Published online May 14, 2017. doi: 10.3748/wjg.v23.i18.3338
Peer-review started: February 8, 2017
First decision: March 3, 2017
Revised: March 21, 2017
Accepted: April 12, 2017
Article in press: April 12, 2017
Published online: May 14, 2017
To investigate the reproducibility of the in vivo endoscopic ultrasound (EUS) - guided needle based confocal endomicroscopy (nCLE) image patterns in an ex vivo setting and compare these to surgical histopathology for characterizing pancreatic cystic lesions (PCLs).
In a prospective study evaluating EUS-nCLE for evaluation of PCLs, 10 subjects underwent an in vivo nCLE (AQ-Flex nCLE miniprobe; Cellvizio, MaunaKea, Paris, France) during EUS and ex vivo probe based CLE (pCLE) of the PCL (Gastroflex ultrahigh definition probe, Cellvizio) after surgical resection. Biopsies were obtained from ex vivo CLE-imaged areas for comparative histopathology. All subjects received intravenous fluorescein prior to EUS and pancreatic surgery for in vivo and ex vivo CLE imaging respectively.
A total of 10 subjects (mean age 53 ± 12 years; 5 female) with a mean PCL size of 34.8 ± 14.3 mm were enrolled. Surgical histopathology confirmed 2 intraductal papillary mucinous neoplasms (IPMNs), 3 mucinous cystic neoplasms (MCNs), 2 cystic neuroendocrine tumors (cystic-NETs), 1 serous cystadenoma (SCA), and 2 squamous lined PCLs. Characteristic in vivo nCLE image patterns included papillary projections for IPMNs, horizon-type epithelial bands for MCNs, nests and trabeculae of cells for cystic-NETs, and a “fern pattern” of vascularity for SCA. Identical image patterns were observed during ex vivo pCLE imaging of the surgically resected PCLs. Both in vivo and ex vivo CLE imaging findings correlated with surgical histopathology.
In vivo nCLE patterns are reproducible in ex vivo pCLE for all major neoplastic PCLs. These findings add further support the application of EUS-nCLE as an imaging biomarker in the diagnosis of PCLs.
Core tip: We performed a prospective study to investigate the reproducibility of in vivo endoscopic ultrasound (EUS) - guided needle based confocal endomicroscopy (nCLE) image patterns in an ex vivo setting and compare these to surgical histopathology for pancreatic cystic lesions (PCLs). A total of 10 subjects underwent in vivo EUS-nCLE and subsequently ex vivo CLE of the PCL following surgical resection. Biopsies were obtained from ex vivo CLE-imaged areas for comparative histopathology. We found that characteristic in vivo nCLE patterns were observed during ex vivo pCLE of resected PCLs. Both in vivo and ex vivo CLE imaging findings correlated with surgical histopathology. These findings support the application of EUS-nCLE in the diagnosis of PCLs.
- Citation: Krishna SG, Modi RM, Kamboj AK, Swanson BJ, Hart PA, Dillhoff ME, Manilchuk A, Schmidt CR, Conwell DL. In vivo and ex vivo confocal endomicroscopy of pancreatic cystic lesions: A prospective study. World J Gastroenterol 2017; 23(18): 3338-3348
- URL: https://www.wjgnet.com/1007-9327/full/v23/i18/3338.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i18.3338
Pancreatic cancer is projected to move from the third to second leading cause of cancer mortality before 2020[1]. In contrast to the steady survival increase for most other cancers, advances in management for pancreatic cancer have been less than modest with the 5-year relative survival rate is currently 8%[1]. The primary reason for the low survival is difficulty in early identification of pancreatic cancer. While pancreatic cystic lesions (PCLs) provide an opportunity for early cancer detection as many have malignant potential. There has been a surge in incidental detection of PCLs due to increasing utilization of cross-sectional imaging, but diagnostic differentiation of these lesions remains challenging[2,3].
Despite judicious utilization of endoscopic ultrasound (EUS), fine needle aspiration (FNA), cyst fluid analysis, and cytology, it is challenging to accurately classify PCLs into non-mucinous [serous cystadenomas (SCA), pseudocysts], pre-malignant mucinous [intraductal papillary mucinous neoplasm (IPMN) and mucinous cystic neoplasms (MCN)], and neoplastic [cystic-neuroendocrine tumors (NET), pseudopapillary tumor] PCLs. This is highlighted by a recent study at a large tertiary center involving 851 resected cystic tumors where the associated etiology for one in five cases was benign in nature[4].
Confocal laser endomicroscopy (CLE) offers real-time microscopic imaging of tissue where the system provides tissue-sequences with high resolution (1-3.5 μm) facilitating in vivo histopathology. Needle-based CLE (nCLE) is a new technology to evaluate PCLs where the device is pre-loaded in a 19-guage FNA needle for evaluation of the intracystic epithelium. Recent major trials have established reference standards and have all assessed the safety profile and feasibility of diagnostic capabilities of EUS-guided nCLE in patients with PCLs[5-8]. More over in vivo nCLE image patterns for PCLs have been internally and externally validated among independent observers[7-10].
While ex vivo confirmation of in vivo CLE findings has been demonstrated in diagnosis of Barrett’s esophagus and gastric adenocarcinoma, there are no human studies corroborating CLE findings of PCLs using ex-vivo examination and surgical histopathology[11-13]. We have previously published the technique and individual case reports of IPMN, MCN, SCA, and cystic-NET demonstrating potential feasibility for correlation between in vivo and ex vivo nCLE imaging with surgical histopathology[14-20].
The aim of this study was to validate the in vivo EUS-nCLE image patterns of specific types of PCLs by reproducing identical images in ex vivo pCLE examination and correlation with surgical histopathology.
The Institutional Review Board approved this prospective study, which was conducted at The Ohio State University Wexner Medical Center. From June 2015 to December 2016, all consenting subjects who underwent EUS-nCLE with subsequent surgical resection were enrolled in the INDEX study (Comparison of confocal laser endomicroscopic in vivo Diagnosis and ex vivo examination against surgical histopathology of cystic pancreatic lesions; ClinicalTrials.gov NCT02516488). An informed consent was obtained for both aspects (in vivo and ex vivo) aspects of the study. Our criteria for using EUS-nCLE included: (1) ≥ 18 years of age; (2) a PCL lesion size of ≥ 20 mm (determined by cross-sectional imaging studies); and (3) evaluation for surgical removal based on recommended international consensus guidelines[21]. Exclusion criteria were: (1) women with known pregnancy at the time of procedure; (2) coagulopathy (INR > 1.5 and/or platelets < 50000/mL); and (3) known allergy to fluorescein. Ex vivo pCLE of PCLs was performed on representative cases of common types of PCLs.
Demographics, history of present illness, laboratory data, and image findings were collected using a standardized data collection form. Imaging data were compiled with those from EUS to describe: location, number and size of the PCLs, lesion characteristics, evidence of dilation of the main pancreatic duct, and presence of communication with the pancreatic duct. One gastrointestinal pathologist reviewed all surgical histopathology specimens and the biopsies obtained during ex vivo CLE examination.
All EUS examinations were performed at The Ohio State University Medical Center using a linear echoendoscope (Olympus America, Center Valley, PA, United States). All EUS examinations were performed under the direction of an anesthesiologist utilizing intravenous (IV) propofol. Fluorescein (5 mL; 10% fluorescein sodium) was intravenously injected 2 to 3 min prior to CLE imaging. The AQ-Flex nCLE miniprobe (Cellvizio, Mauna Kea Technologies, Paris, France) was then advanced through the locking device into the 19-gauge (g) needle (Flex needle, Boston Scientific, Natick, MA, United States). The preloaded 19-g needle was advanced under EUS-guidance into the PCL. The tip of the nCLE probe was negotiated until it opposed the intracystic epithelium. Intracystic endomicroscopic images (video) were captured with permissible angulation of the 19 g needle using the elevator of the echoendoscope. After image acquisition, the nCLE probe was withdrawn and the PCL was aspirated. Antibiotic (quinolone) prophylaxis was administered via IV route on the day of procedure followed by 3 d of oral therapy.
We have recently published a video manual of the ex vivo pCLE imaging technique[19]. Immediately prior to resection of the part of the pancreas with the cystic lesion and under the direction of the surgeon and anesthetist, fluorescein (10%, 5 mL) was intravenously injected before ligation of blood vessels supplying the pancreas. Following resection, the specimen was transported to the pathology-processing laboratory for immediate processing as the pre-ligation IV fluorescein is retained for a maximum of one hour after injection. The pathologist then incised the cyst along the long axis using their standard processing technique. The epithelium of the PCL was then imaged using a Gastroflex ultrahigh definition probe (UHD) probe (Cellvizio, Mauna Kea Technologies, Paris, France) at 3-5 random areas based on the size of the cyst. Site-specific biopsies were then obtained at the pCLE-imaged areas using standard endoscopy biopsy forceps (Radial Jaw 4, Boston Scientific, Natick, MA, United States). The ex vivo biopsies were obtained from the PCL sites providing the clearest pCLE image.
The specific characteristics of the CLE probes used for the study are described in Table 1. The AQ-Flex nCLE probe was used during in vivo EUS-guided approach while the Gastroflex-UHD pCLE probe with high-definition image acquisition was used during ex vivo post-surgical cyst interrogation. The larger GastroFlex-UHD miniprobe has increased number of fiber optics and provides higher magnification and improved resolution; however, due to its size, the probe cannot be accommodated through the working channel of 19-g EUS needle. This concept of comparing in vivo to ex vivo imaging is derived from a prior study showing enhanced image acquisition of Gastroflex when compared to CholangioFlex miniprobe (Cellvizio, Mauna Kea Technologies, Paris, France) for assessing indeterminate biliary strictures where individual structures were more easily identified[22]. The Gastroflex-UHD probe has a superior lateral resolution of 1 μm, compared to 3.5 μm for the Cholangioflex probe. The lateral resolutions of the AQ-Flex and Cholangioflex probes are identical[23].
| Device | Channel size (mm) | Field of view (μm) | Resolution (μm) | Confocal depth (μm) | |
| GastroflexTM UHD | Probe based | ≥ 2.8 | 240 | 1.00 | 55-65 |
| AQ-FlexTM 19 | Needle based | ≥ 0.91 | 325 | 3.50 | 40-70 |
| CholangioflexTM | Probe based | ≥ 1.0 | 325 | 3.50 | 40-70 |
| Standard microscopy × 20 | Microscope | NA | NA | 0.70 | NA |
| Standard microscopy × 40 | Microscope | NA | NA | 0.45 | NA |
The resected specimen underwent standard histopathologic processing. The biopsies obtained from pCLE imaged specific sites also underwent standard processing under the supervision of a gastroenterologist pathologist.
A dedicated software (Cellvizio Viewer, version 1.6.1; Mauna Kea Technologies, Paris, France) was used to review all in vivo and ex vivo CLE videos and images. A diligent frame-by-frame review of the videos was performed multiple times to document the most illustrative image patterns. The various image patterns observed during CLE examination of the PCLs are described in Table 2. There was no blinded assessment of the pre-and postoperative CLE images.
| Variable | Explanation of patterns | Cyst type where pattern is observed |
| Papillae | A papilla is a finger-like projection of variable length consisting of an overlying epithelium and underlying vascular core | IPMN |
| Epithelial bands | Epithelial bands are either single or multiple layers of epithelium without a papillary configuration. These bands demonstrated layering or a horizon-type configuration | MCN |
| Trabecular pattern | Nests of cells separated by blood vessels of fibrous bands | Cystic-NET |
| Fern pattern | A concentrated network of parallel vessels emanating from a central vessel similar to a fern-leaf | SCA |
CLE imaging offers an “en-face” view and has resolutions ranging from 1 μm (Gastroflex probe) to 3.5 μm (AQ-Flex probe). In comparison, standard biopsy or surgical resection and histopathology reveals a “transverse view”, but offers a much higher resolution than CLE imaging, which increases with magnification (Table 1).
A total of 10 subjects (mean age 53 ± 12 years [SD]; 5 female) underwent EUS-nCLE with surgical resection and subsequent ex vivo imaging (Table 3). The mean size of PCLs was 34.8 ± 14.3 mm with the majority of lesions located in the tail (n = 5) when compared to head/uncinate (n = 2) or neck/body region (n = 3). No adverse events occurred during the surgical resection that impacted the quality of the specimen.
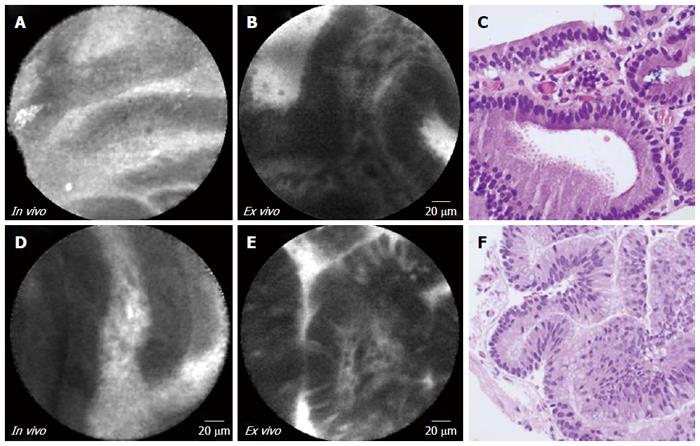
| Subject | Corresponding Figure | Gender | Age | Abdominal symptoms | Size (mm) | Location | MPD communication | MPD dilation | Cyst CEA(ng/dL) | Final diagnosis | Pathological features |
| 1 | 1A-C | Female | 67 | Symptomatic | 21 | Head/uncinate | Yes | Yes | 188 | IPMN | Gastric subtype |
| High grade dysplasia | |||||||||||
| 2 | 1D-F | Male | 71 | Incidental | 40 | Head/uncinate | Yes | Yes | Very viscous | IPMN | Intestinal subtype |
| High grade dysplasia | |||||||||||
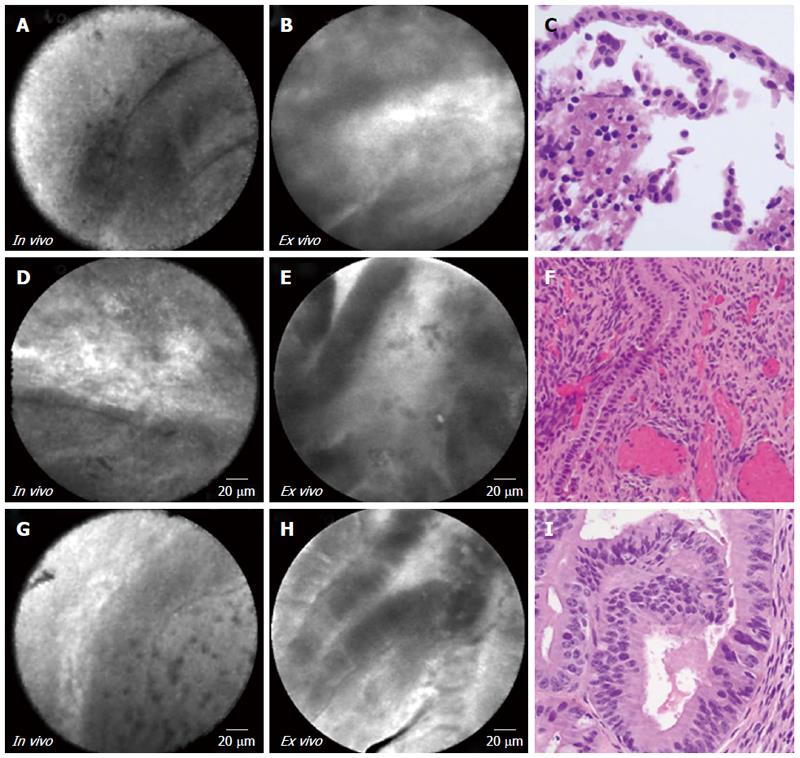
| 4 | 2A-C | Female | 47 | Incidental | 28 | Neck/body | No | No | 6512 | Mucinous | Low grade |
| cystadenoma | |||||||||||
| 3 | 2D-F | Female | 51 | Symptomatic | 41 | Neck/body | No | Yes | 76 | Mucinous | Low grade |
| cystadenoma | |||||||||||
| 5 | 2G-I | Female | 45 | Symptomatic | 24 | Neck/body | No | Yes | 2400 | Mucinous | Low to moderate grade |
| cystadenoma | |||||||||||
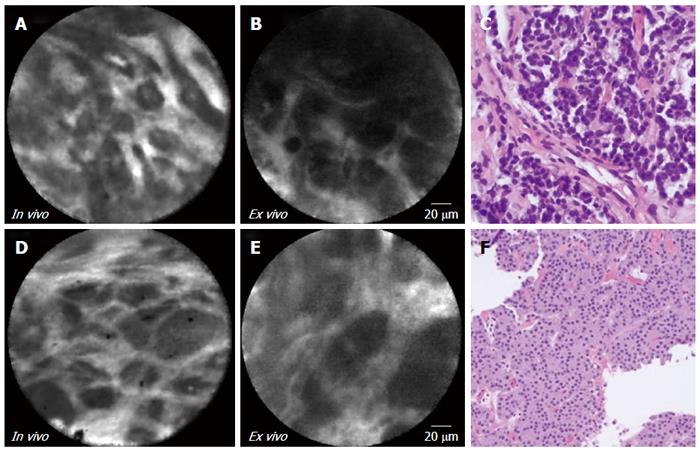
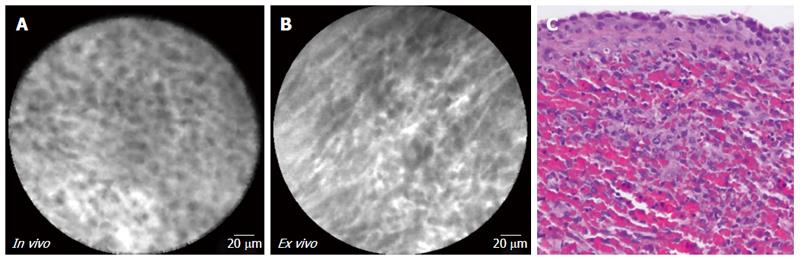
| 6 | 3A-C | Male | 44 | Symptomatic | 57 | Tail | No | No | 1.5 | Cystic-NET | |
| 7 | 3D-F | Male | 30 | Incidental | 21 | Tail | No | No | 4.7 | Cystic-NET | |
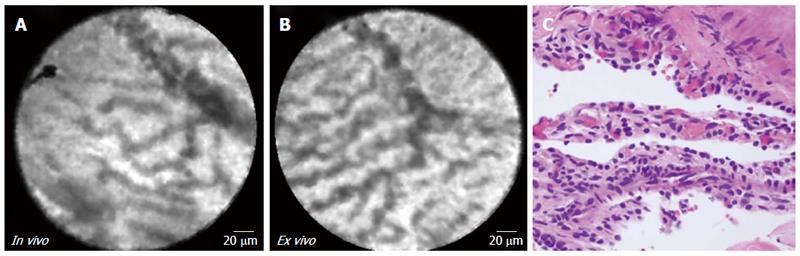
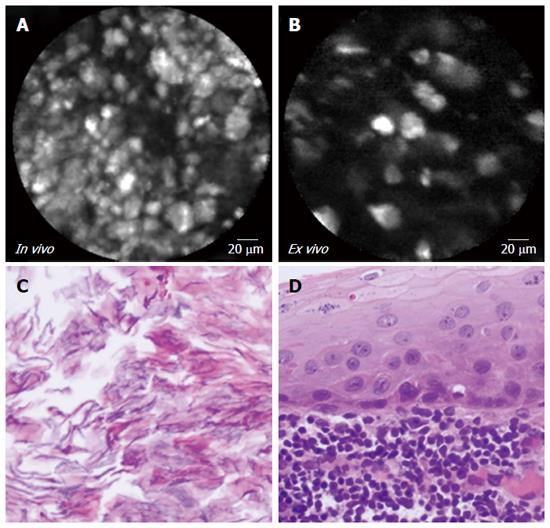
| 8 | 4A-C | Female | 59 | Incidental | 60 | Tail | No | No | 0.5 | Serous cystadenoma | |
| 9 | 5A-C | Male | 52 | Incidental | 31 | Tail | No | No | Pasty aspirate | Lymphoepithelial cyst | |
| 10 | 6A-D | Male | 62 | Incidental | 25 | Tail | No | No | 2664 | Epidermoid cyst |
Complete “finger-like” papillary projections were seen on in vivo EUS-nCLE and ex vivo pCLE imaging for both patients with IPMNs (Figure 1). The vascular cores (lamina propria) of the papillae were better defined in the ex vivo imaging. There was no difference in in vivo or ex vivo imaging between the different subtypes (gastric vs intestinal) of IPMN lesions. The CLE images and histopathology were similar.
EUS-nCLE demonstrated horizon-type epithelial bands of variable thickness without papillary conformation (Figure 2 and Table 3). Ex vivo imaging showed thicker epithelial bands with improved definition. MCN with moderate grade dysplasia (Figure 2, panels G to I) revealed a thicker epithelial band. During in vivo nCLE, MCNs revealed areas of denuded epithelium as evidenced by lack of visualizing any epithelial bands. Few foci of dark background with bright particles (auto-fluorescent inflammatory cells) were also observed representing areas of chronic inflammation. While the characteristic “ovarian stroma” was detected in histopathology, no corresponding CLE features were observed.
In vivo and ex vivo imaging (Figure 3 and Table 3) revealed dark clusters or trabeculae of cells separated by bright vascular spaces. Corroborating this finding, corresponding biopsies from the cystic-NETs revealed characteristic well-differentiated NETs, which were confirmed by immunostaining.
Both EUS-nCLE and ex vivo imaging (Figure 4 and Table 3) depict a “fern pattern” of vascularity that is best described as a concentrated parallel or interconnected network of vessels emanating from a larger vessel (similar to a fern leaf). However, the vascular pattern observed in CLE imaging is not represented in histopathology. Characteristic histology of SCAs include multiple cystic spaces lined by cuboidal/flat epithelial cells. The pathology image in Figure 4 (panel C) revealed flattened cystic spaces lined by cuboidal epithelial cells.
Two distinct benign cysts, epidermoid cyst of intra pancreatic accessory spleen (IPAS) and lymphoepithelial cyst, were included in this study. The epidermoid cyst of IPAS demonstrated cords of cells suggestive of ectopic splenic tissue. Pathology confirmed these findings revealing a thin squamous epithelium and underlying splenic red pulp (Figure 5 and Table 3). On the other hand, the lymphoepithelial cyst had clusters of bright particles that correlated to keratinous debris seen on pathologic slides (Figure 6 and Table 3). Microscopy demonstrated keratin flakes and the cyst wall was lined by squamous epithelium bordered by abundant lymphoid tissue.
This study confirms the reproducibility of in vivo EUS-nCLE image patterns in ex vivo pCLE examination of surgically resected PCLs. The histopathology from CLE imaged site-specific biopsies were comparable to CLE patterns. Some variations in histological views can be explained by higher resolution and the plane of image reproduction. While EUS-nCLE produces en-face microscopic imaging of the epithelium of PCLs, tissue histology reveals transverse views. To our knowledge, this is the largest study describing in vivo and ex vivo CLE findings in definitively diagnosed PCLs. We have correlated for the first time, CLE image patterns with surgical histopathology among common PCLs. These promising findings and growing body of literature lend support to further investigation of EUS-nCLE in the management of PCLs.
The management of PCLs continues to pose challenges. Suboptimal classification and risk stratification of PCLs can lead to inappropriate surgeries or false reassurances. The current guidelines for management of PCLs are not robust since the diagnostic accuracy of current standard of care (cyst fluid CEA, cytology) is inadequate. There is an increasing need for tools to accurately diagnose PCLs. Over the last 5 years, there is an accumulative body of evidence of applying EUS-nCLE or novel cyst fluid molecular makers in diagnosing PCLs[6-10,24].
Imaging data from three major clinical trials have recognized specific nCLE image patterns for diagnosing PCLs[5-8]. We have recently validated (internally and externally, inter-and intraobserver) nCLE image patterns of common PCLs[9,10]. We have also published on the technique of in vivo and ex vivo CLE imaging of PCLs, and individual nCLE video reports of IPMNs, MCNs, SCAs, Cystic-NETs, and squamous lined cysts[15-18,20]. In this study, we performed ex vivo CLE examination of resected PCLs from subjects enrolled in a prospective study.
For EUS-nCLE aided diagnosis, IPMNs were diagnosed by the presence of finger-like papillae. Although the nCLE image patterns for MCNs were slightly insufficient, they contained a characteristic either single or multiple (layered) band-like epithelium[8,9]. Thus, the presence of complete papillae or single/multiple band-like epithelium was diagnostic of a mucinous PCL. The diagnosis of IPMNs tends to be easier than that of MCNs since the latter demonstrate relatively flat or horizon-type epithelium which can be patchy with atrophic areas and foci of inflammation[25,26]. We have observed that some MCNs can demonstrate bright inflammatory cells on a dark background suggestive of chronic inflammation similar to pseudocysts[7,8].
A characteristic “superficial vascular network” or “fern pattern” has been observed in SCAs where the specificity approached 100%[5-10]. Comparable image reproduction in ex vivo CLE imaging and corresponding histopathological image supports evidence from current studies. The nCLE imaging of cystic-NETs and comparable ex vivo image patterns and correlative histopathology confirm published reports[8,15]. Some rare types of PCLs can be difficult to distinguish by cross sectional imaging and are often evaluated for malignant potential[27]. Thus, awareness of in vivo EUS-nCLE image patterns of rare squamous lined PCLs (lymphoepithelial cyst and epidermoid cyst of IPAS) is useful as it may help avoid unnecessary surgical resection.
The small sample size (n = 10) is not suitable for statistical analysis and the images observed in this study may not fully characterize all patients with the examined cyst types. As with other novel diagnostic modalities, we anticipate further refinement of technical aspects and additional characterization of nCLE imaging patterns in the future. Although our ex vivo nCLE image findings were not externally validated, we have previously performed both internal and external validation of the in vivo nCLE image patterns[9,10]. Lastly, since surgical resection of pseudocysts rarely performed, we did not perform ex vivo imaging of these lesions within the study period.
In conclusion, the evaluation of PCLs continues to pose a challenge. In uncertain clinical situations, a composite approach including clinical features, imaging characteristics, cyst fluid CEA, cytological examination, and nCLE is necessary. The correlation of histopathology and the reproducibility of in vivo and ex vivo CLE imaging patterns supports the application of EUS-nCLE as an imaging biomarker in the diagnosis of PCLs. Multicenter prospective studies are needed to confirm whether EUS-nCLE alone or in combination with cyst fluid molecular markers can facilitate desirable outcomes in managing pancreatic cystic lesions.
Endoscopic ultrasound (EUS) and fine needle aspiration (FNA) are standard of care for evaluation of pancreatic cystic lesions (PCLs). Needle-based CLE (nCLE) is a new technology that offers real-time microscopic imaging of tissue facilitating in vivo histopathology. The authors have previously published the technique of in vivo and ex vivo CLE imaging of PCLs. The aim of this study was to validate the in vivo EUS-nCLE image patterns of specific types of PCLs by reproducing identical images in ex vivo pCLE examination and correlation with surgical histopathology.
The current guidelines for management of PCLs are not robust since the diagnostic accuracy of current standard of care is inadequate. There is an increasing need for novel technology to accurately diagnose PCLs. Over the last 5 years, there is an accumulative body of evidence of applying EUS-nCLE in diagnosing PCLs.
This study confirms the reproducibility of in vivo EUS-nCLE image patterns in ex vivo pCLE examination of surgically resected PCLs. The histopathology from CLE imaged site-specific biopsies were comparable to CLE patterns. These promising findings lend support to the application of EUS-nCLE in the management of PCLs.
Confocal laser endomicroscopy: A novel endoscopic technology that offers real-time microscopic imaging of tissue where the system provides tissue-sequences with high resolution (1-3.5 μm) facilitating in vivo histopathology.
This new endoscopic technique which is based on confocal microscopy seems very interesting. It allows a pathological diagnosis by acquisition of images that are pathognomonic of the various pancreatic cystic lesions examinated by the authors.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kleeff J, Tonelli F S- Editor: Ma YJ L- Editor: A E- Editor: Zhang FF
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12135] [Cited by in F6Publishing: 12706] [Article Influence: 1588.3] [Reference Citation Analysis (2)] |
| 2. | de Jong K, Nio CY, Hermans JJ, Dijkgraaf MG, Gouma DJ, van Eijck CH, van Heel E, Klass G, Fockens P, Bruno MJ. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol. 2010;8:806-811. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 367] [Cited by in F6Publishing: 346] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 3. | Laffan TA, Horton KM, Klein AP, Berlanstein B, Siegelman SS, Kawamoto S, Johnson PT, Fishman EK, Hruban RH. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802-807. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 616] [Cited by in F6Publishing: 617] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 4. | Valsangkar NP, Morales-Oyarvide V, Thayer SP, Ferrone CR, Wargo JA, Warshaw AL, Fernández-del Castillo C. 851 resected cystic tumors of the pancreas: a 33-year experience at the Massachusetts General Hospital. Surgery. 2012;152:S4-S12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 284] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 5. | Konda VJ, Meining A, Jamil LH, Giovannini M, Hwang JH, Wallace MB, Chang KJ, Siddiqui UD, Hart J, Lo SK. A pilot study of in vivo identification of pancreatic cystic neoplasms with needle-based confocal laser endomicroscopy under endosonographic guidance. Endoscopy. 2013;45:1006-1013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 132] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 6. | Nakai Y, Iwashita T, Park DH, Samarasena JB, Lee JG, Chang KJ. Diagnosis of pancreatic cysts: EUS-guided, through-the-needle confocal laser-induced endomicroscopy and cystoscopy trial: DETECT study. Gastrointest Endosc. 2015;81:1204-1214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 122] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 7. | Napoléon B, Lemaistre AI, Pujol B, Caillol F, Lucidarme D, Bourdariat R, Morellon-Mialhe B, Fumex F, Lefort C, Lepilliez V. A novel approach to the diagnosis of pancreatic serous cystadenoma: needle-based confocal laser endomicroscopy. Endoscopy. 2015;47:26-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 8. | Napoleon B, Lemaistre AI, Pujol B, Caillol F, Lucidarme D, Bourdariat R, Morellon-Mialhe B, Fumex F, Lefort C, Lepilliez V. In vivo characterization of pancreatic cystic lesions by needle-based confocal laser endomicroscopy (nCLE): proposition of a comprehensive nCLE classification confirmed by an external retrospective evaluation. Surg Endosc. 2016;30:2603-2612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 9. | Krishna SG, Brugge WR, Dewitt JM, Kongkam P, Napoleon B, Robles-Medranda C, Tan D, El-Dika S, McCarthy S, Walker J. Needle-based confocal laser endomicroscopy for the diagnosis of pancreatic cystic lesions: an international external interobserver and intraobserver study (with videos). Gastrointest Endosc. 2017; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 10. | Krishna SG, Swanson B, Hart PA, El-Dika S, Walker JP, McCarthy ST, Malli A, Shah ZK, Conwell DL. Validation of diagnostic characteristics of needle based confocal laser endomicroscopy in differentiation of pancreatic cystic lesions. Endosc Int Open. 2016;4:E1124-E1135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Kakeji Y, Yamaguchi S, Yoshida D, Tanoue K, Ueda M, Masunari A, Utsunomiya T, Imamura M, Honda H, Maehara Y. Development and assessment of morphologic criteria for diagnosing gastric cancer using confocal endomicroscopy: an ex vivo and in vivo study. Endoscopy. 2006;38:886-890. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Lim LG, Yeoh KG, Salto-Tellez M, Khor CJ, Teh M, Chan YH, So JB, Rajnakova A, Shen E, Srivastava S. Experienced versus inexperienced confocal endoscopists in the diagnosis of gastric adenocarcinoma and intestinal metaplasia on confocal images. Gastrointest Endosc. 2011;73:1141-1147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Leggett CL, Gorospe EC, Chan DK, Muppa P, Owens V, Smyrk TC, Anderson M, Lutzke LS, Tearney G, Wang KK. Comparative diagnostic performance of volumetric laser endomicroscopy and confocal laser endomicroscopy in the detection of dysplasia associated with Barrett’s esophagus. Gastrointest Endosc. 2016;83:880-888.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 14. | Modi RM, Swanson B, Muscarella P, Conwell DL, Krishna SG. Novel techniques for diagnosis of serous cystadenoma: fern pattern of vascularity confirmed by in vivo and ex vivo confocal laser endomicroscopy. Gastrointest Endosc. 2017;85:258-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Kamboj AK, Swanson B, Dillhoff ME, Conwell DL, Krishna SG. Cystic pancreatic neuroendocrine tumors: correlation of in vivo needle-based confocal endomicroscopic findings by ex vivo analysis. Gastrointest Endosc. 2017;85:259-260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Modi RM, Kamboj AK, Swanson B, Conwell DL, Krishna SG. Novel technique for diagnosis of mucinous cystic neoplasms: in-vivo and ex-vivo confocal laser endomicroscopy. VideoGIE. 2016;2:55-56. [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Modi RM, Kamboj AK, Swanson B, Conwell DL, Krishna SG. Epidermoid cyst within an intrapancreatic accessory spleen: endosonography and confocal endomicroscopy of an unusual pancreatic cystic lesion. Endoscopy. 2016;48:E332-E333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Li F, El-Dika S, Modi RM, Chen W, Krishna SG. Poorly differentiated pancreatic carcinoma with sarcomatoid differentiation: confocal endomicroscopy of an uncommon pancreatic cystic lesion. Endoscopy. 2016;48:E363-E364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Kamboj AK, Modi RM, Swanson B, Conwell DL, Krishna SG. A comprehensive examination of the novel techniques used for in vivo and ex vivo confocal laser endomicroscopy of pancreatic cystic lesions. VideoGIE. 2016;1:6-7. [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Krishna SG, Swanson B, Conwell DL, Muscarella P. In vivo and ex vivo needle-based confocal endomicroscopy of intraductal papillary mucinous neoplasm of the pancreas. Gastrointest Endosc. 2015;82:571-572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183-197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1714] [Cited by in F6Publishing: 1540] [Article Influence: 128.3] [Reference Citation Analysis (0)] |
| 22. | Shieh FK, Drumm H, Nathanson MH, Jamidar PA. High-definition confocal endomicroscopy of the common bile duct. J Clin Gastroenterol. 2012;46:401-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Nakai Y, Isayama H, Shinoura S, Iwashita T, Samarasena JB, Chang KJ, Koike K. Confocal laser endomicroscopy in gastrointestinal and pancreatobiliary diseases. Dig Endosc. 2014;26 Suppl 1:86-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 24. | Theisen BK, Wald AI, Singhi AD. Molecular Diagnostics in the Evaluation of Pancreatic Cysts. Surg Pathol Clin. 2016;9:441-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Volkan Adsay N. Cystic lesions of the pancreas. Mod Pathol. 2007;20 Suppl 1:S71-S93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 146] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 26. | Gómez V, Majumder S, Smyrk TC, Topazian MD, Chari ST, Gleeson FC, Harmsen WS, Enders FT, Abu Dayyeh BK, Iyer PG. Pancreatic cyst epithelial denudation: a natural phenomenon in the absence of treatment. Gastrointest Endosc. 2016;84:788-793. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Uchiyama S, Chijiiwa K, Hiyoshi M, Ohuchida J, Imamura N, Nagano M, Hidaka H, Yorita K, Akiyama Y, Nishiura M. Intrapancreatic accessory spleen mimicking endocrine tumor of the pancreas: case report and review of the literature. J Gastrointest Surg. 2008;12:1471-1473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |