Published online Feb 21, 2018. doi: 10.3748/wjg.v24.i7.794
Peer-review started: December 2, 2017
First decision: December 20, 2017
Revised: January 14, 2018
Accepted: January 18, 2018
Article in press: January 18, 2018
Published online: February 21, 2018
To assess the viability of orthotopic and heterotopic patient-derived pancreatic cancer xenografts implanted into nude mice.
This study presents a prospective experimental analytical follow-up of the development of tumours in mice upon implantation of human pancreatic adenocarcinoma samples. Specimens were obtained surgically from patients with a pathological diagnosis of pancreatic adenocarcinoma. Tumour samples from pancreatic cancer patients were transplanted into nude mice in three different locations (intraperitoneal, subcutaneous and pancreatic). Histological analysis (haematoxylin-eosin and Masson’s trichrome staining) and immunohistochemical assessment of apoptosis (TUNEL), proliferation (Ki-67), angiogenesis (CD31) and fibrogenesis (α-SMA) were performed. When a tumour xenograft reached the target size, it was re-implanted in a new nude mouse. Three sequential tumour xenograft generations were generated (F1, F2 and F3).
The overall tumour engraftment rate was 61.1%. The subcutaneous model was most effective in terms of tissue growth (69.9%), followed by intraperitoneal (57.6%) and pancreatic (55%) models. Tumour development was faster in the subcutaneous model (17.7 ± 2.6 wk) compared with the pancreatic (23.1 ± 2.3 wk) and intraperitoneal (25.0 ± 2.7 wk) models (P = 0.064). There was a progressive increase in the tumour engraftment rate over successive generations for all three models (F1 28.1% vs F2 71.4% vs F3 80.9%, P < 0.001). There were no significant differences in tumour xenograft differentiation and cell proliferation between human samples and the three experimental models among the sequential generations of tumour xenografts. However, a progressive decrease in fibrosis, fibrogenesis, tumour vascularisation and apoptosis was observed in the three experimental models compared with the human samples. All three pancreatic patient-derived xenograft models presented similar histological and immunohistochemical characteristics.
In our experience, the faster development and greatest number of viable xenografts could make the subcutaneous model the best option for experimentation in pancreatic cancer.
Core tip: Several investigations have established patient-derived xenograft models for breast, renal, head and neck cancer, and hepatocellular tumours. Some of these models have predicted the clinical response of a specific type of tumour to different chemotherapeutic agents. However, the morphological and histological features of human pancreatic cancer xenografts in experimental models have been poorly studied. In the present study, the effectiveness of three experimental models based on the implantation of patient pancreatic cancer in three different locations (subcutaneous, intraperitoneal and pancreatic) have been assessed for the first time.
- Citation: Rubio-Manzanares Dorado M, Marín Gómez LM, Aparicio Sánchez D, Pereira Arenas S, Praena-Fernández JM, Borrero Martín JJ, Farfán López F, Gómez Bravo MÁ, Muntané Relat J, Padillo Ruiz J. Translational pancreatic cancer research: A comparative study on patient-derived xenograft models. World J Gastroenterol 2018; 24(7): 794-809
- URL: https://www.wjgnet.com/1007-9327/full/v24/i7/794.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i7.794
The development of chemotherapeutic agents for pancreatic cancer is closely related to the evolution of experimental models. Over the past 50 years, subcutaneous xenografts derived from cancer cell lines grown in vitro have been widely used[1-3]. A multitude of anticancer drugs have been tested using these preclinical models[4]. However, drugs that demonstrate benefits in animal models are not necessarily effective in humans[3,5-7].
Since the 1970s, human cancer samples obtained by biopsy or surgery have been implanted directly into mice[5,8-10]. These patient-derived xenografts (PDX), also known as tumour xenografts, were initially abandoned because of their high rejection rate[10]. Recently, this line of research has been reinitiated due to the development of genetically-modified immunodeficient mice, which has increased the success rate of grafting[5,11-13].
This PDX model has become competitive in the study of pancreatic cancer, particularly for predicting the clinical response to chemotherapy, owing to the better clinical predictive ability of this model[5]. Our group has successfully applied this procedure for the implantation of colorectal cancer patient-derived xenografts into nude mice[14]. In the present study, we assessed for the first time the effectiveness of three experimental models based on the implantation of pancreatic cancer PDX in different locations (subcutaneous, intraperitoneal and pancreatic). To date, no studies have investigated the characteristics of a series of pancreatic tumour xenografts placed orthotopically. The aim of this study was to assess the viability of orthotopic (intrapancreatic) and heterotopic (intraperitoneal and subcutaneous) PDX of human pancreatic cancer implanted into nude mice, and to determine which location better maintains human histological and immunohistochemical characteristics.
This study presents a prospective experimental analytical follow-up of the development of tumours in mice upon implantation of human pancreatic adenocarcinoma samples. Specimens were obtained surgically from patients with a pathological diagnosis of pancreatic adenocarcinoma. Human cancer samples were implanted as tumour xenografts in three experimental models. All animal experimentation procedures were approved by the Animal Research Committee of the University of Seville, Spain (protocol number CEEA-US2014-013/5).
We included patients diagnosed with pancreatic adenocarcinoma who underwent an exploratory laparotomy from May 17, 2012 to February 13, 2014 at the Virgen del Rocío University Hospital in Seville, Spain. All patients signed a written informed consent form for inclusion in the study, which had been previously approved by the Ethics Committee. The exclusion criteria for the study were tumours not identified as pancreatic adenocarcinoma, patients under 18 years old, or those who had not given written consent for the use of their samples.
Eleven samples were obtained from a total of 10 patients. Samples were obtained by cephalic pancreatoduodenectomy (n = 9) and distal pancreatectomy (n = 1). Two samples were obtained from the same patient (H4), comprising an interaortocaval lymph node metastasis and a sample extracted from the pancreatic tumour (Table 1). Samples were immediately dissected from the surgical specimen by a pathologist, who confirmed the pathological diagnosis of pancreatic adenocarcinoma.
| Code | Sex/Age (yr) | Localization | Surgery | Histology | T | N | M | Stage | Histological grade | Perineural invasion | Neoadjuvant therapy | Subcutaneous engraftment | Intraperitoneal engraftment | Pancreatic engraftment |
| H1 | F/75 | Ph | PC | DAC | 4 | X | 1 | IV | G2 | NO | NO | NO | NO | YES |
| H2 | F/77 | Ph | PC | Periampullary adenocarcinoma | 4 | 0 | 0 | III | G2 | YES | NO | YES | YES | YES |
| H3 | M/57 | Ph | PC | DAC | 3 | 0 | 0 | IIA | G2 | NO | NO | NO | NO | NO |
| H4 | F/71 | Ph and periaortic node | PC | DAC | X | 1 | 1 | IV | G2 | NO | YES | YES | NO | NO |
| (nab + paclitaxel + gemcitabine) | ||||||||||||||
| H5 | M/71 | Ph | PC | DAC | 3 | 1 | 0 | IIB | G2 | NO | NO | YES | NO | NO |
| H6 | F/73 | Ph | PC | DAC | 3 | 1 | 0 | IIB | G2 | YES | NO | NO | NO | NO |
| H7 | M/50 | Ph | PC | DAC | 3 | 0 | 0 | IIA | G2 | YES | NO | NO | NO | NO |
| H8 | M/70 | Ph | PC | Periampullary adenocarcinoma | 3 | 1 | 0 | IIB | G2 | NO | NO | YES | YES | NO |
| H9 | M/81 | Pt | DP | DAC | 3 | 0 | 0 | IIA | G2 | NO | NO | NO | NO | NO |
| H10 | F/78 | Ph | PC | DAC | 3 | 1 | 0 | IIB | G2 | NO | NO | NO | NO | NO |
The surgical samples obtained from each patient were divided into five equally sized portions of 3 mm × 3 mm × 3 mm. Samples were placed immediately into culture medium (Nutrient mixture F-10 Ham; Sigma-Aldrich, St. Louis, MO, United States) containing 20% foetal bovine serum that had been previously maintained at 2-8 °C. Samples were maintained on ice until implantation (from 30 min to 2 h). Three specimens were used as tumour xenograft implants at the subcutaneous, pancreatic and intraperitoneal locations. The remaining two specimens were used for anatomopathological and immunohistochemical analyses.
Male Hsd:Athymic Nude-Foxn1nu mice (severe combined immunodeficient) aged 6 to 8 wk and weighing 20 to 25 g were purchased from Harlan Laboratories (Barcelona, Spain). Male mice were used to avoid hormonal interference. Mice were anesthetized in a laminar flow cabinet using ketamine (80 mg/kg), xylazine (10 mg/kg) and droperidol (100 mg/kg) intraperitoneally under sterile conditions.
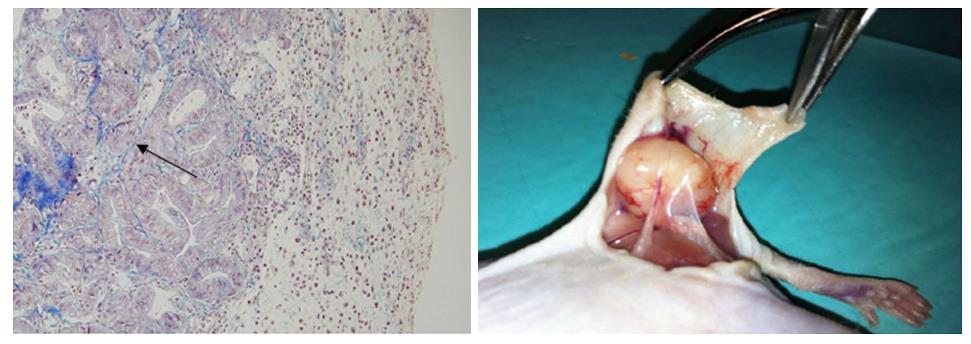
Three experimental models of tissue implantation were developed: (1) Subcutaneous model: A 2–3-mm incision was made with a scalpel on the back of the nude mouse approximately 10 mm from the base of the tail. A subcutaneous pouch was dissected with scissors. The tumour tissue was gently introduced into the corresponding pouch, which was then closed by separated sutures using 3/0 silk (Figure 1); (2) Pancreatic model: The nude mouse was placed in the right lateral decubitus position and a 3-mm left subcostal incision was made 1 mm from the rib cage. The tail of the pancreas was completely exposed using a cotton swab, which helped us to locate the spleen. Using a resorbable 4/0 suture, the tumour xenograft was sutured onto the tail of the mouse pancreas; and (3) Intraperitoneal model: A 3-cm medium laparotomy was performed about 10 mm from the pubis. The musculature was pulled with the dissecting clamp to prevent an accidental enterotomy. The corresponding tumour xenograft was placed intraperitoneally, and the abdominal wall was immediately closed.
Mice were monitored daily for discomfort or distress and for tumour growth. Tumours were observed until they reached a maximum length of 10 to 15 mm. At this time, mice were anesthetized and then euthanized by cervical dislocation. All personnel had been properly trained and consistently applied the technique humanely and effectively. The tumour was then harvested under sterile conditions. Animals that showed no tumour growth were euthanised 20 wk after the implantation. An explorative laparotomy was performed to evaluate tumour growth. Tumour xenografts were successively re-implanted into a new nude mouse over three sequential generations (F1, F2 and F3) or cryopreserved in a freezer. In this way, tumour fragments from an F1 generation mouse were re-implanted into three mice at the three different locations in order to establish the F2 generation. Donor mice (bearing F1 tumours) were euthanised by cervical dislocation, and five tumour samples with a size of 3 mm × 3 mm × 3 mm were obtained, of which one was immediately fixed in 4% paraformaldehyde for histological analysis, done was frozen in liquid nitrogen for immunohistochemical analysis, and the other three were maintained in culture medium for re-implantation. Necropsy was performed on donor mice. This process was repeated until implantation of the F3 generation tumour xenograft into the nude mice.
The harvested xenograft tumours were fixed in a 10% formalin solution and then embedded in paraffin. The tissue was stained with haematoxylin and eosin and Masson’s trichrome stain. For the assessment of samples stained with haematoxylin and eosin and Masson’s stain, two different pathologists reviewed all samples twice with a week between each measurement. Measurements were always performed early in the morning.
Tumour differentiation was evaluated with haematoxylin and eosin staining. Samples were classified as either well differentiated (well-formed tumour glands with a small nucleus relative to the cytoplasm), moderately differentiated (irregular tumour glands with higher cellular atypia and pleomorphic nuclei) and less differentiated (very poorly defined tumour glands, which sometimes acquired a solid pattern, greater architectural and cellular atypia, pleomorphic and large nuclei relative to the cytoplasm and a visible nucleolus).
The World Health Organisation guidelines state that well-differentiated tumours have fewer than five mitoses in 10 high-growth fields; moderately differentiated tumours have from 5 to 10 mitoses; and less differentiated tumours have more than 10 mitoses[15]. The number of mitoses was calculated by counting the number of cells undergoing mitosis in 10 high-magnification fields at 40 × magnification. The same samples were previously examined at 10 × magnification to identify the area in which cells undergoing mitosis were concentrated, and counting began in these areas. Of the two measurements of mitosis for each sample, the highest value was taken.
The degree of fibrosis in the intra- and peritumoral areas was evaluated with Masson’s trichrome staining on a scale from 1 to 4, with 1 corresponding to the minimum and 4 to the maximum degree of fibrosis between the tumour glands. There is no published classification system to assess the degree of fibrosis in pancreatic tumours. There is a subjectivity component in this assessment, which can lead to a degree of inter-observer variability[13].
Different tumour characteristics were assessed by immunohistochemistry, specifically cell proliferation (Ki67), cell death (TUNEL), angiogenesis (CD31) and fibrogenesis (α-smooth muscle actin, or alpha-SMA).
This process involves the use of specific primary antibodies for the detection of Ki-67 (FLEX monoclonal mouse anti-human Ki-67 antigen, clone MIB-1, ref IR626; DAKO Denmark A/S), TUNEL (TACSTM TdT kit, TA4625; R&D Systems, Inc., MN, United States), CD31 (polyclonal anti-CD31, ab28364; Abcam, Cambridge, United States) and α-SMA (polyclonal anti-alpha-SMA, ab5694; Abcam, Cambridge, United States). The fluorescence emitted by a secondary antibody (Alexa 488 anti-rabbit/goat/mouse IgG; Abcam, Cambridge, United States) that was common to all proteins studied was measured using a fluorescence microscope (BX61, Olympus America Inc.) in a darkened room to avoid loss of fluorescence, and analysis was performed using the Cell Sens Dimensions software (Olympus America Inc.). We determined the cut-off level of expression of each protein studied using the Cut-off Finder software version 2.1[16,17].
We analysed tumour development (tumour growth greater than 1-1.5 cm or the presence of metastases), disease-free time (time in weeks from tumour xenograft implantation until the tumour had reached the target size), mortality (mice that died spontaneously without intervention by the investigators), postoperative mortality (death during the first 24 h after surgery or on subsequent days as the result of direct failure of the surgical technique) and body weight (weekly weight of the implanted mice measured in grams).
The variables related to histological assessment included differentiation of the tumour and evaluation of the stroma. The variables related to the immunohistochemistry analysis were tumour cell proliferation and stromal cell activation, which was assessed by measuring fibrogenesis, angiogenesis and apoptosis.
Statistical analyses were carried out using SPSS® for Windows software version 21.0 (SPSS Inc., Chicago, IL, United States). Quantitative variables are presented as the mean ± SE. Qualitative variables are expressed as frequencies and percentage. The chi-square exact test or the Fisher’s exact test were used for the evaluation of tumour development, pathology and immunohistochemistry. A P-value ≤ 0.05 was considered significant. The Bonferroni correction was applied for post hoc analysis. The Kaplan-Meir with Log Rank Test was used to analyse disease-free time and tumour development time.
The demographic and tumour characteristics of the patients, as well as the information regarding F1 engraftment, are summarised in Table 1[18].
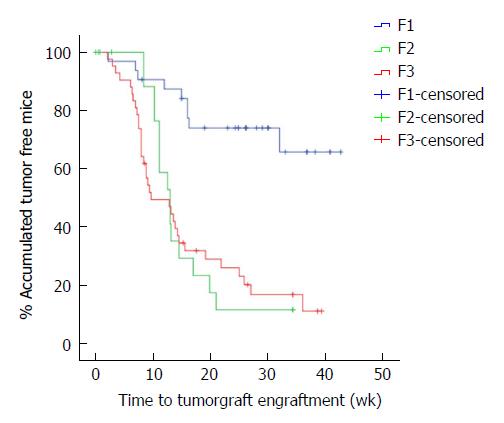
The overall tumour engraftment rate was 61.1% (58/95) over the three generations. Tumour xenograft development at F1 (from human to mouse) was significantly lower (28.1%) than in successive implantations, in which the tumour xenograft transplantation was performed from mouse to mouse (F2 71.4% and F3 80.9%; P < 0.001). We also observed faster tumour xenograft development in the transplantation between mice (F2 and F3) when compared to the implant from humans to mice (F1 33.7 ± 2.5 wk vs F2 15.5 ± 1.8 wk vs F3 16.1 ± 1.9 wk, P < 0.001, Figure 2).
Analysis of tumour engraftment in the three models showed that the subcutaneous model was the most prolific (69.9%), followed by the intraperitoneal (57.6%) and pancreatic (55.0%) models.
The overall mortality of mice was 13.6%. Postoperative mortality was 4.2%. Mortality was also analysed for each model, for which the lowest mortality was 9.1% in the subcutaneous model, followed by 15.2% in the intraperitoneal model and 17.2% in the pancreatic model.
The time taken to reach the target size of the tumour xenograft (1.5 cm) in the three models was analysed. We observed that the time taken was shorter in the subcutaneous model (17.7 ± 2.6 wk) than in the intraperitoneal (25.0 ± 2.7 wk) and pancreatic (23.1 ± 2.3 wk) models, although there was no significant difference between them (P = 0.063).
The probability that a certain patient characteristic could be related to successful engraftment in mice was evaluated (Table 2). Our analysis did not identify any correlation between the progression of engraftment with patient age or gender, tumour stage, tumour differentiation status (histology) or tumour location (head, body or tail of the pancreas or metastases), nor with the presence of lymph nodes metastases, distant metastasis or perineural invasion.
| Variables | Number of patients | Number of F1 tumours produced | Impact of clinical characteristics on tumour take rate (P1 value) |
| Age (yr) | |||
| < 70 | 2 | 0 (0) | 0.444 |
| ≥ 70 | 8 | 5 (62.5) | |
| Gender | |||
| Male | 5 | 2 (40) | 0.999 |
| Female | 5 | 3 (60) | |
| Histology | |||
| ADCP | 9 | 3 (33.3) | 0.180 |
| Ampulloma | 2 | 2 (100) | |
| Differentiation | |||
| G1 | 0 | 0 | <0.001 |
| G2 + G3 | 10 | 5 (50) | |
| Staging | |||
| ≤ II (I or II) | 7 | 2 (28.6) | 0.160 |
| > II (III or IV) | 3 | 3 (100) | |
| Tumour origin | |||
| Head | 9 | 4 (50) | Not available |
| Tail | 1 | 0 (0) | |
| Body | 0 | 0 | |
| Metastases | 1 | 1 (100) | |
| Perineural invasion | |||
| Yes | 3 | 1 (33.3) | 0.999 |
| No | 7 | 4 (57.1) | |
| Lymph node metastasis | |||
| Yes | 6 | 3 (50) | 0.999 |
| No | 4 | 2 (50) | |
| Distant metastases | |||
| Yes | 2 | 1 (50) | 0.999 |
| No | 8 | 4 (50) | |
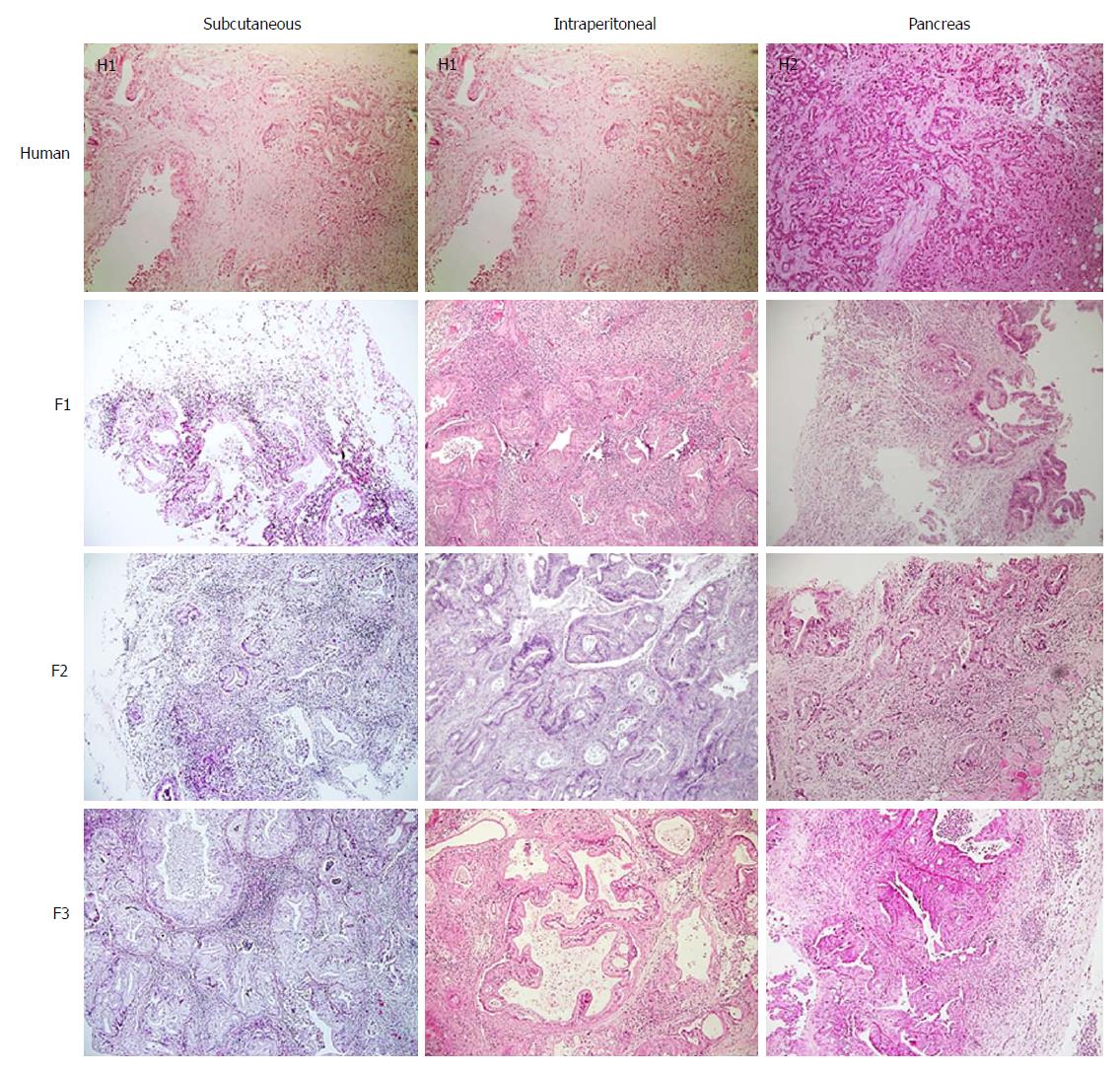
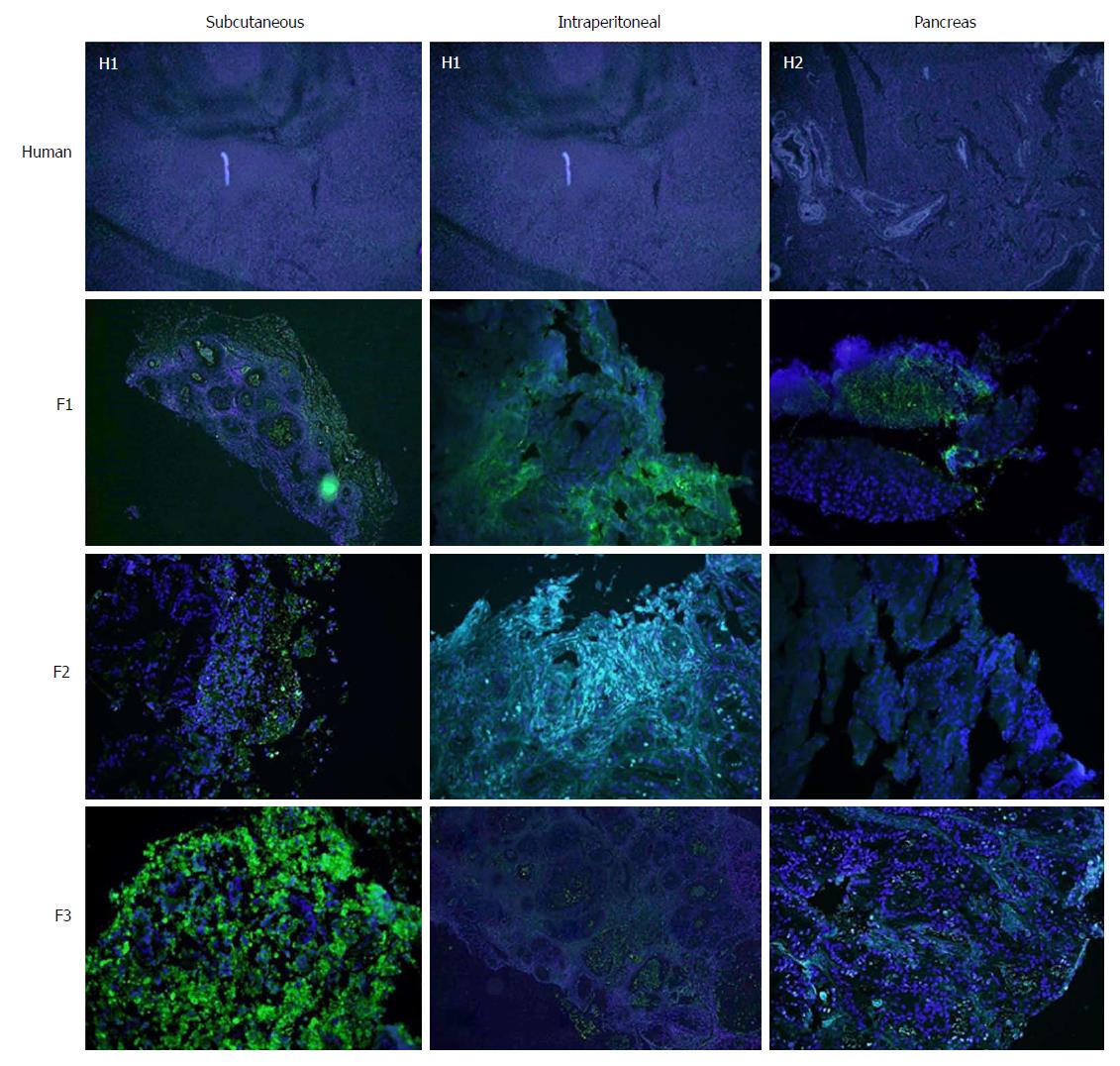
Tumour differentiation was analysed in all three models. Most of the human samples were moderately differentiated (72.7%) (Figure 3). No significant differences were observed between human pancreatic cancer samples and any of three tumour xenograft models (Table 3).
| Human (n = 11) | Subcutaneous (n = 23) | Intraperitoneal (n = 17) | Pancreas (n = 16) | P1 value | |
| Impact of differentiation | |||||
| Differentiated | 2 (18.2) | 7 (30.4) | 5 (29.4) | 3 (18.8) | 0.205 |
| Moderately differentiated | 8 (72.7) | 7 (30.4) | 4 (23.5) | 8 (50) | |
| Undifferentiated | 1 (9.1) | 9 (39.1) | 8 (47.1) | 5 (31.3) | |
| Impact of fibrosis | |||||
| Mild | 4 (36.3) | 12 (52.1) | 6 (35.2) | 6 (37.5) | 0.044 |
| Moderate | 3 (27.2) | 5 (21.7) | 9 (52.9) | 3 (18.8) | |
| High | 0 (0) | 4 (17.3) | 2 (11.7) | 5 (31.3) | |
| Very high | 4 (36.3) | 2 (8.6) | 0 (0) | 2 (12.5) | |
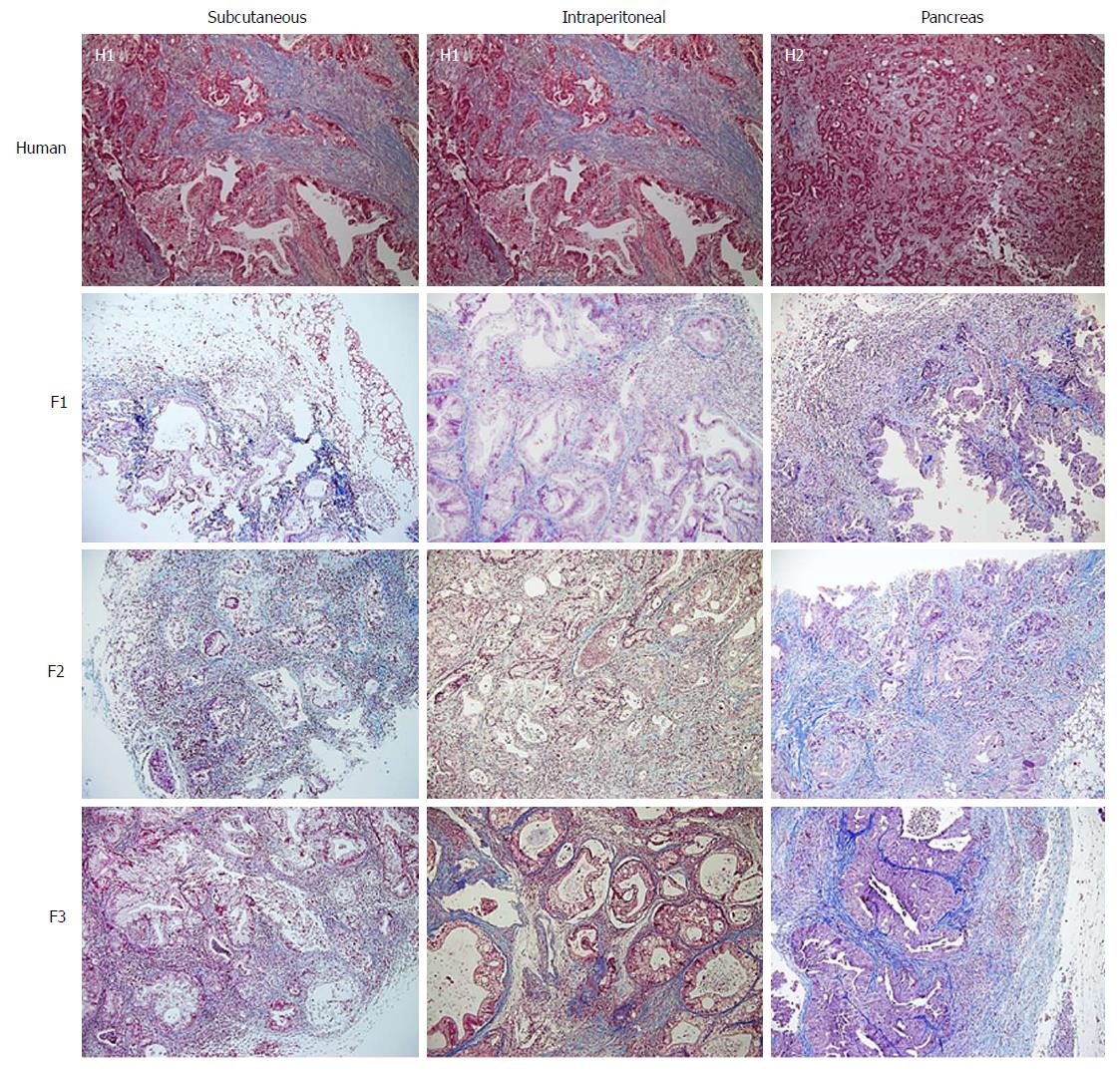
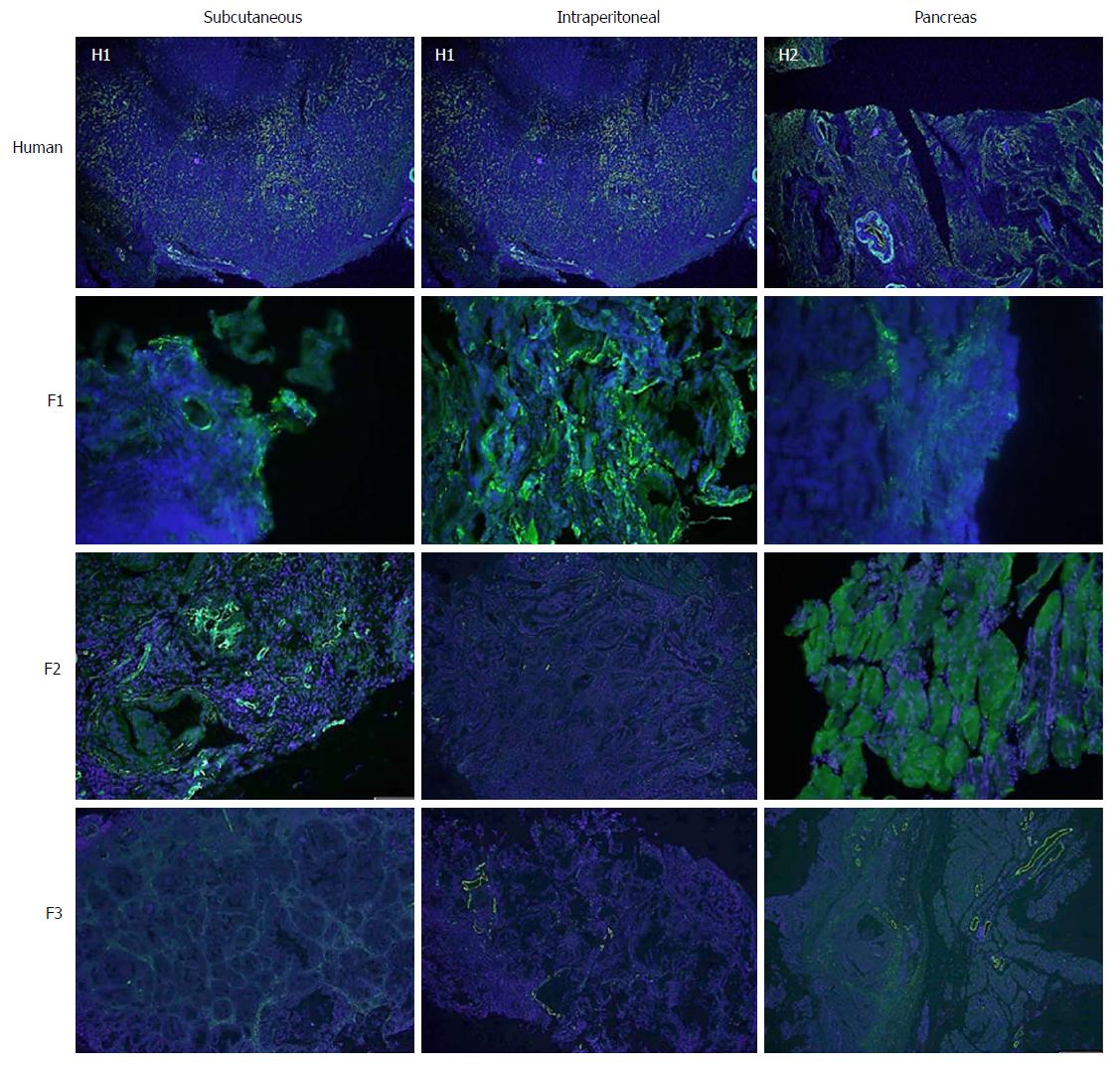
With regard to the assessment of stromal tissue, the human samples showed higher fibrosis than the mice samples (P = 0.044). More than one third (36.3%) of the human samples displayed very high fibrosis (Figure 4). When the experimental models were analysed individually, no significant differences were observed compared with the human samples, although the intraperitoneal model seemed to show a trend towards a lower degree of fibrosis (Table 3).
The results of the immunohistochemistry assessment are summarised in Table 4.
| Human (n = 11) | Subcutaneous (n = 23) | Intraperitoneal (n = 17) | Pancreas (n = 16) | P1 value | |
| Impact of cell proliferation (Ki67) | |||||
| Mild | 4 (36.4) | 13 (56.5) | 10 (58.8) | 8 (50) | 0.650 |
| High | 7 (63.3) | 10 (43.5) | 7 (41.2) | 8 (50) | |
| Impact of fibrogenesis (α-SMA) | |||||
| Mild | 0 (0) | 16 (69.6) | 11 (64.7) | 14 (87.5) | < 0.001 |
| High | 11 (100) | 7 (30.4) | 6 (35.3) | 2 (12.5) | |
| Impact of angiogenesis (CD31) | |||||
| Mild | 2 (18.2) | 15 (65.2) | 11 (64.7) | 14 (87.5) | < 0.001 |
| High | 9 (81.8) | 8 (34.8) | 6 (35.3) | 2 (12.5) | |
| Impact of apoptosis (TUNEL) | |||||
| Mild | 3 (27.3) | 16 (69.6) | 14 (82.4) | 15 (93.8) | < 0.001 |
| High | 8 (72.7) | 7 (30.4) | 3 (17.6) | 1 (6.3) | |
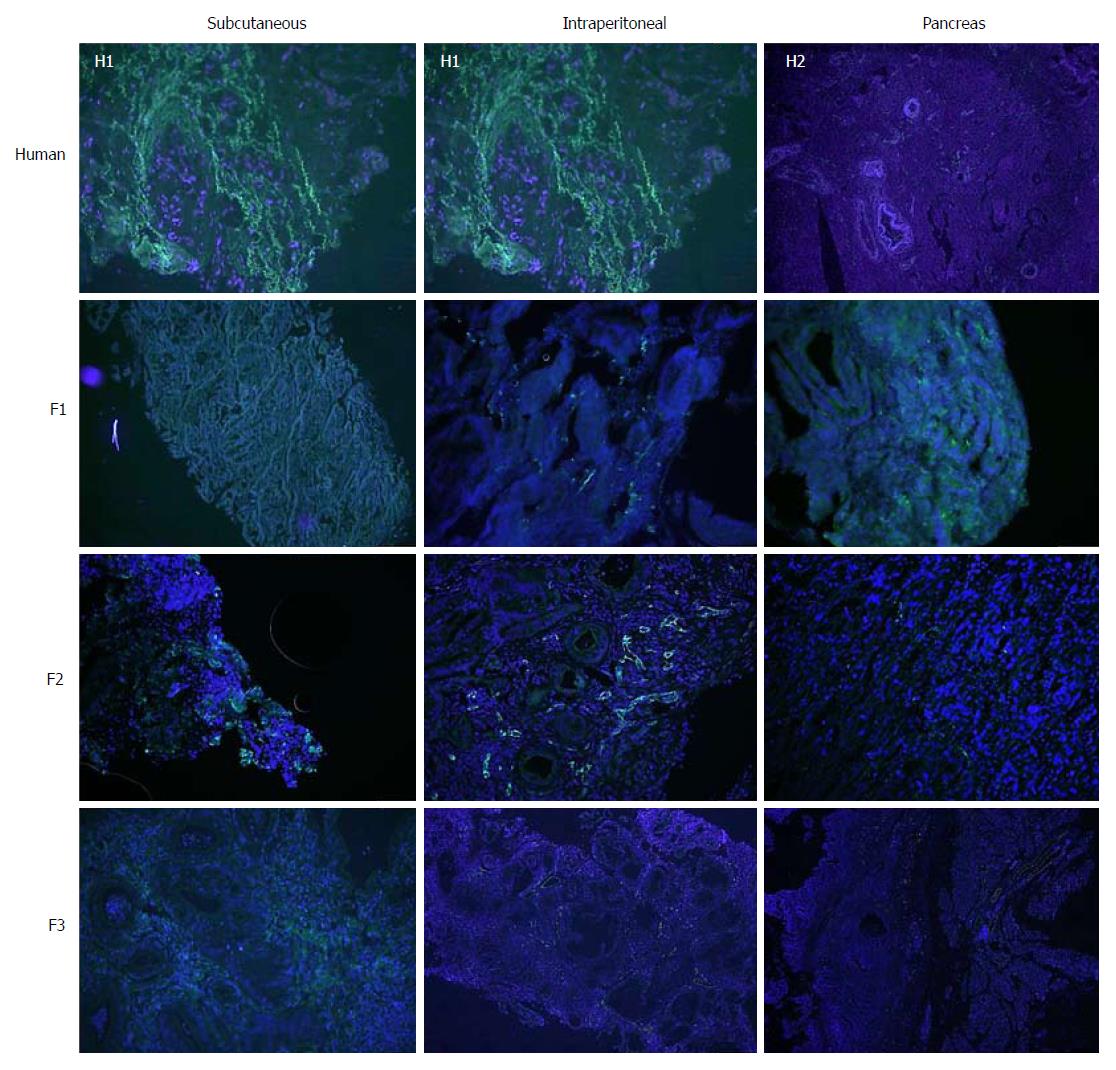
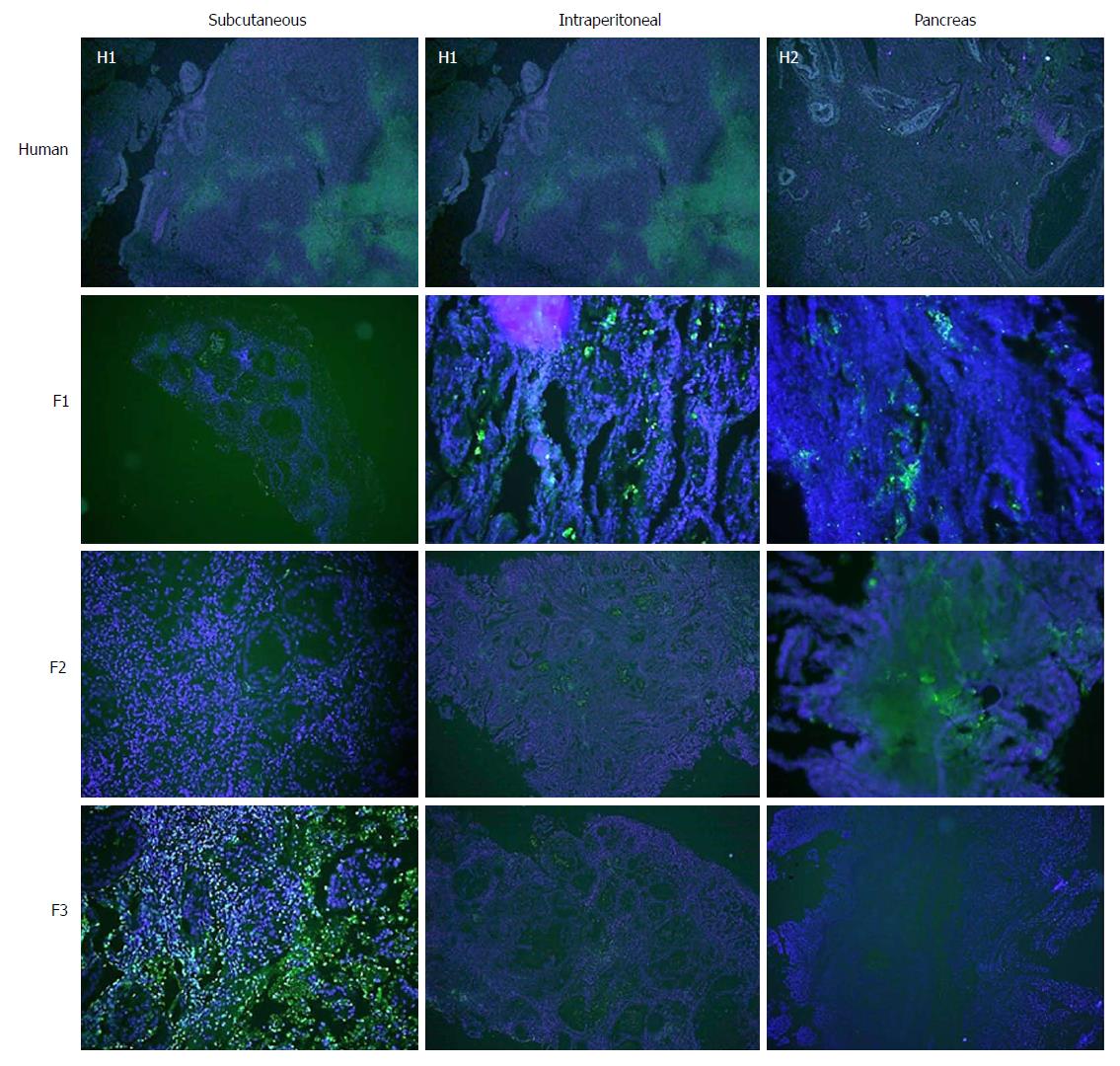
Concerning cell proliferation, as indicated by Ki67 staining, no differences were observed between the three experimental models (Figure 5). However, significant differences in fibrogenesis were detected between the human samples and the animal models (P < 0.001). All human samples showed high fibrogenesis (n = 11) in clear contrast to the PDX models, which showed a lower degree of fibrogenesis (Figure 6). In addition, up to 87.5% of the pancreatic model samples presented low α-SMA expression (Table 4). Similarly, a decrease in angiogenesis was observed in all models, but the reduction was more pronounced in the pancreatic model (P = 0.0027; Table 4 and Figure 7). A significant difference in apoptosis was found between the human samples and the animal models (round to P = 0.001). Most of the human samples showed elevated apoptosis (72.7%), which was in contrast to the experimental models for which most samples presented only mild apoptosis (Figure 8). In addition, up to 93.8% of the pancreatic model samples presented slight apoptosis (Table 4).
This study was designed to examine the usefulness of a pancreatic tumour xenograft model by focusing on the pathological and immunohistochemical features of subcutaneous, intraperitoneal and pancreatic implantation of human pancreatic cancer tissue fragments into mice. The main findings were: (1) the engraftment rate between mice (F2 and F3) was higher than the engraftment rate from human to mouse (F1); (2) the subcutaneous model developed a higher number of implants, although there were no significant differences in favour of any model; and (3) the tumour xenografts of the three models maintained some human characteristics including differentiation and cell proliferation. While these tumour xenografts presented reduced fibrosis and fibrogenesis, two other features of pancreatic cancer, hypovascularisation and apoptosis, were enhanced.
Our study differs from others published in the literature as we extracted the sample directly from the surgical site[19-21], and tumour cells were not cultured in vitro. Neither collagenase nor Matrigel were used to facilitate their implantation[22,23]. These steps, along with rapid implantation, facilitates the procedure and eliminates the influence of factors related to the processing of tumour cells such as DNA aberrations and cell death[24,25].
There was no correlation between the development of F1 tumour xenografts and the clinical characteristics of the patients or the pathological anatomy of the human tumour samples that were implanted into mice (differentiation, fibrosis, cell proliferation, number, fibrogenesis, angiogenesis and apoptosis). The apparent lack of correlation between any of these parameters, together with the rate of engraftment, may reflect a biological phenomenon, or it may be simply be due to an insufficient number of human tumours in some groups. However, similar results have been obtained in other studies[25]. This could be one of the limitations of this study. For this reason, new studies with higher numbers of participants are required. In addition, a genetic assessment of the samples could have enriched the study and might have explained this phenomenon.
Faster tumour development and a greater number of successful engraftments were observed for mouse-to-mouse compared to human-to-mouse transplants. This is probably a result of decreased apoptosis and fibrogenesis with progressing passage, which facilitates tumour development[6,26]. The three experimental models have similar characteristics in terms of differentiation, fibrosis, cell proliferation, fibrogenesis, angiogenesis and apoptosis. Tumour development appeared to occur earlier in the subcutaneous model; however, tumour xenografts that were implanted into the peritoneum or orthotopically in the pancreas were harder to visualise as they were grafted into a distensible cavity. Imaging tests such as nuclear magnetic resonance would probably evidence similar growth to the subcutaneous model[27]. While evaluating the cost-effectiveness of the tumour xenograft was not the purpose of this paper, the shorter time taken for the subcutaneous PDX to reach the target size may reduce the cost of maintenance and experimental duration when compared to other models[6]. The practical utility of tumour xenografts needs to be addressed in terms of engraftment speed and reproducibility. In fact, tumour xenografts show a rather limited engraftment rate and slow tumour growth. However, those models are readily applicable for drug experiment because, once a xenograft has been successfully engrafted, it can be used after several freeze-thaw cycles and several passages[5].
The intraperitoneal and pancreatic models showed a tendency toward higher mortality. Two factors may explain this trend in these two models: firstly, the laparotomy performed in the intraperitoneal model and the subcostal incision in the pancreatic model are more aggressive procedures and more prone to complications (evisceration, unnoticed enterotomy, solid viscera lesion and pancreatitis); and secondly, in both models the tumour xenografts developed inside a cavity where the tumour growth could not be observed. Tumour xenografts could infiltrate vital structures, unnoticed by the researcher, resulting in the death of the mouse. Pancreatic and intraperitoneal models are more invasive and thus might include more suffering/physiological burden in a mouse. That might be one of reasons why the tumour xenograft grows better subcutaneously.
We did not find any significant differences between the differentiation and proliferation of human samples compared to any of the experimental models. Therefore, tumour xenografts in the tested experimental models appear to reproduce human characteristics independent of the model in which it is implanted (subcutaneous, intraperitoneal or pancreas), suggesting that tumour xenografts could be valid models for the study of chemotherapy[22,25,28,29].
Some authors suggest that the interstitial matrix may act as a physical barrier to the penetration of chemotherapeutics into deeper areas of the tumour[5,30]. These studies conclude that the tumour stroma of tumour xenografts is larger and more similar to human tumour samples than models in which isolated cells are implanted from the human tumour or from in vitro cultures[5]. The diffusion of chemotherapeutics in the latter model could be higher than in human tumours; therefore, it is expected that the tumour xenografts will be a more realistic model.
The election in our study of alpha SMA as a marker for fibrogenesis is based on the intense desmoplasia presented by pancreatic tumours. The pancreatic stellate cells involved in tumour desmoplasia are characterized by expressing α-SMA and by the synthesis of procollagen α-1T which are the main components of the extracellular matrix that constitute desmoplasia[31,32].
We did not observe any differences in stromal fibrosis in successive tumour xenografts, although there was a tendency toward reduced fibrosis compared to human samples. However, when we analysed fibrogenesis or α-SMA expression, a progressive decline in fibrogenesis was observed throughout the three passages. In the F1 tumour xenografts, there was no difference in fibrogenesis compared to the human specimen; however, in the F2 tumour xenograft there was a significant decrease, and in F3 the decrease was remarkable. This phenomenon may explain the increase in tumorigenesis in successive re-implants[22,23,26].
Finally, by comparing histological fibrosis measured by Masson’s staining and α-SMA expression, a decrease in stromal cell activation through successive re-implants was evidenced in all experimental models. The advent of anti-tumour drugs for cancer niches is determined by a variety of factors including drug penetration or diffusion, which is dependent on vascularisation of the tumour and the stromal matrix. Therefore, the distance from cancer cells to the vessels will determine the efficacy of the chemotherapeutic drug[5]. Although a decrease in the activation of myoblasts and fibroblasts may facilitate tumour implantation, we must also consider that this may translate into reduced fibrosis of the tumour stroma. The chemotherapeutic agent may show better diffusion, and thus greater effectiveness, in the animal model than in humans. As such, we must be cautious when testing the pharmacokinetics of chemotherapy drugs, as they may be more effective in animal models than in humans. Hence, further pharmacological studies that compare all three models are mandatory.
Regarding tumour angiogenesis, the evaluation of tumour vascularisation is important in the investigation of pancreatic cancer. The extension of the vascular network is fundamental to assess the response to the treatment of anti-tumour drugs. Multiple authors use a microvessel density analysis system by CD31 expression in pancreatic PDX similar to ours[5,33,34].
A study by Akashi et al[5] compared grafts that had been extracted directly from the tumour specimen with those derived from cultured cell lines. The authors observed a higher density of microvessels in the periphery of the tumour xenografts derived from cell lines. In contrast, tumour xenografts derived directly from humans presented areas of low microvessel density, and thus, maintained similar features to those of human basal tumours. Hypovascularisation is a characteristic feature of pancreatic cancer which makes this tumour chemoresistant[5,34,35]. This characteristic was potentiated in our experimental tumour xenografts indicating that these models are representative of human tumours for the purposes of drug testing. Although hypovascularisation is a hallmark of pancreatic cancer, further chemotherapeutic studies are required to determine how tumour xenografts behave against anticancer drugs in order to avoid resistance to chemotherapeutic drugs in preclinical models due to the significant decrease in angiogenesis in tumour xenografts.
In addition to a tendency toward hypovascularisation and the development of a strong desmoplastic stroma, human pancreatic cancer has a relatively low apoptosis rate that makes it chemoresistant[36,37]. In our study, there was a trend toward decreasing TUNEL expression with subsequent re-implants for all three experimental models, which was more remarkable in the orthotopic implant and pancreatic model.
In conclusion, The three PDX models have similar characteristics in terms of differentiation, fibrosis, cell proliferation, fibrogenesis, angiogenesis and apoptosis, but tumour development was detected earlier in the subcutaneous model. Intraperitoneal and pancreatic PDX models presented a greater morbidity and mortality than subcutaneous model. There was also a higher number of viable tumour xenografts with the progression of sequential implants in this experimental model. For these reasons, the subcutaneous model may represent the best option for the investigation of anticancer drugs with tumour xenografts.
Currently, a dozen experimental models are available. The molecular characteristics of these models can vary substantially with the morphology of the lesion, and thus, the selection of the model is not trivial. Multiple laboratories have established models of breast xenografts, head and neck cancer, and hepatocellular tumours that maintain the characteristics of the primary tumour from which they come. Some of these models have predicted the clinical response of a specific type of tumour to different chemotherapeutic agents. The main advantage of human tumour xenografts in the mouse is that they seem to preserve the pathological and immunohistochemical characteristics of the primary tumour, which may allow us to experiment with drugs with human-like pancreatic cancer models. However, the degree to which periampullary carcinoma implants and ductal adenocarcinoma (DAC) reflect the morphological and histological characteristics of their tumours of origin have been poorly studied and there are few publications on the subject.
Periampullary carcinomas, and especially DAC, are characterised by early vascular, lymphatic and perineural dissemination, so that some authors consider it to be a systemic disease from the beginning. This implies criteria of unresectability and justifies the ominous prognosis of the disease. At the time of diagnosis, 85% of patients present a macroscopic disease beyond the limits of the organ. The large epidemiological series approximate the incidence of pancreas cancer to their annual mortality. Given the unfortunate prognosis of this disease, the development of new chemotherapy drugs with systemic action that complement the local surgical treatment is fundamental. One of the main problems that researchers find in developing new molecules is the lack of animal models that faithfully reproduce the characteristics of human pancreatic cancer. Both the models developed in genetically modified mice (GEMM) and those induced by carcinogenic substances have facilitated the understanding at the molecular level and the appearance of new anti-tumour drugs with in vitro activity. Unfortunately, these treatment lines are often ineffective in humans.
The appearance of immunocompromised nude mice has allowed the resumption of animal models with human xenografts, whose main limitation was the high rejection rate. In this way, we can develop experimental models of human periampullary tumours that preserve the original genotypic and phenotypic characteristics.
The morphological and histological characteristics derived from the xenografts of pancreatic cancer in the experimental models have been poorly studied. In order to make animal models that are more similar to cancer in humans, we have developed this study.
Following this line of work, we have developed three experimental models through the use of xenografts: subcutaneous, intraperitoneal and pancreatic. The main objective of this study is to assess the viability of orthotopic (intrapancreatic) and heterotopic (intraabdominal and subcutaneous) xenografts of human pancreas cancers implanted in nude mice.
This work is part of a more ambitious line of research that in the future intends to identify molecules or combinations of these with the help of these models to rescue patients for surgery.
A prospective experimental analytical follow-up of the development of tumours in mice upon implantation of human pancreatic adenocarcinoma samples was presents. Specimens surgically from patients with a pathological diagnosis of pancreas adenocarcinoma were obtained. Human cancer samples were implanted as tumour xenografts in three experimental models. The surgical samples were divided into five equally sized portions of 3 mm × 3 mm × 3 mm. Three specimens were used as tumour xenograft implants at the subcutaneous, pancreatic and intraperitoneal locations in nude mice. To date, no study comparing the implantation of a heterotopic pancreatic cancer xenograft with an orthotopic tumour xenograft has been published.
Histological analysis and immunohistochemical assessment of apoptosis, proliferation, angiogenesis and fibrogenesis were performed. When a tumour xenograft got the target size, it was re-implanted in a new nude mouse. Three sequential tumour xenograft generations (F1, F2 and F3) were generated.
The main findings of this study were: (1) the engraftment rate between mice was higher than the engraftment rate from human to mouse; (2) the subcutaneous model developed a higher number of implants, although there were no significant differences in favour of any model; and (3) the tumour xenograft of the three models maintained some human characteristics including differentiation and cell proliferation. While these tumour xenografts presented reduced fibrosis and fibrogenesis, two other features of pancreatic cancer, hypovascularisation and apoptosis, were enhanced.
The practical utility of tumour grafts needs to be addressed in terms of engraftment speed and reproducibility. In fact, tumour xenografts show a rather limited engraftment rate and slow tumour growth. However, those models are readily applicable for drug experiment because, once a xenograft has been successfully engrafted, it can be used after several freeze-thaw cycles and several passages.
Although these models can develop tumours that are very similar to humans, they are not an exact reflection of them. In this way, chemotherapeutic agents could be more effective in pancreatic PDX models than in human tumours. For this reason, new preclinical studies with chemotherapeutic agents are mandatory in those models.
In the establishment of patient-derived xenograft models, samples of primary tumours were implanted in immunodepressed mice subcutaneously, intraperitoneally or orthotopically, with no intermediate step of in vitro propagation. Although the subcutaneous model is easy to perform through an incision with the scalpel on the back of the mouse, the microenvironment of the tumour is not exactly the same. We have considered one step further by designing a new intraperitoneal and pancreatic model that may reproduce the natural conditions of human pancreatic cancer. However, in our study, implanted subcutaneous xenografts maintain pathological and immunohistochemical characteristics of the primary tumour from which they derive similarly to the other two developed models.
To date, there has been no study on pancreatic cancer that has determined the best location to develop xenografts in animal models. In our study, the detection of tumour development is earlier in the subcutaneous model, which implies a lower cost compared to the other models. In addition, the subcutaneous model is the one with the highest number of viable xenografts developed throughout the different re-implantations. Taking into account that the three models developed have similar anatomopathological and immunohistochemical characteristics, the subcutaneous model could be the best option for the investigation of anticancer drugs with xenografts. However, more studies are needed to confirm this theory.
The majority of works that strive to broaden our knowledge about the diagnosis and treatment of pancreas cancer, are based on xenografts from cell lines cultured in vitro. Chemotherapy agents which have good results in these experimental models do not have the same results when they are used in human tumours. In our case, we have established three models of pancreas tumours directly derived from patients and we have compared the morphological and immunological characteristics of the xenografts with the human tumours in order to establish which is the model that most faithfully reflected human tumour characteristics.
In our experience, due to the earliest development and the highest number of viable xenografts, as well as being the experimental model with the lowest morbidity, the subcutaneous model may be the best model for experimentation in pancreatic cancer.
Our intention is to select the best implant route in order to use these models in the future to detect biomarkers of pancreatic cancer and to develop specific chemotherapeutic regimens for each patient.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Sukocheva OA S- Editor: Wang JL L- Editor: A E- Editor: Huang Y
| 1. | Morton JJ, Bird G, Refaeli Y, Jimeno A. Humanized Mouse Xenograft Models: Narrowing the Tumor-Microenvironment Gap. Cancer Res. 2016;76:6153-6158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 166] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 2. | Jackson SJ, Thomas GJ. Human tissue models in cancer research: looking beyond the mouse. Dis Model Mech. 2017;10:939-942. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Voskoglou-Nomikos T, Pater JL, Seymour L. Clinical predictive value of the in vitro cell line, human xenograft, and mouse allograft preclinical cancer models. Clin Cancer Res. 2003;9:4227-4239. [PubMed] [Cited in This Article: ] |
| 4. | Huynh AS, Abrahams DF, Torres MS, Baldwin MK, Gillies RJ, Morse DL. Development of an orthotopic human pancreatic cancer xenograft model using ultrasound guided injection of cells. PLoS One. 2011;6:e20330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Akashi Y, Oda T, Ohara Y, Miyamoto R, Hashimoto S, Enomoto T, Yamada K, Kobayashi A, Fukunaga K, Ohkochi N. Histological advantages of the tumor graft: a murine model involving transplantation of human pancreatic cancer tissue fragments. Pancreas. 2013;42:1275-1282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457-1461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2272] [Cited by in F6Publishing: 2409] [Article Influence: 160.6] [Reference Citation Analysis (0)] |
| 7. | Johnson JI, Decker S, Zaharevitz D, Rubinstein LV, Venditti JM, Schepartz S, Kalyandrug S, Christian M, Arbuck S, Hollingshead M. Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br J Cancer. 2001;84:1424-1431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 624] [Cited by in F6Publishing: 592] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 8. | Garrido-Laguna I, Uson M, Rajeshkumar NV, Tan AC, de Oliveira E, Karikari C, Villaroel MC, Salomon A, Taylor G, Sharma R. Tumor engraftment in nude mice and enrichment in stroma- related gene pathways predict poor survival and resistance to gemcitabine in patients with pancreatic cancer. Clin Cancer Res. 2011;17:5793-5800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 179] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 9. | Whiteford CC, Bilke S, Greer BT, Chen Q, Braunschweig TA, Cenacchi N, Wei JS, Smith MA, Houghton P, Morton C. Credentialing preclinical pediatric xenograft models using gene expression and tissue microarray analysis. Cancer Res. 2007;67:32-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Capellá G, Farré L, Villanueva A, Reyes G, García C, Tarafa G, Lluís F. Orthotopic models of human pancreatic cancer. Ann N Y Acad Sci. 1999;880:103-109. [PubMed] [Cited in This Article: ] |
| 11. | Morton CL, Houghton PJ. Establishment of human tumor xenografts in immunodeficient mice. Nat Protoc. 2007;2:247-250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 321] [Cited by in F6Publishing: 317] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 12. | Smith V, Wirth GJ, Fiebig HH, Burger AM. Tissue microarrays of human tumor xenografts: characterization of proteins involved in migration and angiogenesis for applications in the development of targeted anticancer agents. Cancer Genomics Proteomics. 2008;5:263-273. [PubMed] [Cited in This Article: ] |
| 13. | Galuschka C, Proynova R, Roth B, Augustin HG, Müller-Decker K. Models in Translational Oncology: A Public Resource Database for Preclinical Cancer Research. Cancer Res. 2017;77:2557-2563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Perez M, Lucena-Cacace A, Marín-Gómez LM, Padillo-Ruiz J, Robles-Frias MJ, Saez C, Garcia-Carbonero R, Carnero A. Dasatinib, a Src inhibitor, sensitizes liver metastatic colorectal carcinoma to oxaliplatin in tumors with high levels of phospho-Src. Oncotarget. 2016;7:33111-33124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. Geneva: World Health Organisation; 2010; . [Cited in This Article: ] |
| 16. | Budczies J, Klauschen F, Sinn BV, Győrffy B, Schmitt WD, Darb-Esfahani S, Denkert C. Cutoff Finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS One. 2012;7:e51862. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 783] [Cited by in F6Publishing: 922] [Article Influence: 76.8] [Reference Citation Analysis (0)] |
| 17. | Striefler JK, Sinn M, Pelzer U, Jühling A, Wislocka L, Bahra M, Sinn BV, Denkert C, Dörken B, Oettle H. P53 overexpression and Ki67-index are associated with outcome in ductal pancreatic adenocarcinoma with adjuvant gemcitabine treatment. Pathol Res Pract. 2016;212:726-734. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471-1474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5537] [Cited by in F6Publishing: 6112] [Article Influence: 436.6] [Reference Citation Analysis (0)] |
| 19. | Arjona-Sánchez A, Ruiz-Rabelo J, Perea MD, Vázquez R, Cruz A, Muñoz Mdel C, Túnez I, Muntané J, Padillo FJ. Effects of capecitabine and celecoxib in experimental pancreatic cancer. Pancreatology. 2010;10:641-647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Ruiz-Rabelo JF, Vázquez R, Perea MD, Cruz A, González R, Romero A, Muñoz-Villanueva MC, Túnez I, Montilla P, Muntané J. Beneficial properties of melatonin in an experimental model of pancreatic cancer. J Pineal Res. 2007;43:270-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Padillo FJ, Ruiz-Rabelo JF, Cruz A, Perea MD, Tasset I, Montilla P, Túnez I, Muntané J. Melatonin and celecoxib improve the outcomes in hamsters with experimental pancreatic cancer. J Pineal Res. 2010;49:264-270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Jiang YJ, Lee CL, Wang Q, Zhou ZW, Yang F, Jin C, Fu DL. Establishment of an orthotopic pancreatic cancer mouse model: cells suspended and injected in Matrigel. World J Gastroenterol. 2014;20:9476-9485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 36] [Cited by in F6Publishing: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | Partecke LI, Sendler M, Kaeding A, Weiss FU, Mayerle J, Dummer A, Nguyen TD, Albers N, Speerforck S, Lerch MM. A syngeneic orthotopic murine model of pancreatic adenocarcinoma in the C57/BL6 mouse using the Panc02 and 6606PDA cell lines. Eur Surg Res. 2011;47:98-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Monsma DJ, Monks NR, Cherba DM, Dylewski D, Eugster E, Jahn H, Srikanth S, Scott SB, Richardson PJ, Everts RE. Genomic characterization of explant tumorgraft models derived from fresh patient tumor tissue. J Transl Med. 2012;10:125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Garcia PL, Council LN, Christein JD, Arnoletti JP, Heslin MJ, Gamblin TL, Richardson JH, Bjornsti MA, Yoon KJ. Development and histopathological characterization of tumorgraft models of pancreatic ductal adenocarcinoma. PLoS One. 2013;8:e78183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Grippo PJ, Tuveson DA. Deploying mouse models of pancreatic cancer for chemoprevention studies. Cancer Prev Res (Phila). 2010;3:1382-1387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Qiu W, Su GH. Development of orthotopic pancreatic tumor mouse models. Methods Mol Biol. 2013;980:215-223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 28. | Hruban RH, Adsay NV, Albores-Saavedra J, Anver MR, Biankin AV, Boivin GP, Furth EE, Furukawa T, Klein A, Klimstra DS. Pathology of genetically engineered mouse models of pancreatic exocrine cancer: consensus report and recommendations. Cancer Res. 2006;66:95-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 301] [Cited by in F6Publishing: 299] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 29. | Hidalgo M, Amant F, Biankin AV, Budinská E, Byrne AT, Caldas C, Clarke RB, de Jong S, Jonkers J, Mælandsmo GM. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. 2014;4:998-1013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1230] [Cited by in F6Publishing: 1119] [Article Influence: 111.9] [Reference Citation Analysis (0)] |
| 30. | Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. 2010;7:653-664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2184] [Cited by in F6Publishing: 2128] [Article Influence: 152.0] [Reference Citation Analysis (0)] |
| 31. | Ernsting MJ, Hoang B, Lohse I, Undzys E, Cao P, Do T, Gill B, Pintilie M, Hedley D, Li SD. Targeting of metastasis-promoting tumor-associated fibroblasts and modulation of pancreatic tumor-associated stroma with a carboxymethylcellulose-docetaxel nanoparticle. J Control Release. 2015;206:122-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 32. | Xu Q, Wang Z, Chen X, Duan W, Lei J, Zong L, Li X, Sheng L, Ma J, Han L. Stromal-derived factor-1α/CXCL12-CXCR4 chemotactic pathway promotes perineural invasion in pancreatic cancer. Oncotarget. 2015;6:4717-4732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 33. | Li JA, Xu XF, Han X, Fang Y, Shi CY, Jin DY, Lou WH. Nab-Paclitaxel Plus S-1 Shows Increased Antitumor Activity in Patient-Derived Pancreatic Cancer Xenograft Mouse Models. Pancreas. 2016;45:425-433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Saito K, Matsuo Y, Imafuji H, Okubo T, Maeda Y, Sato T, Shamoto T, Tsuboi K, Morimoto M, Takahashi H. Xanthohumol inhibits angiogenesis by suppressing nuclear factor-κB activation in pancreatic cancer. Cancer Sci. 2018;109:132-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 35. | Baxter LT, Jain RK. Transport of fluid and macromolecules in tumors. II. Role of heterogeneous perfusion and lymphatics. Microvasc Res. 1990;40:246-263. [PubMed] [Cited in This Article: ] |
| 36. | Baish JW, Gazit Y, Berk DA, Nozue M, Baxter LT, Jain RK. Role of tumor vascular architecture in nutrient and drug delivery: an invasion percolation-based network model. Microvasc Res. 1996;51:327-346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 184] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 37. | Beyer K, Partecke LI, Roetz F, Fluhr H, Weiss FU, Heidecke CD, von Bernstorff W. LPS promotes resistance to TRAIL-induced apoptosis in pancreatic cancer. Infect Agent Cancer. 2017;12:30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |