRI, including RE (calculated as [SIHBP-SIUEP]/SIUEP of the liver parenchyma), liver-to-portal vein contrast ratio (calculated by dividing the liver parenchyma SI by the portal vein SI on HBP images), and liver-to-spleen contrast ratio (calculated by dividing the liver parenchyma SI by the spleen SI on HBP images)[30-32]. In previous reports, the signals of the portal vein and spleen were integrated with plasma or extracellular extravascular space exposure to the contrast agent, thus showing that the liver-to-portal vein contrast ratio and liver-to-spleen contrast ratio were more strongly related to liver function than to liver fibrosis[12,33]. Adjustment of the signal of the paraspinal muscle for SIliver on the same slice was performed to normalize the shimming influences and correct for technical bias. Compared with other organs, SImuscle was more stable and less influenced by age and liver function. Jang et al[23] also validated this view. A few articles have shown similar diagnostic accuracies for RE and the CEI. In our study, RE was mildly negatively associated with liver inflammation (r = -0.57, P = 0.007) and fibrosis (r = -0.44, P = 0.043) (unreported), possibly because the SI of the liver after injecting Gd-EOB-DTPA changes, as the window level and width differ in the images[34].
There were several limitations in our study. First, limited by the inclusion criteria, the sample size was small, and the CEI% of patients with fibrosis regression was close to 1. We will explore further by expanding the sample size. Second, we did not use MR elastography (MRE) or T1-mapping to predict liver fibrosis, which have a superior diagnostic value in predicting liver fibrosis than Gd-EOB-DTPA-enhanced MRI[35,36]. However, except for the influence of body mass index and ascites on TE, the sequencing method in MRE is not unified, and the threshold value changes across different methods[26]. In recent years, MR relaxometry in the form of T1 mapping has been considered promising as a non-invasive method for characterizing hepatic fibrosis using the look-locker technique for measurement. Our patients were enrolled between 2014 and 2016, when T1 mapping was not in use. Third, although histology is a gold standard procedure, the tissues used in our study were all from liver biopsies rather than from hepatectomy, and there may have been misjudgment regarding the histological changes. Fourth, the mean follow-up duration after achieving SVR was only 6.2 mo, and a longer follow-up period is warranted.
In conclusion, the CEI of Gd-EOB-DTPA-enhanced MRI can be used to diagnose liver fibrosis in patients with CHC. The change of the CEI can be used to monitor fibrosis regression post SVR by DAA therapy.
The histological change and non-invasive method surveillance after hepatitis C virus (HCV) eradication by direct acting antiviral (DAA) therapy have not been elucidated. As using a liver-specific magnetic resonance imaging (MRI) contrast, whether Gadolinium-ethoxybenzyl-diethylenetriamine penta-acetic acid (Gd-EOB-DTPA) enhanced MRI can be used to diagnose and follow-up the liver fibrosis in patients with chronic hepatitis C (CHC) has not been investigated.
The key issues are whether Gd-EOB-DTPA enhanced MRI can be used in diagnosing and following-up in patients with CHC. The result will provide important information on non-invasive method selection for dynamic assessment of liver fibrosis in patients with CHC and histology change after achieving SVR treated by DAAs.
To investigated the diagnostic and follow-up values of Gd-EOB-DTPA-enhanced MRI for hepatic histology in patients with CHC. We further explore the value of Gd-EOB-DTPA enhanced MRI in evaluating fibrosis regression in patients with CHC after achieving sustained virological response (SVR) treated by DAAs.
Chronic HCV infected patients with paired liver biopsy and Gd-EOB-DTPA enhanced MRI before and after DAA treated was included. Contrast enhancement index (CEI) was calculated according with signal intensity via MRI, and the correlation between CEI and histology change was evaluated. Fibrosis regression was defined as a ≥ 1-point decrease in the Ishak fibrosis score. The diagnostic and follow-up values of the CEI, liver stiffness measurements (LSM), aminotransferase (AST)-to-platelet ratio (APRI) and Fibrosis-4 (FIB-4) were compared.
Thirty-nine patients with CHC were enrolled, with average age of 42.3 ± 14.4 years and 20/39 (51.3%) were male. Twenty-one enrolled patients had eligible paired Gd-EOB-DTPA-enhanced MRI and liver tissues after achieving SVR. According to correlation and the hierarchical analysis, the CEI mainly decreased with the progression of liver fibrosis. Compared with LSM, APRI and FIB-4, the CEI is more useful for liver fibrosis diagnosis, the correlation between the CEI and fibrosis stage was relatively stable and was not related to the treatment state. In paired analysis using liver pathology and CEI before and after treatment, only the dynamic change in the CEI can be used to evaluate fibrosis regression after achieving SVR.
The CEI of Gd-EOB-DTPA-enhanced MRI can be used as a non-invasive method to diagnose liver fibrosis in patients with CHC. The dynamic change of the CEI can be used to monitor fibrosis regression post SVR in patients with CHC after DAA therapy.
Larger and longer-term prospective studies in patients with CHC should be performed in future studies.
We are sincerely grateful to the radiologists Dr. Xiao-Xuan Jia and Dr. Xin-Yu Zhang for their involvement in the MRI analysis. We also thank Prof. Aileen Wee and Guang-De Zhou for their involvement in the liver pathology confirmation. We also thank Hui-Xin Liu, MD, for help with the statistical review.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mogahed EA, Egypt S-Editor: Fan JR L-Editor: A P-Editor: Chen YX
| 1. | WHO. Global hepatitis report, 2017. Geneva: World Health Organization, 2017. [cited 10 October 2021]. Available from: https://www.who.int/. [Cited in This Article: ] |
| 2. | Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743-800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4439] [Cited by in F6Publishing: 4332] [Article Influence: 481.3] [Reference Citation Analysis (0)] |
| 3. | Lohmann V, Bartenschlager R. Indelibly Stamped by Hepatitis C Virus Infection: Persistent Epigenetic Signatures Increasing Liver Cancer Risk. Gastroenterology. 2019;156:2130-2133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Ahumada A, Rayón L, Usón C, Bañares R, Alonso Lopez S. Hepatocellular carcinoma risk after viral response in hepatitis C virus-advanced fibrosis: Who to screen and for how long? World J Gastroenterol. 2021;27:6737-6749. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 7] [Cited by in F6Publishing: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Knop V, Hoppe D, Welzel T, Vermehren J, Herrmann E, Vermehren A, Friedrich-Rust M, Sarrazin C, Zeuzem S, Welker MW. Regression of fibrosis and portal hypertension in HCV-associated cirrhosis and sustained virologic response after interferon-free antiviral therapy. J Viral Hepat. 2016;23:994-1002. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 6. | Laursen TL, Siggaard CB, Kazankov K, Sandahl TD, Møller HJ, Tarp B, Kristensen LH, Laursen AL, Leutscher P, Grønbaek H. Time-dependent improvement of liver inflammation, fibrosis and metabolic liver function after successful direct-acting antiviral therapy of chronic hepatitis C. J Viral Hepat. 2020;27:28-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 7. | Jayaswal ANA, Levick C, Collier J, Tunnicliffe EM, Kelly MD, Neubauer S, Barnes E, Pavlides M. Liver cT1 decreases following direct-acting antiviral therapy in patients with chronic hepatitis C virus. Abdom Radiol (NY). 2021;46:1947-1957. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Bluemke DA, Sahani D, Amendola M, Balzer T, Breuer J, Brown JJ, Casalino DD, Davis PL, Francis IR, Krinsky G, Lee FT Jr, Lu D, Paulson EK, Schwartz LH, Siegelman ES, Small WC, Weber TM, Welber A, Shamsi K. Efficacy and safety of MR imaging with liver-specific contrast agent: U.S. multicenter phase III study. Radiology. 2005;237:89-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 195] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 9. | Kim T, Murakami T, Hasuike Y, Gotoh M, Kato N, Takahashi M, Miyazawa T, Narumi Y, Monden M, Nakamura H. Experimental hepatic dysfunction: evaluation by MRI with Gd-EOB-DTPA. J Magn Reson Imaging. 1997;7:683-688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 84] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Kitao A, Matsui O, Yoneda N, Kozaka K, Kobayashi S, Koda W, Inoue D, Ogi T, Yoshida K, Gabata T. Gadoxetic acid-enhanced MR imaging for hepatocellular carcinoma: molecular and genetic background. Eur Radiol. 2020;30:3438-3447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Schulze J, Lenzen H, Hinrichs JB, Ringe B, Manns MP, Wacker F, Ringe KI. An Imaging Biomarker for Assessing Hepatic Function in Patients With Primary Sclerosing Cholangitis. Clin Gastroenterol Hepatol. 2019;17:192-199.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Yang M, Zhang Y, Zhao W, Cheng W, Wang H, Guo S. Evaluation of liver function using liver parenchyma, spleen and portal vein signal intensities during the hepatobiliary phase in Gd-EOB-D TPA-enhanced MRI. BMC Med Imaging. 2020;20:119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Kim SW, Kim YR, Choi KH, Cho EY, Song JS, Kim JE, Kim TH, Lee YH, Yoon KH. Staging of Liver Fibrosis by Means of Semiautomatic Measurement of Liver Surface Nodularity in MRI. AJR Am J Roentgenol. 2020;215:624-630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Verloh N, Probst U, Utpatel K, Zeman F, Brennfleck F, Werner JM, Fellner C, Stroszczynski C, Evert M, Wiggermann P, Haimerl M. Influence of hepatic fibrosis and inflammation: Correlation between histopathological changes and Gd-EOB-DTPA-enhanced MR imaging. PLoS One. 2019;14:e0215752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Chuang YH, Ou HY, Lazo MZ, Chen CL, Chen MH, Weng CC, Cheng YF. Predicting post-hepatectomy liver failure by combined volumetric, functional MR image and laboratory analysis. Liver Int. 2018;38:868-874. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Mauro E, Crespo G, Montironi C, Londoño MC, Hernández-Gea V, Ruiz P, Sastre L, Lombardo J, Mariño Z, Díaz A, Colmenero J, Rimola A, Garcia-Pagán JC, Brunet M, Forns X, Navasa M. Portal pressure and liver stiffness measurements in the prediction of fibrosis regression after sustained virological response in recurrent hepatitis C. Hepatology. 2018;67:1683-1694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 109] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 17. | Choi YR, Lee JM, Yoon JH, Han JK, Choi BI. Comparison of magnetic resonance elastography and gadoxetate disodium-enhanced magnetic resonance imaging for the evaluation of hepatic fibrosis. Invest Radiol. 2013;48:607-613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Goodman ZD. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J Hepatol. 2007;47:598-607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 548] [Cited by in F6Publishing: 597] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 19. | Rozario R, Ramakrishna B. Histopathological study of chronic hepatitis B and C: a comparison of two scoring systems. J Hepatol. 2003;38:223-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Huang R, Rao H, Yang M, Gao Y, Wang J, Jin Q, Ma D, Wei L. Noninvasive Measurements Predict Liver Fibrosis Well in Hepatitis C Virus Patients After Direct-Acting Antiviral Therapy. Dig Dis Sci. 2020;65:1491-1500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2762] [Cited by in F6Publishing: 3079] [Article Influence: 146.6] [Reference Citation Analysis (0)] |
| 22. | Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, Messinger D, Nelson M; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317-1325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2633] [Cited by in F6Publishing: 3194] [Article Influence: 177.4] [Reference Citation Analysis (0)] |
| 23. | Jang HJ, Min JH, Lee JE, Shin KS, Kim KH, Choi SY. Assessment of liver fibrosis with gadoxetic acid-enhanced MRI: comparisons with transient elastography, ElastPQ, and serologic fibrosis markers. Abdom Radiol (NY). 2019;44:2769-2780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Hsu WF, Lai HC, Su WP, Lin CH, Chuang PH, Chen SH, Chen HY, Wang HW, Huang GT, Peng CY. Rapid decline of noninvasive fibrosis index values in patients with hepatitis C receiving treatment with direct-acting antiviral agents. BMC Gastroenterol. 2019;19:63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 25. | Ioannou GN, Feld JJ. What Are the Benefits of a Sustained Virologic Response to Direct-Acting Antiviral Therapy for Hepatitis C Virus Infection? Gastroenterology. 2019;156:446-460.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 132] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 26. | Patel K, Sebastiani G. Limitations of non-invasive tests for assessment of liver fibrosis. JHEP Rep. 2020;2:100067. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 140] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 27. | Loomba R, Adams LA. Advances in non-invasive assessment of hepatic fibrosis. Gut. 2020;69:1343-1352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 192] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 28. | Pan JJ, Bao F, Du E, Skillin C, Frenette CT, Waalen J, Alaparthi L, Goodman ZD, Pockros PJ. Morphometry Confirms Fibrosis Regression From Sustained Virologic Response to Direct-Acting Antivirals for Hepatitis C. Hepatol Commun. 2018;2:1320-1330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 29. | Poynard T, Munteanu M, Colombo M, Bruix J, Schiff E, Terg R, Flamm S, Moreno-Otero R, Carrilho F, Schmidt W, Berg T, McGarrity T, Heathcote EJ, Gonçales F, Diago M, Craxi A, Silva M, Boparai N, Griffel L, Burroughs M, Brass C, Albrecht J. FibroTest is an independent predictor of virologic response in chronic hepatitis C patients retreated with pegylated interferon alfa-2b and ribavirin in the EPIC³ program. J Hepatol. 2011;54:227-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Watanabe H, Kanematsu M, Goshima S, Kondo H, Onozuka M, Moriyama N, Bae KT. Staging hepatic fibrosis: comparison of gadoxetate disodium-enhanced and diffusion-weighted MR imaging--preliminary observations. Radiology. 2011;259:142-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 154] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 31. | Verloh N, Utpatel K, Haimerl M, Zeman F, Fellner C, Fichtner-Feigl S, Teufel A, Stroszczynski C, Evert M, Wiggermann P. Liver fibrosis and Gd-EOB-DTPA-enhanced MRI: A histopathologic correlation. Sci Rep. 2015;5:15408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 32. | Hako R, Kristian P, Jarčuška P, Haková I, Hockicková I, Schréter I, Janičko M. Noninvasive Assessment of Liver Fibrosis in Patients with Chronic Hepatitis B or C by Contrast-Enhanced Magnetic Resonance Imaging. Can J Gastroenterol Hepatol. 2019;2019:3024630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Dahlqvist Leinhard O, Dahlström N, Kihlberg J, Sandström P, Brismar TB, Smedby O, Lundberg P. Quantifying differences in hepatic uptake of the liver specific contrast agents Gd-EOB-DTPA and Gd-BOPTA: a pilot study. Eur Radiol. 2012;22:642-653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 34. | Katsube T, Okada M, Kumano S, Imaoka I, Kagawa Y, Hori M, Ishii K, Tanigawa N, Imai Y, Kudo M, Murakami T. Estimation of liver function using T2* mapping on gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid enhanced magnetic resonance imaging. Eur J Radiol. 2012;81:1460-1464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Wu WP, Hoi CI, Chen RC, Lin CP, Chou CT. Comparison of the efficacy of Gd-EOB-DTPA-enhanced magnetic resonance imaging and magnetic resonance elastography in the detection and staging of hepatic fibrosis. Medicine (Baltimore). 2017;96:e8339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Obmann VC, Berzigotti A, Catucci D, Ebner L, Gräni C, Heverhagen JT, Christe A, Huber AT. T1 mapping of the liver and the spleen in patients with liver fibrosis-does normalization to the blood pool increase the predictive value? Eur Radiol. 2021;31:4308-4318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |