Published online Nov 28, 2017. doi: 10.3748/wjg.v23.i44.7930
Peer-review started: February 9, 2017
First decision: April 17, 2017
Revised: May 15, 2017
Accepted: June 18, 2017
Article in press: June 18, 2017
Published online: November 28, 2017
The underlying pathophysiology of liver dysfunction in urea cycle disorders (UCDs) is still largely elusive. There is some evidence that the accumulation of urea cycle (UC) intermediates are toxic for hepatocyte mitochondria. It is possible that liver injury is directly caused by the toxicity of ammonia. The rarity of UCDs, the lack of checking of iron level in these patients, superficial knowledge of UC and an underestimation of the metabolic role of fumaric acid, are the main reasons that are responsible for the incomprehension of the mechanism of liver injury in patients suffering from UCDs. Owing to our routine clinical practice to screen for iron overload in severely ill neonates, with the focus on the newborns suffering from acute liver failure, we report a case of citrullinemia with neonatal liver failure and high blood parameters of iron overload. We hypothesize that the key is in the decreased-deficient fumaric acid production in the course of UC in UCDs that causes several sequentially intertwined metabolic disturbances with final result of liver iron overload. The presented hypothesis could be easily tested by examining the patients suffering from UCDs, for liver iron overload. This could be easily performed in countries with a high population and comprehensive national register for inborn errors of metabolism. Conclusion: Providing the hypothesis is correct, neonatal liver damage in patients having UCD can be prevented by the supplementation of pregnant women with fumaric or succinic acid, prepared in the form of iron supplementation pills. After birth, liver damage in patients having UCDs can be prevented by supplementation of these patients with zinc fumarate or zinc succinylate, as well.
Core tip: Underlying pathophysiology of liver dysfunction in urea cycle disorders (UCDs) is still largely elusive. We hypothesize that the key is deficient fumaric acid production in urea cycle in UCDs, which causes several sequentially intertwined metabolic disturbances with the final result of liver iron overload. Providing the hypothesis is correct, neonatal liver damage in patients having UCD can be prevented by the supplementation of pregnant women with fumaric or succinic acid, prepared in the form of iron supplementation pills. After birth, liver damage in patients having UCDs can be prevented by supplementation of these patients with zinc fumarate or zinc succinylate.
- Citation: Ivanovski I, Ješić M, Ivanovski A, Garavelli L, Ivanovski P. Metabolically based liver damage pathophysiology in patients with urea cycle disorders - A new hypothesis. World J Gastroenterol 2017; 23(44): 7930-7938
- URL: https://www.wjgnet.com/1007-9327/full/v23/i44/7930.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i44.7930
Urea cycle defects (UCDs) occur in approximately 1 of 30000 live births[1]. The clinical hepatic presentation of the UCDs may include acute liver failure (ALF), liver dysfunction, and hepatocellular injury. The UCDs are not considered as significant metabolic diseases that cause severe liver dysfunction and ALF[2,3]. However, according to the previous reports, people affected by hepatocellular injury and ALF are also diagnosed as having ornithine transcarbamylase (OTC) deficiency[4,5], the most common UCD. Other UCDs are also associated with hepatocellular injury and liver failure[6-14]. Although liver dysfunctions and histopathological liver changes in patients with the UCDs have been reported since the late 1970s[15], the underlying pathophysiology of hepatic dysfunction has remained unknown. There is some evidence that the accumulation of urea cycle intermediates is toxic for hepatocyte mitochondria[16]. Recently it was shown, in vitro, that liver injury could be caused by direct toxicity of ammonia[17]. The toxicity of carbamoyl phosphate has also been proposed, but this is conjectural[18,19]. A variety of mitochondrial changes have been demonstrated on the electron microscopy of liver samples of patients with UCDs: An enlarged mitochondria with a swollen cristae and a dense matrix and numerous electron dense bodies of different sizes in the mitochondrial matrix[20]. Notably, these mitochondrial changes in the reported patients, were present exclusively in liver cells.
Neonatal hemochromatosis is another disorder followed by severe hepatic injury and associated with extrahepatic siderosis. NH is one of the most commonly recognized causes of liver failure in a neonate[21]. The presence of the UCDs and NH among neonates have very similar clinical and laboratory presentations. While the mechanism of liver damage in the NH is known[22], the mechanism of liver damage in the UCDs has not still been discovered.
Herein we present a case of a newborn affected by severe liver and central nervous system (CNS) toxicity. Furthermore, we aimed to provide a brand new and metabolically based view on the pathophysiology of liver failure among patients diagnosed with the UCDs, with the prospect of its prevention.
The propositus was a 2-day-old male, born at 39 gestation weeks, weighing 3610 g. He was the sixth child of non-consanguineous parents. All previous pregnancies were normal, and all children are healthy. No previous abortions have occurred. The family history was negative for metabolic diseases. During the first hours of life, the health of the newborn male worsened due to vomiting, lethargy and focal seizures. He was immediately transported from a local hospital to University Children’s Hospital for further examinations and treatment. Initial laboratory analyses showed an absence of hypoglycemia, total bilirubin level 42 μmol/L, conjugated fraction 28 μmol/L, alanine aminotransferase (ALT) level of 987 IU/L, aspartate aminotransferase (AST) level of 4165 IU/L and lactate dehydrogenase level 4760 IU/L. Ammonia level was 823 μmol/L. The coagulation test displayed extended partial thromboplastin time (PTT) of 59.5 s, extended prothrombin time (PT) of 22.2 s and international normalized ratio (INR) of 1.75. Fibrinogen level was normal (2.24 g/L). Having completed the routine screening of iron concentration (performed for neonatal iron overload), the results showed elevated iron serum concentration (30.7 μmol/L), very high ferritin (7145.6 ng/L), decreased serum transferrin concentration (1.35 g/L), TIBC 34.1 μmol/L and transferrin saturation 90% accordingly. A biopsy of submucosal oral salivary glands was done as well. Without knowing of histological findings of biopsy specimen, the patient was diagnosed as having NH. It was decided to treat the patient with blood exchange transfusion and intravenous immunoglobulin, but there was no improvement. Two days later, the pathologist reported that the biopsy specimen stained for iron deposits, was negative. The diagnosis of NH was immediately suspended and patient’s blood and urine samples were urgently sent to another medical institution for metabolic testing. The results of that testing showed: High citrulline (328 μmol/L, normal 10-21 μmol/L) and high alanine (847 μmol/L, normal range 274-384 μmol/L) levels, as well as high urine orotic acid excretion (0.9, normal < 0.14). This metabolic profile finding gave the final diagnosis of citrullinemia type I, a defect in the urea cycle, caused by the deficiency of argininosuccinate synthetase. The treatment was continued according to the rules for the treatment of acute hypeammomiemia[23]. It included prompt removal of ammonia from the body and providing the organism with adequate calories and essential amino acids to halt further breakdown of endogenous proteins. The patient showed visible clinical and laboratory improvement. The ammonia level fell to 46 μmol/L, ALT level 70 IU/L, AST 86 IU/L and LDH level 538 IU/L. Unfortunately, on the 20th day of the recovery process in the intensive care unit, the patient contracted sepsis caused by the multi-resistant hospital species of Enterobacter. Despite vigorous antimicrobial and supportive therapy, the patient started to suffer from gastrointestinal, intracranial and pulmonary hemorrhage, accompanied by the failure of vital functions. Ten days later the patient died.
Clinical presentation, laboratory tests findings and course of the disease indicate that the patient had UCD (i.e., citrullinemia). This is in accordance with diagnostic criteria used by Urea Cycle Disorders Consortium of the Rare Diseases Clinical Research Network[24], as well. In addition, we think that the patient had another very serious disease, a form of high level of iron concentration, which caused severe liver failure. Due to the absence of siderosis in the biopsy specimen of the oral salivary submucosal glands, neonatal hemochromatosis phenotype as a cause of the high level of iron concentration in the blood, hyperferritinemia and very high transferrin saturation was excluded. Besides in the above mentioned report, the following data did not match with the diagnosis of NH: (1) five previously born healthy children; (2) absence of late second and third trimester fetal loss[25]; (3) health of the patient immediately after birth; and (4) lack of improvement during the specific therapy applied in case of NH, i.e., intravenous immunoglobulin and exchange transfusion, as well.
Owing to the clinic’s routine practice of using screening for checking the high concentration of iron in neonates’ bodies, with the focus on the newborn children who suffer from acute liver failure, the presented case is, to our best knowledge, the first report of the high concentration of iron in newborns with UCD. No other cases of UCDs with high concentration of iron have been reported in the literature to date.
CNS toxicity manifested by lethargy and seizures can be explained by ammonia toxicity. Namely, the ammonia in CNS is detoxified using its own possibility to be accepted by α-keto glutarate and form glutamate. This produces the lack of α-keto glutarate in Krebs cycle in CNS cells, which become energy deprived. Liver toxicity in all existing cases of UCDs was explained in a speculative way[15,16,18-20]. Acute liver failure, i.e., liver toxicity in patients having UCD can be logically explained on a basis of an “isolated hepatic iron overload”. According to our postulation, this specific iron overload, is an inevitable occurrence in lives of many patients suffering from UCD. It is, probably, the cause of the liver damage that is seen among many UCD patients in their post neonatal life, as well.
There are several reasons which are responsible for the incomprehension of the mechanism of the liver injury in patients suffering from the UCDs. Firstly, there is a rarity of the UCDs. Secondly, there is a lack of checking of iron levels in these patients. The third reason is the superficial knowledge of the urea cycle (UC) and the underestimation of the metabolic role of fumaric acid (FA) which is a byproduct in the UC and its physiologic importance. During the preparation of this work we even found one scientific article in which the metabolic map of the UC was depicted without FA[26]. It is worth mentioning that several of the components and reactions of citric acid cycle were analyzed the 1930s in the research of the Nobel laureate Albert Szent-Györgyi. He received the Nobel Prize in 1937 for his discoveries pertaining to fumaric acid, a key component of the cycle[27].
For better understanding of our theory, at this point we have to remind the readers that the liver is the predominant hematopoietic organ through the period of weeks 20th to 24th week of gestation[28]. According to our theory, there are compounds whose metabolism is the key for understanding and elucidation of the mechanism of hepatic injury in the UCDs. These are succinyl-CoA, i.e., succinic acid (SA) and fumaric acid (FA), their intertwined pathways and their physiological roles.
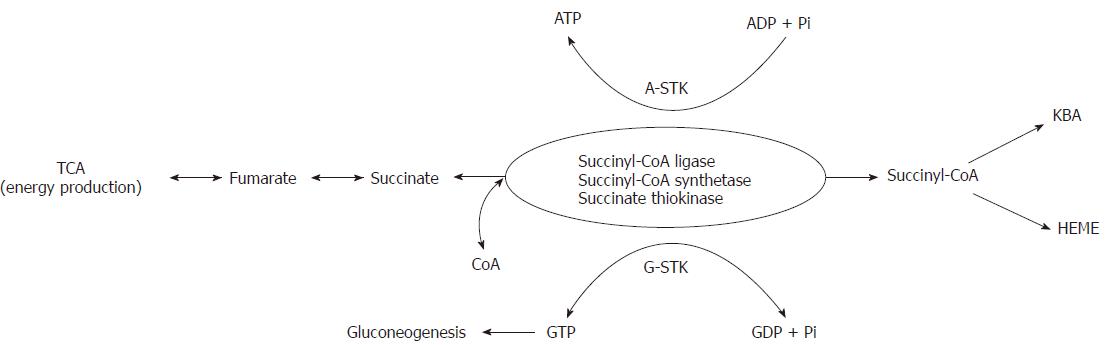
The sources of SA are exclusively located in mitochondria. SA is produced from α-ketoglutarate, methionine, isoleucine, valine, cholesterol and from β-oxidation of odd-chain fatty acids in the form of activated succinate, i.e., succinyl-CoA. Succinyl-CoA, which is easily converted to succinic acid and vice versa (via the catalytic activity of succinyl-CoA synthetase, also known as succinate thiokinase or succinyl-CoA ligase) has three metabolic roles. The first one is the energy production, through the course of TCA, in the form of nucleoside triphosphates (ATP and GTP). The second role is also related to the energy production via the ketone body activation (KBA) and utilization. The third metabolic role of succinyl-CoA is heme formation. These three physiological functions of SA are basically enabled by the catalytic activity of succinate thiokinase (STK). Mammalian cells have two distinct STKs. One STK is specific for GDP/GTP (G-STK) and the other STK is specific for ADP/ATP (A-STK). A-STK functions in TCA in the direction of succinyl-CoA breakdown for energy production in the form of ATP. G-STK has two metabolical roles. The first one is for the energy production in the form of GTP which is used for glucose production via gluconeogenesis. The second role presents reversing of SA into succinyl-CoA, at the expense of GTP, for ketone body activation and heme biosynthesis (Figure 1)[29-31].
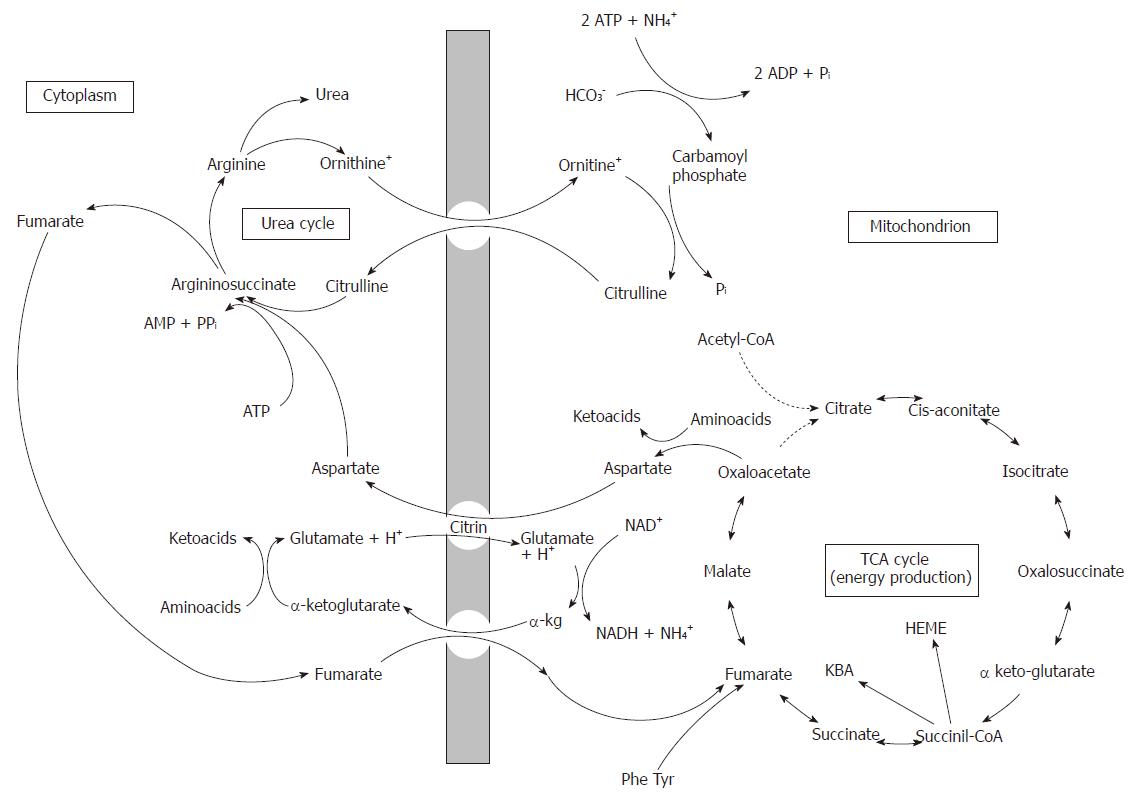
While SA is produced exclusively in mitochondria, FA is produced both, in mitochondria and in the cytosol. In mitochondria FA is gained from SA upon the catalytic action of succinate dehydrogenase, an enzyme located in mitochondria. The second mitochondrial production of FA is through the catabolism of phenylalanine (Phe) and tyrosine (Tyr). FA produced in mitochondria is primarily used for the energy production by converting to malate, and then to oxaloacetate. However, in mitochondria FA can be easily converted to succinate (upon catalytic activity of succinate dehydrogenase) and then to succinyl-CoA (via the activity of G-STK, for ketone body utilization and heme production). The cytosolic production of FA comes from the UC through the division of argininosuccinate to arginine and fumarate, by the action of argininosuccinate liase (or argininosuccinase). After adding water to fumarate forms L-malate, and subsequent NAD+-dependent oxidation converts L-malate to oxaloacetate. These two reactions are analogous to the reactions of the citric acid cycle, but are catalyzed by cytosolic fumarase and malate dehydrogenase. Oxaloacetate transforms by glutamate aminotransferase, then re-forms aspartate, which is used for the synthesis of citrulline[32]. Judging from the rate of gluconeogenesis[33], it could be concluded that the need for aspartate in the cytosol largely depends upon the supply from the mitochondria[34]. Therefore, it is reasonable to believe that most of the FA produced in the cytosol by the division of argininosuccinate is transferred into the mitochondria where it is metabolized by fumarase and malate dehydrogenase to oxaloacetate. In mitochondria, oxaloacetate is converted in aspartate which is, then, exported into the cytosol by citrin and is reused for urea synthesis (Figure 2).
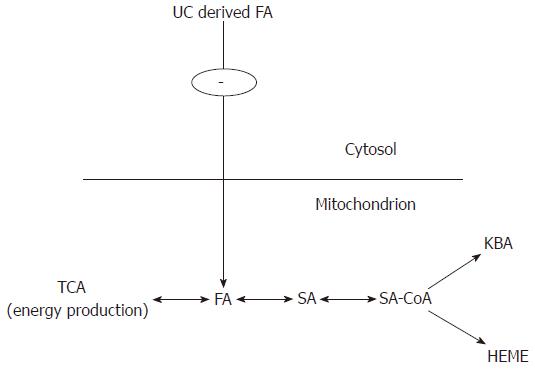
In conclusion, in accordance with our theory, FA and SA form a basic, mitochondrial dynamic exchangeable pool. Depending on the metabolical needs of the cell (energy production, ketone body activation and heme production) the chemical equilibrium can be directed to the left or to the right (Figure 3).
Considering the law of chemical equilibrium reactions (the law of Guldberg-Waage, the law of reversible chemical reactions, A + B ↔ A’ + B’) in the case of the deficiency of a compound involved in the reaction, the equilibrium is directed toward the deficiency. In the presented case (citrullinemia), this means that because of complete deficiency of cytosolic FA production, the equilibrium is directed to the left, i.e., toward FA, with the consequence of less SA available for ketone body activation and heme production. Compromised heme synthesis leads to compromised hemoglobin production. As a consequence, the decreased heme and hemoglobin production is “understood” (because evolution is still not perfect) as iron deficiency by the fetal hematopoietic hepatic tissue (“liver bone marrow”). An identical mechanism operates in the cases of thalassemias and other anemias with ineffective hematopoiesis, such as the congenital sideroblastic anemia and congenital dyserythropoietic anemia, where the defect is recognized as iron deficiency. In all these situations because of ″iron deficiency″ there is an increased iron influx because of decreased hepcidin production[35]. During fetal life placenta tightly controls the movement of iron from the mother to the fetus by mechanisms that are similar to those controlling the absorption of dietary iron from the intestine[36]. Therefore, in fetal deficient hematopoiesis, which is wrongly recognized as iron deficiency, expression of hepcidin is down regulated with consequential increase of placental iron influx. An excess of iron was transferred across the placenta through the hepcidin receptor ferroportin, bound to transferrin and most of this iron was transported directly into the liver, the main hematopoietic fetal organ. This could be a physiologic event, since humans possess various transferrin isoforms, that differ in their degree of glycosylation[37,38]. It is possible that each transferrin isoform refers to a specific cell/tissue/organ tropism. We argue that probably one of low glycosylated transferrin isoforms, known as carbohydrate-deficient transferrin, was involved in the transportation of the placental iron exclusively into the hepatic hematopoietic tissue. This argumentation could explain the absence of extrahepatic siderosis and the existence of high concentration of isolated hepatic iron and hepatic injury in the presented case. Not being used by erythroblasts for heme/hemoglobin synthesis because of the lack of FA, i.e., SA, the excess of the iron is deposited in hepatocytes and reticuloendothelial von Kupffer cells. In hepatocytes, the excess of iron is stored in the mitochondria (Fe++) and ferritin (Fe+++).
After birth, human being lives in the oxygen-enriched atmosphere. Our bodies require oxygen for many metabolic processes. However, oxygen is highly reactive and its interaction with non-chelated, i.e., free iron potentiates its toxicity. Normal cellular reactions, including respiration and “respiratory burst” generate reactive oxygen intermediates - superoxide free radical (O2-) and hydrogen peroxide (H2O2). Superoxide free radical is promptly dismutated into less toxic hydrogen peroxide, via the catalytic activity of superoxide dismutase. Superoxide and secondary reactive oxygen intermediates (H2O2), are potent antimicrobial agents. When the production of reactive oxygen species exceeds the processing capacity of the body, oxidative stress appears. Under these circumstances, reactive oxygen intermediates may be converted to much damaging radicals by the iron-catalyzed Fenton reaction[39], which is depicted bellow.
Fe2+ + H2O2 → Fe3+ + HO• + OH-
Fe3+ + H2O2 → Fe2+ + HOO• + H+
Hydroxyl radicals (HO•) and hydroperoxyl radicals (HOO•) promote the peroxidation of proteins, DNA and membrane lipids, problems that are exacerbated by the high concentration of iron. Certain organelles are particularly susceptible to the iron-dependent peroxidation. In the cells with the high concentration of iron, the injured mitochondria and lysosomes become leaky[40]. The mitochondrial damage and release of lysosomal proteases cause further cell injury and may ultimately lead to the cell death. This process causes severe tissue damage in the liver, heart, and endocrine organs of patients who have disorders due to the high concentration of iron. These deleterious properties of iron are threatening only when the element, i.e., iron is in a “free” state or in an abnormal form within the cell. This happens in the cases of its high quantity and if this surplus is accompanied by life in oxygen-enriched atmosphere. During fetal life, a fetus lives in a sterile atmosphere of the uterine cavity and in relatively low oxygen pressure. It is well known that oxygen physiologically diffuses down decreasing rate of partial pressure from air in the lungs PO2 (150 mmHg), pulmonary capillary vessels (105 mmHg), arterial blood (95 mmHg), placental vessels (30-40 mmHg), umbilical venous fetal blood (20-30 mmHg), equilibrate PO2 with the fetal tissues at PO2 of around 10-20 mmHg in a term fetus. Therefore, during the fetal life there is no peroxidation reaction and need for the “respiratory burst”. As a consequence of low oxygen partial pressure, there is no generation of reactive oxygen intermediates, superoxide free radical and hydrogen peroxide. In that way, it is reasonable to speculate that iron accumulated in the fetal body does not exert its toxicity via the catalytic Fenton reaction properties. Problems start immediately after birth with the first breath and activation of the pulmonary function, reaching the arterial oxygen partial pressure between 75 mmHg and 100 mmHg, as well as the bacterial invasion of the newborn and start of the “respiratory burst”.
UCDs with neonatal presentation and NH have very similar clinical and laboratory characteristics. The main difference is the timing of the hepatic damage that conducts the mode of presentation at birth. Newborns with UCDs, immediately after birth, are healthy. The problems commence after the activation of the pulmonary function and bacterial invasion, with the activation of iron toxicity, too. On contrary, in case of the NH, the liver injury starts during the intrauterine life and is complement-mediated. At birth, the child having NH is already severely ill because of the liver injury with further deterioration of the hepatic function. It is complemented with the injury of extrahepatic organs which already contain high concentration of iron. After birth and development of the pulmonary function and bacterial colonization of the newborn, accumulated iron starts to exert its toxicity via the generation of injurious radicals (hydroxyl radicals (HO•) and hydroperoxyl radicals (HOO•) by iron-catalyzed Fenton reaction. The severity of liver damage in the UCDs is proportional to the amount of iron excess and the degree of FA deficiency, which is in a correlation with the degree of enzyme deficiency. Complete enzyme deficiency results in very severe, life threatening neonatal liver disease, urgent for an instant liver transplant.
Presented hypothesis could be easily tested by the examining of patients suffering from UCDs, the newborns and the patients with post neonatal, late presentation, for liver iron overload by using specific magnetic resonance imagination (MRI) for pathologic iron deposition in their livers. Having in mind the rarity of UCDs, i.e., its incidence of only 1: 30000 live births[1], follows that this could be easily performed in countries with a high population and comprehensive national register for inborn errors of metabolism. The countries of choice for this purpose are the United States, Republic of China, Japan, Italy, France, Great Britain. In the United States with its 300000000 inhabitants, and approximately 3000000 of births per year, and UCD incidence of 1:30000 live births, each year approximately a hundred children have UCD. These children should be screened by MRI for liver iron overload. Provided the presented theory is correct, these children should have positive MRI for liver iron overload and would have a prospect for physiologically based mode of prevention.
The presented theory, provided correct, offers a prospect for the prevention of hepatic damage in UCDs, during the fetal life and after birth, throughout the whole life, as well. During the fetal life prevention could be achieved by the gestational fumarate supplementation of pregnant women, as it is a routine practice for pregnant women to be supplied with iron because of higher need for iron during the pregnancy. Proscribed iron according to the postulated theory, should be in the form of ferrous fumarate (or succinate). Taking into account Avogadro’s law number (6.022 × 1023), it could be easily calculated that one ferrous fumarate pill of 350 mg contains about 200 mg of FA, i.e., 1.0344 × 1021 FA molecules. These molecules can be converted into the same number of SA and succinyl-CoA subsequently via the catalytic activity of G-STK, in mitochondria. On the other hand, it is well known that one red blood cell contains about 280 million (28 × 107) hemoglobin molecules and that for the synthesis of one heme molecule there is a need of 8 SA molecules in the form of active succinate (succinyl-CoA)[41]. Since one hemoglobin molecule contains 4 globin chains and each globin chain binds one heme, it can be stated that for the synthesis of one hemoglobin molecule there is a need for 32 succinates, and for the synthesis of 280 million of hemoglobin molecules there is a need for 32 × 28 × 107 succinate molecules (i.e., 8.96 × 109 fumaric acid molecules). Thus, only one ferrous fumarate pill of 350 mg, of which 200 mg belong to FA, is sufficient for the production of 1.15 × 1011 red blood cells (1.0344 × 1021 FA molecules/8.96 × 109 FA molecules = 1.15 × 1011 red blood cells). When it comes to the fetus, at the 25th week of gestation, it has approximately 60 mL of blood volume[42] and a total of about 4.20 × 1011 fetal red blood cells (60 mL = 6 × 104 mm3, multiplied by the fetal red blood cell number about 7 × 106/mm3). In terms of the aforementioned points, the prevention of the fetal liver damage in fetuses having the UCDs, can be realized by the daily supplementation with 200 mg of fumaric acid in iron supplement formula during pregnancy.
Throughout their lives, many patients with the UCDs experience various degrees of the liver damage, without any explanation of the mechanism of the damage. In accordance with the presented theory, the pathophysiology is probably the same. It is caused by the high concentration of hepatic iron, because of the increased iron absorption and its transportation via the specific transferrin isoform into the liver, as a consequence of the compromised hematopoiesis (since the liver retains its genetically imprinted capability of hematopoiesis throughout the life). The postnatal ″liver hematopoiesis″ is compromised because of the fumarate deficiency, caused by the urea cycle enzymatic fumarate deficient production. After birth, the fumarate deficiency is further augmented by the protein restricted diet, which practically excludes protein catabolism and "expels" the UC from the hepatocytes. A physiologically required preventive dose of FA can be calculated upon the following consideration: Almost 1% of the body’s red blood cells are generated each day and the balance between red blood cell production and the removal of aging red blood cells from the circulation is precisely maintained. The ceaseless hematopoietic process replenishes the senescent cells that leave the circulation and produces nearly 200000000000 (2 × 1011) red blood cells per day[43]. As it was previously demonstrated, approximately 1.0344 × 1021 of FA molecules must be used for the heme synthesis of 200000000000 (2 × 1011) red blood cells. This quantity of FA is concentrated in only one ferrous fumarate pill of 350 mg. In this way, adult patients suffering from the UCDs can prevent the high concentration of iron, by daily consumption of 200 mg of fumarate. An identical calculation can be used for the succinate supplement on the basis of ferric-succinate formulas. The prevention of the hepatic iron excess during childhood can be also achieved with the fumarate-succinate supplementation, i.e., doses should be adjusted according to the age dependent erythropoietic kinetic properties. Bearing in mind that UCD patients are always subjected to an increasing risk of high concentrations of iron in their livers, we would suggest these patients to be supplemented with formulae on the basis of zinc-fumarate or zinc-succinate.
Urea cycle defects (UCDs) occur in approximately 1 of 30000 live births[1]. The clinical hepatic presentation of the UCDs may include acute liver failure (ALF), liver dysfunction, and hepatocellular injury.
This metabolic profile finding gave the final diagnosis of citrullinemia type I, a defect in the urea cycle, caused by the deficiency of argininosuccinate synthetase.
The treatment was continued according to the rules for the treatment of acute hypeammomiemia. It included prompt removal of ammonia from the body and providing the organism with adequate calories and essential amino acids to halt further breakdown of endogenous proteins. The patient showed visible clinical and laboratory improvement. The ammonia level fell to 46 μmol/L, alanine aminotransferase level 70 IU/L, aspartate aminotransferase 86 IU/L and LDH level 538 IU/L.
The prevention of the hepatic iron excess during childhood can be also achieved with the fumarate-succinate supplementation, i.e., doses should be adjusted according to the age dependent erythropoietic kinetic properties. Bearing in mind that UCD patients are always subjected to an increasing risk of high concentrations of iron in their livers, we would suggest these patients to be supplemented with formulae on the basis of zinc-fumarate or zinc-succinate.
The manuscript “Metabolically based liver damage pathophysiology in patients with urea cycle disorders-a new hypothesis” is and interested and very detail for the new hypothesis.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Serbia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Huang CM S- Editor: Qi Y L- Editor: A E- Editor: Lu YJ
| 1. | Ah Mew N, Lanpher B, Gropman A, Chapman K, Simspon K, Consortium . UCD, Summar M. Urea Cycle Disorders Overview. GeneReviews. Seattle: University of Washington, 2003- 2015; . [Cited in This Article: ] |
| 2. | Clayton PT. Inborn errors presenting with liver dysfunction. Semin Neonatol 200 2; 7: 49-63. . [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Clayton PT. Diagnosis of inherited disorders of liver metabolism. J Inherit Metab Dis. 2003;26:135-146. [PubMed] [Cited in This Article: ] |
| 4. | Mustafa A, Clarke JT. Ornithine transcarbamoylase deficiency presenting with acute liver failure. J Inherit Metab Dis. 2006;29:586. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Teufel U, Weitz J, Flechtenmacher C, Prietsch V, Schmidt J, Hoffmann GF, Kölker S, Engelmann G. High urgency liver transplantation in ornithine transcarbamylase deficiency presenting with acute liver failure. Pediatr Transplant. 2011;15:E110-E115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 6. | Morrow G, Barness LA, Efron ML. Citrullinemia with defective urea production. Pediatrics. 1967;40:565-574. [PubMed] [Cited in This Article: ] |
| 7. | Ito S, Kurasawa G, Yamamoto K, Furuta I, Ishihara F, Kobayashi K, Saheki T, Matsuura T, Yamauchi M, Kakinoki H. A pregnant patient with fulminant hepatic failure was found to carry a novel missense mutation in the argininosuccinate synthetase gene. J Gastroenterol. 2004;39:1115-1117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 8. | Güçer S, Aşan E, Atilla P, Tokatli A, Cağlar M. Early cirrhosis in a patient with type I citrullinaemia (CTLN1). J Inherit Metab Dis. 2004;27:541-542. [PubMed] [Cited in This Article: ] |
| 9. | de Groot MJ, Cuppen M, Eling M, Verheijen FW, Rings EH, Reijngoud DJ, de Vries MM, van Spronsen FJ. Metabolic investigations prevent liver transplantation in two young children with citrullinemia type I. J Inherit Metab Dis. 2010;33 Suppl 3:S413-S416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Faghfoury H, Baruteau J, de Baulny HO, Häberle J, Schulze A. Transient fulminant liver failure as an initial presentation in citrullinemia type I. Mol Genet Metab. 2011;102:413-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Fecarotta S, Parenti G, Vajro P, Zuppaldi A, Della Casa R, Carbone MT, Correra A, Torre G, Riva S, Dionisi-Vici C. HHH syndrome (hyperornithinaemia, hyperammonaemia, homocitrullinuria), with fulminant hepatitis-like presentation. J Inherit Metab Dis. 2006;29:186-189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Mhanni AA, Chan A, Collison M, Seifert B, Lehotay DC, Sokoro A, Huynh HQ, Greenberg CR. Hyperornithinemia-hyperammonemia-homocitrullinuria syndrome (HHH) presenting with acute fulminant hepatic failure. J Pediatr Gastroenterol Nutr. 2008;46:312-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Mori T, Nagai K, Mori M, Nagao M, Imamura M, Iijima M, Kobayashi K. Progressive liver fibrosis in late-onset argininosuccinate lyase deficiency. Pediatr Dev Pathol. 2002;5:597-601. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Sundaram SS, Alonso EM, Narkewicz MR, Zhang S, Squires RH. Characterization and outcomes of young infants with acute liver failure. J Pediatr. 2011;159:813-818.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 139] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 15. | LaBrecque DR, Latham PS, Riely CA, Hsia YE, Klatskin G. Heritable urea cycle enzyme deficiency-liver disease in 16 patients. J Pediatr. 1979;94:580-587. [PubMed] [Cited in This Article: ] |
| 16. | Wilson JM, Shchelochkov OA, Gallagher RC, Batshaw ML. Hepatocellular carcinoma in a research subject with ornithine transcarbamylase deficiency. Mol Genet Metab. 2012;105:263-265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Laemmle A, Gallagher RC, Keogh A, Stricker T, Gautschi M, Nuoffer JM, Baumgartner MR, Häberle J. Frequency and Pathophysiology of Acute Liver Failure in Ornithine Transcarbamylase Deficiency (OTCD). PLoS One. 2016;11:e0153358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Walker V. Ammonia toxicity and its prevention in inherited defects of the urea cycle. Diabetes Obes Metab. 2009;11:823-835. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Zimmermann A, Bachmann C, Baumgartner R. Severe liver fibrosis in argininosuccinic aciduria. Arch Pathol Lab Med. 1986;110:136-140. [PubMed] [Cited in This Article: ] |
| 20. | Zamora SA, Pinto A, Scott RB, Parsons HG. Mitochondrial abnormalities of liver in two children with citrullinaemia. J Inherit Metab Dis. 1997;20:509-516. [PubMed] [Cited in This Article: ] |
| 21. | Durand P, Debray D, Mandel R, Baujard C, Branchereau S, Gauthier F, Jacquemin E, Devictor D. Acute liver failure in infancy: a 14-year experience of a pediatric liver transplantation center. J Pediatr. 2001;139:871-876. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 165] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 22. | Feldman AG, Whitington PF. Neonatal hemochromatosis. J Clin Exp Hepatol. 2013;3:313-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 23. | Rezvani I. Urea Cycle and Hyperammonemia (Arginine, Citrulline, Ornithine). Nelson Textbook of Pediatrics 19th ed. WB Sounders. 2011;424-429. [Cited in This Article: ] |
| 24. | Tuchman M, Lee B, Lichter-Konecki U, Summar ML, Yudkoff M, Cederbaum SD, Kerr DS, Diaz GA, Seashore MR, Lee HS. Cross-sectional multicenter study of patients with urea cycle disorders in the United States. Mol Genet Metab. 2008;94:397-402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 150] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 25. | Whitington PF, Hibbard JU. High-dose immunoglobulin during pregnancy for recurrent neonatal haemochromatosis. Lancet. 2004;364:1690-1698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Tamamori A, Okano Y, Ozaki H, Fujimoto A, Kajiwara M, Fukuda K, Kobayashi K, Saheki T, Tagami Y, Yamano T. Neonatal intrahepatic cholestasis caused by citrin deficiency: severe hepatic dysfunction in an infant requiring liver transplantation. Eur J Pediatr. 2002;161:609-613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | “The Nobel Prize in Physiology or Medicine 1937” The Nobel Foundation. Retrieved 2011-10-26. . [Cited in This Article: ] |
| 28. | Fernández KS, de Alarcón PA. Development of the hematopoietic system and disorders of hematopoiesis that present during infancy and early childhood. Pediatr Clin North Am. 2013;60:1273-1289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Jenkins TM, Weitzman PD. Physiological roles of animal succinate thiokinases. Specific association of the guanine nucleotide-linked enzyme with haem biosynthesis. FEBS Lett. 1988;230:6-8. [PubMed] [Cited in This Article: ] |
| 30. | Jenkins TM, Weitzman PD. Distinct physiological roles of animal succinate thiokinases. Association of guanine nucleotide-linked succinate thiokinase with ketone body utilization. FEBS Lett. 1986;205:215-218. [PubMed] [Cited in This Article: ] |
| 31. | Johnson JD, Muhonen WW, Lambeth DO. Characterization of the ATP- and GTP-specific succinyl-CoA synthetases in pigeon. The enzymes incorporate the same alpha-subunit. J Biol Chem. 1998;273:27573-27579. [PubMed] [Cited in This Article: ] |
| 32. | Murray RK, Bender DA, Botham KM, Kennelly PJ, Rodwell VW, Anthony Weil P. Harper’s Illustrated Biochemistry, 28e > Chapter 28. USA: Catabolism of Proteins & of Amino Acid Nitrogen > by The McGraw-Hill Companies, Inc 2009; . [Cited in This Article: ] |
| 33. | Ross BD, Hems R, Krebs HA. The rate of gluconeogenesis from various precursors in the perfused rat liver. Biochem J. 1967;102:942-951. [PubMed] [Cited in This Article: ] |
| 34. | Saheki T, Kobayashi K. Mitochondrial aspartate glutamate carrier (citrin) deficiency as the cause of adult-onset type II citrullinemia (CTLN2) and idiopathic neonatal hepatitis (NICCD). J Hum Genet. 2002;47:333-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 222] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 35. | Fleming RE, Ponka P. Iron overload in human disease. N Engl J Med. 2012;366:348-359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 390] [Cited by in F6Publishing: 388] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 36. | Fleming RE, Bacon BR. Orchestration of iron homeostasis. N Engl J Med. 2005;352:1741-1744. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 151] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 37. | Arndt T, Stanzel S, Sewell AC. Paediatric age-dependent serum transferrin isoform distribution studied by HPLC. Clin Lab. 2007;53:575-582. [PubMed] [Cited in This Article: ] |
| 38. | Bortolotti F, De Paoli G, Tagliaro F. Carbohydrate-deficient transferrin (CDT) as a marker of alcohol abuse: a critical review of the literature 2001-2005. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;841:96-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 127] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 39. | Winterbourn CC. Toxicity of iron and hydrogen peroxide: the Fenton reaction. Toxicol Lett. 1995;82:969-974. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 813] [Cited by in F6Publishing: 825] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 40. | Frigerio R, Mela Q, Passiu G, Cacace E, La Nasa G, Perpignano G, Carcassi UE. Iron overload and lysosomal stability in beta zero-thalassaemia intermedia and trait: correlation between serum ferritin and serum N-acetyl-beta-D-glucosaminidase levels. Scand J Haematol. 1984;33:252-255. [PubMed] [Cited in This Article: ] |
| 41. | Murray RK, Bender DA, Botham KM, Kennelly PJ, Rodwell VW, Anthony Weil P. Harper’s Illustrated Biochemistry, 28e > Chapter 31. USA: Porphyrins & Bile Pigments > by The McGraw-Hill Companies, Inc. Copyright © 2009; . [Cited in This Article: ] |
| 42. | Smith GC, Cameron AD. Estimating human fetal blood volume on the basis of gestational age and fetal abdominal circumference. BJOG. 2002;109:721-722. [PubMed] [Cited in This Article: ] |
| 43. | Erslev AJ. Production of erythrocytes. In: Williams WJ, Beutler E, Erslev AJ, Lichtman MA. New York, NY, McGraw-Hill 1983; 365. [Cited in This Article: ] |