Published online Mar 21, 2017. doi: 10.3748/wjg.v23.i11.2002
Peer-review started: November 13, 2016
First decision: December 19, 2016
Revised: January 7, 2017
Accepted: February 17, 2017
Article in press: February 17, 2017
Published online: March 21, 2017
Processing time: 129 Days and 23.7 Hours
To explore the effect of interleukin (IL)-22 on in vitro model of alcoholic liver fibrosis hepatic stellate cells (HSCs), and whether this is related to regulation of Nrf2-keap1-ARE.
HSC-T6 cells were incubated with 25, 50, 100, 200 and 400 μmol/L acetaldehyde. After 24 and 48 h, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to detect proliferation of HSCs to choose the best concentration and action time. We used the optimal concentration of acetaldehyde (200 μmol/L) to stimulate HSCs for 24 h, and treated the cells with a final concentration of 10, 20 or 50 ng/mL IL-22. The cell proliferation rate was detected by MTT assay. The cell cycle was analyzed by flow cytometry. The expression of nuclear factor-related factor (Nrf)2 and α-smooth muscle antigen was detected by western blotting and immunocytochemistry. The levels of malondialdehyde (MDA) and glutathione (GSH) were measured by spectrophotometry.
In the MTT assay, when HSCs were incubated with acetaldehyde, activity and proliferation were higher than in the control group, and were most obvious after 48 h treatment with 200 μmol/L acetaldehyde. The number of cells in G0/G1 phases was decreased and the number in S phase was increased in comparison with the control group. When treated with different concentrations of IL-22, HSC-T6 cell activity and proliferation rate were markedly decreased in a dose-dependent manner, and cell cycle progression was arrested from G1 to S phase. Western blotting and immunocytochemistry demonstrated that expression of Nrf2 total protein was not significantly affected. Expression of Nrf2 nuclear protein was low in the control group, increased slightly in the model group (or acetaldehyde-stimulated group), and increased more obviously in the IL-22 intervention groups. The levels of MDA and GSH in the model group were significantly enhanced in comparison with those in the control group. In cells treated with IL-22, the MDA level was attenuated but the GSH level was further increased. These changes were dose-dependent.
IL-22 inhibits acetaldehyde-induced HSC activation and proliferation, which may be related to nuclear translocation of Nrf2 and increased activity of the antioxidant axis Nrf2-keap1-ARE.
Core tip: We successfully established an in vitro cell model of alcoholic liver fibrosis (ALF). We investigated the influence of interleukin (IL)-22 on ALF and the possible mechanism involved. To our knowledge, this is the first study to confirm the inhibitory effect of IL-22 on ALF at the cellular level. We found that the effect was at least partly related to promotion of nuclear translocation of nuclear factor-related factor (Nrf)2 and increased activity of the antioxidant axis Nrf2-keap1-ARE. We aimed to provide a new target for research on ALF and new drug development.
- Citation: Ni YH, Huo LJ, Li TT. Antioxidant axis Nrf2-keap1-ARE in inhibition of alcoholic liver fibrosis by IL-22. World J Gastroenterol 2017; 23(11): 2002-2011
- URL: https://www.wjgnet.com/1007-9327/full/v23/i11/2002.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i11.2002
Alcoholic liver fibrosis (ALF) is a wound-healing response to prolonged alcoholic liver injury, which is characterized by excessive extracellular matrix accumulation. ALF is a turning point in the development of alcoholic liver disease (ALD), so treatment of ALF has become the focus of clinical research on ALD[1,2]. As well as other causes of liver fibrosis, activation and proliferation of hepatic stellate cells (HSCs) are key factors in fibrogenesis. Previous studies have found that acetaldehyde, the most harmful metabolite of alcohol, can trigger HSC activation and proliferation in alcoholic liver injury via inducing oxidative stress[3,4], whereas inhibiting oxidative stress or enhancing antioxidant capacity can reverse the activation and proliferation of HSCs induced by acetaldehyde.
Nuclear factor-related factor (Nrf)2 has a molecular weight of 66 kDa and was discovered by Moi et al[5] in 1994. Under normal conditions, cytoplasmic Nrf2 mostly combines with its binding protein Kelch-like ECH-associated protein (keap1) and is in an inactive state. Oxidative stress or stimulation of nucleophilic substances may trigger dissociation of Nrf2 from keap1 and release free Nrf2. Following dissociation, Nrf2 is rapidly translocated to the nucleus and transactivates the antioxidant response element (ARE) in the promoter region of many antioxidant genes, triggering transcription of downstream target genes (e.g., GSH and HO-1). Thus, Nrf2 is the key regulatory factor in oxidative stress[6,7]. A significant body of evidence suggests that upregulation of Nrf2 or promotion of Nrf2 nuclear translocation can delay the progression of ALF[8,9]. Nrf2 promises to be one of the most important areas of research investigating the formation of ALF.
Interleukin (IL)-22 (also known as IL-10-related T-cell-inducible factor) is a member of the IL-10 cytokine family. It is secreted by T helper (Th)1, Th17 and Th22 cells and natural killer (NK)/NKT cells. By binding to a heterodimeric receptor complex IL-22R1/IL-10R2, IL-22 initiates the JAK/STAT3 signaling pathway[10]. IL-22 is one of the major inflammatory mediators associating with organ fibrosis, especially in the lungs and kidneys[10,11]. Previous animal and clinical research has shown that IL-22 and IL-22R1 expression is significantly increased in ALF, suggesting that IL-22 is involved in the process of ALF. This effect could be related to the promotion of liver progenitor cell/hepatocyte proliferation, inhibition of hepatocyte apoptosis, upregulation of metallothionein and glutathione (GSH) expression[12-14]. However, the activity of the antioxidant axis Nrf2-keap1-ARE in HSCs has not been reported to date.
Therefore, in the present study we used acetaldehyde as a stimulator of HSC-T6 cells to establish a model of ALF in vitro. Different concentrations of IL-22 were added to the culture, and the proliferation rate and activity of HSCs were detected, as well as nuclear translocation of Nrf2.
HSC-T6 cells were purchased from the cell bank of the Central South University (Hunan, China). Acetaldehyde (40%) was purchased from Tianjin DaMao Chemical Reagents (Tianjin, China). Dulbecco’s modified Eagle’s medium (DMEM) was purchased from Sijiqing (Hangzhou, China). Fetal calf serum (10%) was purchased from Hyclone (Logan, UT, United States). Recombinant mouse IL-22 was purchased from R&D Systems (Minneapolis, MN, United States). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay kit and nuclear-cytoplasmic protein extraction kit were purchased from Boster Bioengineering Company (Wuhan, China). Malondialdehyde (MDA) and GSH kits were purchased from Jiancheng Company (Nanjing, China). α-smooth muscle actin (SMA) and Nrf2 polyclonal antibody were purchased from Abcam (Cambridge, MA, United States). Horseradish peroxidase (HRP)-conjugated anti-rabbit secondary antibodies were purchased from Boster Bioengineering.
Passaged and activated HSCs were seeded into 25-cm2 sealable flasks and grown until the monolayers were 75%-80% confluent. HSCs were cultured in DMEM supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37 °C in a humidified incubator with 5% CO2, until the cells showed adherent growth.
There were five groups of cells: normal control group; model group; and high-, medium- and low-dose IL-22 intervention groups. The normal control group was cultured in conventional DMEM for 48 h; for the model group, 200 μmol/L acetaldehyde was added to the DMEM for 48 h; and the IL-22 intervention groups were co-incubated with 200 μmol/L acetaldehyde and different concentrations (10, 20 or 50 ng/mL) of IL-22 for 24 h after pretreatment with 200 μmol/L acetaldehyde for 24 h.
HSC-T6 cells were seeded at 5 × 104/mL in a 96-well plate and incubated in 100 μL culture medium overnight. Six wells were used for each group. The outer wells were filled with sterile PBS. When the monolayers of HSC-T6 cells were 70%-80% confluent, DMEM with 10% fetal bovine serum was replaced by serum-deprived DMEM to synchronize the cells. We treated HSC-T6 cells with acetaldehyde at 25, 50, 100, 200 or 400 μmol/L for 24 or 48 h to establish an in vitro model of ALF. After treatment with acetaldehyde, 10 μL of 5 mg/mL MTT solution was added to form purple formazan. Subsequently, 100 μL formazan dissolving liquid was added to dissolve the formazan crystals. Results were measured using a microplate reader at an absorbance of 570 nm, and the 50% effective concentration (EC50) value was obtained from the MTT viability growth curve. The HSC proliferation rate was calculated as follows: (OD of treated wells/OD of control wells) × 100%. Experiments were repeated three times and in triplicate.
We used the same method to test the effect of IL-22 on acetaldehyde-stimulated HSC-T6 cells. The cells were co-cultured with IL-22 at a final concentration of 10, 20 or 50 ng/mL after 24 h pretreatment with 200 μmol/L acetaldehyde. The OD was measured at 570 nm. The inhibitory rate was calculated as follows: [1 - (OD of treated wells/OD of model wells)] × 100%. Experiments were repeated three times and in triplicate.
Logarithmic growth phase HSC-T6 cells were inoculated in 6-well plates. When the monolayers were 70%-80% confluent, DMEM with 10% fetal bovine serum was replaced by serum-deprived DMEM to synchronize the cells, and different interventional treatments were added to the culture medium according to the experimental group. After treatment, HSC-T6 cells were trypsinized and resuspended in their original culture medium. The cells were harvested, washed and suspended in PBS twice, fixed in 70% ethanol at -20 °C overnight, and stained in 500 μL PBS containing propidium iodide (PI) (200 mg/mL RNase A + 50 μg/mL PI) at 37 °C for 30 min in the dark. Analysis was performed on a Cytomics FC500 flow cytometer (Beckman Coulter Inc., Brea, CA, United States).
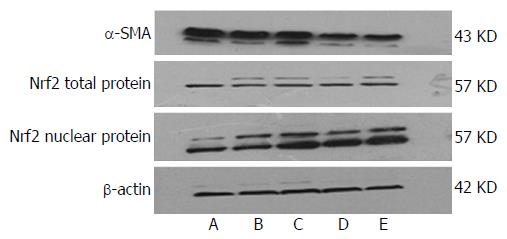
After treatment, HSC-T6 cells were harvested by scraping the cells from the culture dishes. Cell lysates were prepared using nuclear and cytoplasmic extraction kits for the detection of Nrf2 and α-SMA proteins. Protein concentrations were determined by the BCA Protein Assay Kit (Boster Bioengineering Company). After separation by 10% SDS-PAGE (140 V for 55 min), the proteins (60 μg) were transferred onto a PVDF membrane. The blots were incubated overnight at 4 °C with primary antibodies diluted with TBS solution containing 0.05% Tween 20 (TBST) after 5% nonfat milk blocking for 3 h at room temperature. The α-SMA antibody was diluted to 1:500, Nrf2 antibody to 1:1000, and β-actin antibody to 1:6000. On the next day, the membranes were washed with TBST three times and probed for 1 h with HRP-conjugated goat anti-rabbit IgG antibody (1:3000). TBST washing was repeated, and the immunoreactive band intensities were measured by grey intensity analysis using ImageJ software, and the gray values of β-actin protein bands were used to normalize the gray values of each target protein. All experiments were performed at least three times.
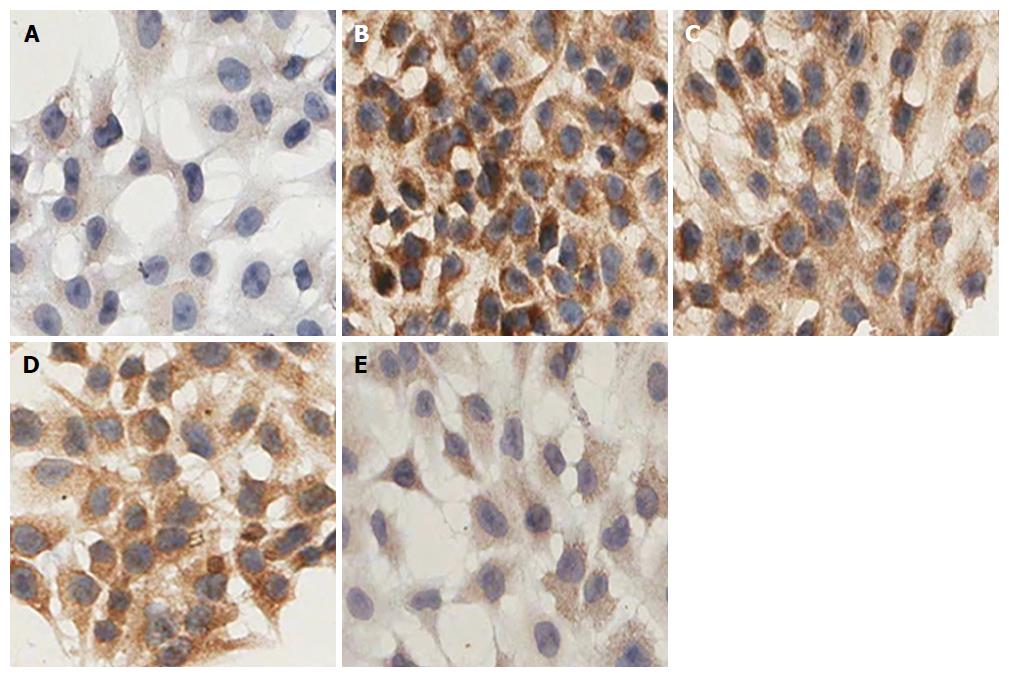
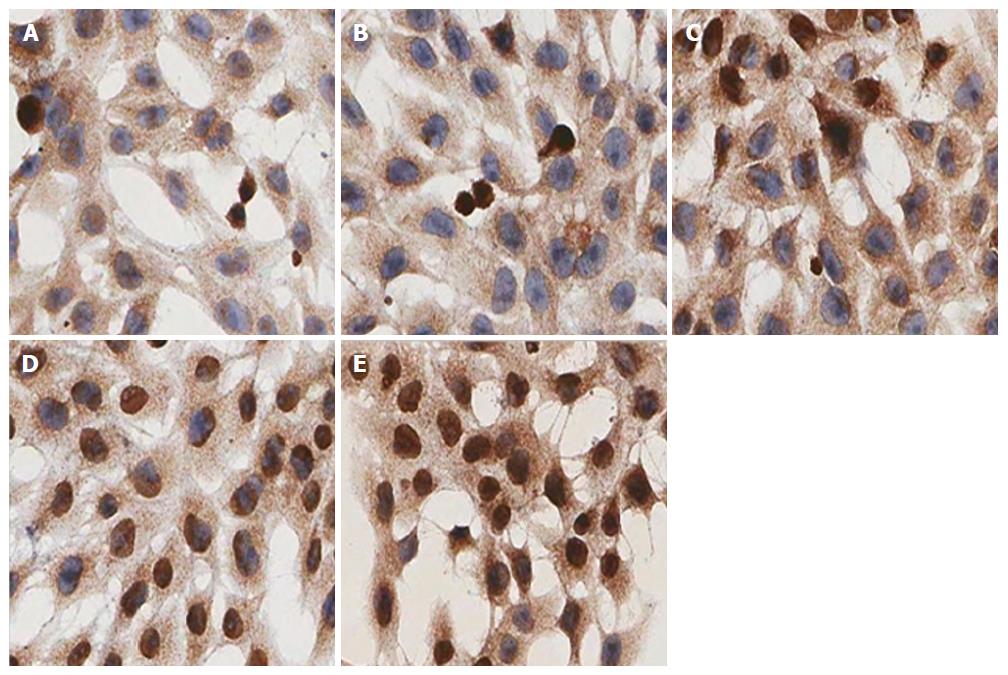
HSC-T6 cells were seeded at 2 × 104/mL, with 0.5 mL/well on coverslips in 24-well plates. Six wells were used for each group. Different interventional treatments were added to the culture medium according to the experimental group. After treatment, coverslips were removed, washed with PBS, fixed in 4% polyformaldehyde, and membranes were disrupted with 0.1% Triton-X100. Then, endogenous peroxidase was blocked by 3% H2O2 and target proteins nonspecific binding by 5% goat serum (every two steps included washing with PBS three times for 2 min each), primary rabbit polyclonal anti-α-SMA (1:100) and anti-Nrf2 (1:200) were applied and incubated in a humidified chamber overnight at 4 °C, followed by biotinylated goat anti-rabbit secondary antibody for 1 h at room temperature. After rinsing with PBS, the coverslips were counterstained by hematoxylin and dehydrated. The signal was visualized by light microscopy and analyzed by measuring the OD of positive staining using the Scanscope Digital Pathology Scanning System (Aperio; Leica Biosystems, Wetzlar, Germany).
Logarithmic growth phase HSC-T6 cells were inoculated in 6-well plates, and different interventional treatments were added to the culture medium according to the experimental group, with each group repeated in three wells. Cell supernatants were collected and centrifuged at 3000 ×g for 10 min. Then blank pipes, standard pipes and experiment pipes were set up according to MDA, GSH kit instruction. The OD value was measured at 532 and 420 nm respectively, and the content of MDA (nmol/L) and GSH (NU/L) were calculated.
SPSS version 17.0 was used for all statistical analysis and data were expressed as mean ± SD. If the data showed a normal distribution and homogeneity of variance, one-way ANOVA was used to assess the significance of differences between the mean values. Multiple pair-wise comparisons were conducted using the least significant difference (LSD). Otherwise, Welch’s test was used to assess the significance of differences between the mean values. Multiple pairwise comparisons were conducted using Dunnett’s T3 test. The difference was statistically significant at P < 0.05.
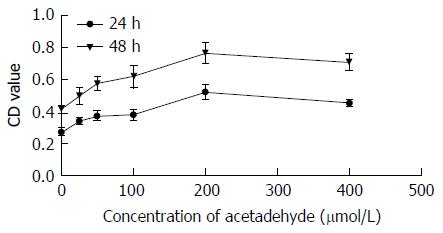
Compared with the control group, OD was significantly increased after stimulation with the graded concentrations of acetaldehyde. According to the growth curve, EC50 = 100 μmol/L was obtained. At this concentration, we found that OD reached a maximum at 48 h after exposure to 200 μmol/L acetaldehyde. Then, we determined the concentration and incubation time that best mimicked HSC activation seen in an in vivo model of ALF (Table 1 and Figure 1). In the experiment group, intervention with different concentrations of IL-22 significantly reduced OD. There was a negative correlation between OD and dose of IL-22 (Table 2 and Figure 2).
| Concentration ofacetaldehyde (μmol/L) | 24 h | 48 h | ||
| OD | Proliferation rate | OD | Proliferation rate | |
| 0 | 0.2743 ± 0.0134 | 0.4154 ± 0.0130 | ||
| 25 | 0.3409 ± 0.0118a | 124% | 0.4983 ± 0.0258a | 120% |
| 50 | 0.3732 ± 0.0152a | 136% | 0.5733 ± 0.0208a | 138% |
| 100 | 0.3807 ± 0.0157a | 139% | 0.6181 ± 0.0351a | 149% |
| 200 | 0.5019 ± 0.0098a | 183% | 0.7649 ± 0.0333a | 184% |
| 400 | 0.4524 ± 0.0104a | 165% | 0.7069 ± 0.0265a | 170% |
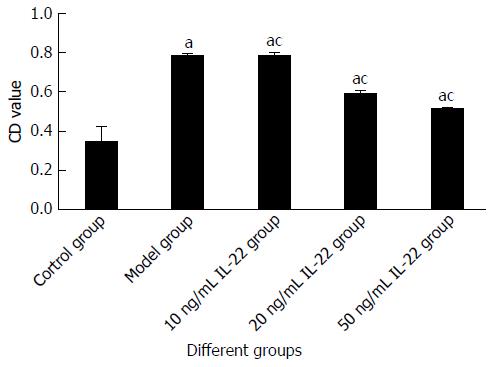
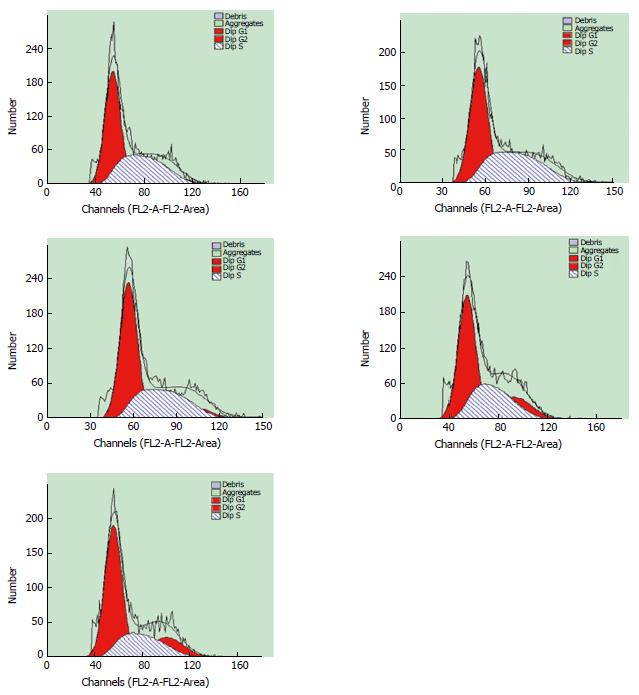
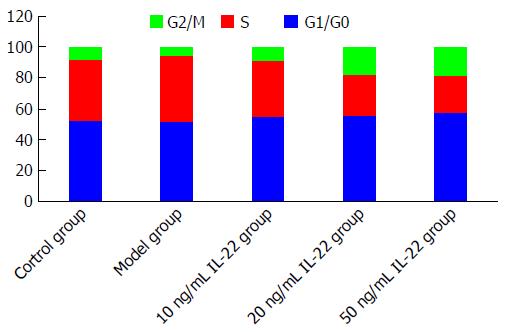
After stimulation with acetaldehyde at the optimal concentration and duration, HSCs were more abundant in S phase (42.94%) and less abundant in G1 phase (51.41%) in comparison with the control group (40.25% and 52.01%, respectively), indicating that HSCs were activated and proliferated. The percentage of HSCs in S phase gradually decreased with increased concentrations of IL-22, while the percentage in G1 phase was gradually increased in a dose-dependent manner when compared with the model group (Figures 3 and 4).
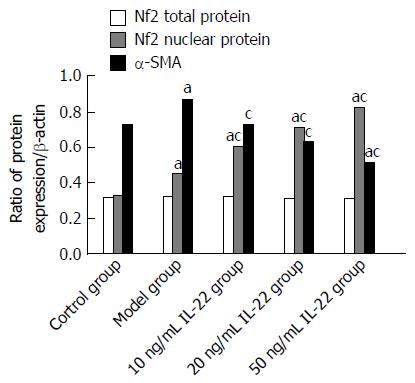
In the control group, there was basic expression of α-SMA. In comparison, the expression of α-SMA was significantly increased in the model group. However, α-SMA expression was gradually reduced as IL-22 concentration increased, and the difference was significant in comparison with the model group.
Expression of Nrf2 total protein showed no significant difference between all the groups. Compared with the control group, expression of Nrf2 nuclear protein was increased slightly in the model group, and increased significantly in the IL-22 intervention groups, in a dose-dependent manner (Figures 5 and 6).
HSC-T6 cells with α-SMA-positive expression had brown granules in the cytoplasm, although the degree of staining was varied. In the control group, the staining of the cytoplasm was light brown, whereas it was obviously deepened in the model group. Treatment with different concentrations of IL-22 gradually reduced the depth of brown staining in the cytoplasm in a dose-dependent manner. The difference was significant (Figure 7).
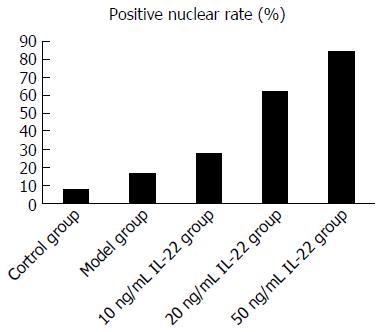
For expression of Nrf2 nuclear protein, immunocytochemistry showed that in the IL-22 intervention groups, the positive rate of nuclear staining was higher than in the model group (27.8%, 62.5% and 84.6% in the low-, middle- and high-dose of IL-22 intervention groups and 16.7% in the model group), whereas there was rarely positive nuclear staining in the control group (7.6%) (Figures 8 and 9).
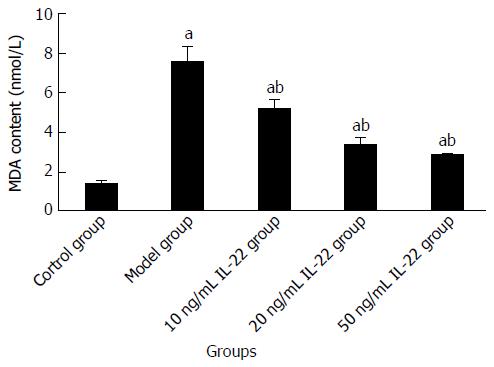
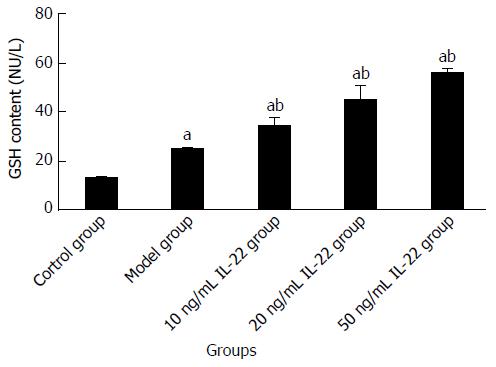
Compared with the control group, the content of MDA and GSH was increased in the model group. When cells were treated with different concentrations of IL-22, the MDA level was attenuated and the content of GSH was further elevated, both in a dose-dependent manner. The differences were significant (Table 3, Figures 10 and 11).
| Group | Acetaldehyde (μmol/L) | IL-22 (ng/mL) | MDA (nmol/mL) | GSH (NU/L) |
| Control group | 0 | 0 | 1.3654 ± 0.1022 | 12.8569 ± 0.3699 |
| Model group | 200 | 0 | 7.5073 ± 0.6126a | 24.6972 ± 0.5043a |
| IL-22 groups | ||||
| Low | 200 | 10 | 5.1256 ± 0.3835ac | 33.8866 ± 2.6506ac |
| Medium | 200 | 20 | 3.3195 ± 0.2963ac | 44.5935 ± 4.1386ac |
| High | 200 | 50 | 2.8141 ± 0.0720ac | 55.6834 ± 1.1671ac |
ALD is a chronic liver injury caused by long-term heavy alcohol drinking. Its pathological stages comprise steatosis (alcoholic fatty liver), steatohepatitis (alcoholic hepatitis) and liver fibrosis/cirrhosis[15-17]. ALF is regarded as a turning point in the development and progression of ALD, so timely treatment of ALF can reverse ALD[1,2]. Previous research has shown that, as the principal metabolite of ethanol, acetaldehyde triggers activation and proliferation of HSCs, which is the central feature of ALF[3,4]. α-SMA is the most important activation marker of HSCs; it is mostly expressed in activated HSCs and can be used to assess the extent of liver fibrosis[18-20]. As our results demonstrated, after stimulation by different concentrations of acetaldehyde for different times, the proliferation rate of HSCs was significantly increased. It was most obvious when 200 μmol/L acetaldehyde was added for 48 h, which agreed with previous studies[21-23] and these conditions were used for later model establishment. We demonstrated by flow cytometry that acetaldehyde markedly decreased HSCs in G0/G1 phases but increased the HSCs in S phase. Western blotting and immunocytochemistry also showed that, in response to acetaldehyde-induced stimulation, expression of α-SMA was obviously increased, adding to a growing body of evidence about the successful establishment of an in vitro model of ALF.
Oxidative stress is one of the clearly important mechanisms of ALF, which is characterized by accumulation of lipid peroxidation product MDA and depletion of endogenous antioxidant GSH[3,4]. A large body of evidence[24,25] has demonstrated that acetaldehyde serves as one of the most important stimulants of ALF by triggering oxidative stress. However, reducing oxidative stress blocks HSC activation and proliferation, as well as prevents ALF. Our results also showed that compared with the control group, the contents of MDA and GSH were significantly increased in the model group. This shows that lipid peroxidation and antioxidant capacity are synchronously activated in the early stage of ALF. As the disease progresses, the antioxidant system is exhausted, which leads to the persistence of oxidative stress and formation of liver fibrosis. So, focusing on the relationship between oxidative stress and fibrogenesis is important to ALF treatment.
Nrf2 is an important transcription factor in the process of antioxidative stress response[6,7]. Under basal conditions, Nrf2 is constitutively sequestered in the cytoplasm by its binding protein keap1, which causes proteasomal degradation of Nrf2. However, under conditions of oxidative stress, the ubiquitination degradation pathway of Nrf2 is inhibited, resulting in cytoplasmic Nrf2 deposition. Free Nrf2, dissociated from keap1, moves into the nucleus, combines with ARE, and serves as a transcriptional factor to regulate the expression of corresponding downstream genes (e.g., GSH and HO-1). This comes with enhanced oxidation resistance and reduced oxidative stress[6,7,26]. So, the means by which to increase the expression of Nrf2 or promote Nrf2 nuclear translocation could be an important target for the treatment and prevention of liver fibrosis.
IL-22 belongs to the IL-10 cytokine superfamily and is secreted by Th1, Th17, Th22 and NK/NKT cells. It mediates various types of biological behavior via a heterodimeric receptor complex IL-22R1/IL-10R2 that is exclusively expressed in epithelial cells and some fibroblasts. Binding of IL-22 to its receptor results in activation of signaling cascades, including JAK/STAT, ERK and JNK. In the liver, the receptor complex is selectively expressed in hepatocytes, liver progenitor cells and HSCs[10,11]. Ki et al[12] demonstrated an antioxidant role of IL-22 in ALF, indicated by the upregulation of antioxidant proteins metallothionein I/II. Xing et al[13] established an animal model of chronic alcoholic hepatitis using high-fat diet, carbonyl and Fe(III) complex factors. Based on this model, they found that IL-22 reduced liver lipid peroxidation, restored the level of GSH, and inhibited oxidative stress. In their later research about acute liver injury induced by D-galactosamine/lipopolysaccharide, they confirmed the inducing effect of IL-22 on expression of Bcl-x, heme oxygenase-1 and redox factor-1, also indicating that IL-22 has liver-protective effects of anti-inflammation, anti-apoptosis and anti-oxidation. And, based on its cell-targeted characteristic so that it would not trigger excessive immune reactions, the clinical toxicity was very slight[12-14,27]. We also showed that IL-22 inhibited activation and proliferation of HSCs in a dose-dependent manner; the population of cells in G0/G1 phases was increased dramatically, while that in S phase was decreased markedly when compared with the number of HSCs maintained in the acetaldehyde control group. This indicates that IL-22 provoked G1/S phase arrest of acetaldehyde-induced HSCs. We used western blotting and immunocytochemistry to explore the possible mechanism. We showed that expression of Nrf2 total protein did not differ among the groups, whereas IL-22 treatment resulted in a dose-dependent increase in Nrf2 nuclear protein. This indicates that IL-22 promotes Nrf2 nuclear translocation, activates the Nrf2-keap1-ARE signaling pathways, increases expression of its downstream target protein GSH, and inhibits oxidative stress and progression of ALF.
The limit to our study is that we only investigated the inhibitory effect of IL-22 on ALF at the cellular level, while animal experiments and clinical research about IL-22 and ALF were not sufficiently performed. Further investigation of the relevant mechanisms is needed.
In summary, we established an in vitro model of acetaldehyde-induced ALF. We further demonstrated that IL-22 effectively inhibited activation and proliferation of HSCs, followed by delayed disease progression of ALF. This may partly be related to promoting Nrf2 nuclear translocation and enhancing the activity of the antioxidant axis Nrf2-keap1-ARE. These results could provide an experimental and theoretical basis for new drug development for treatment of ALF.
Alcoholic liver fibrosis (ALF) is the turning point and research focus of alcoholic liver disease. Oxidative stress-induced hepatic stellate cell (HSC) activation and proliferation have been shown to be the key factors in ALF. In the process, acetaldehyde is the most important effector. Nuclear factor-related factor (Nrf)2 is the key regulatory factor of antioxidant responses, and upregulation of Nrf2 or promotion of Nrf2 nuclear translocation delays the progression of ALF. Interleukin (IL)-22 belongs to the IL-10 cytokine superfamily and has liver-protective effects of anti-inflammation, anti-apoptosis, anti-oxidation and anti-fibrosis. However, whether it regulates the activity of the antioxidant axis Nrf2-keap1-ARE in HSCs has not yet been elucidated.
Upregulation of Nrf2 or promotion of Nrf2 nuclear translocation delays the progression of ALF. IL-22 is a novel organ fibrosis-related cytokine. It delays the progression of liver fibrosis via promotion of liver progenitor cell/hepatocyte proliferation, inhibition of hepatocyte apoptosis, and upregulation of metallothionein (MTI/II) and glutathione (GSH) expression.
This is the first study to confirm the inhibitory effect of IL-22 on ALF at the cellular level. More important, we found that the effect was at least partly related to the promoted nuclear translocation of Nrf2 and the increased activity of antioxidant axis Nrf2-keap1-ARE.
Acetaldehyde (200 μmol/L for 48 h) mimics in vivo HSC activation and proliferation of ALF, indicating the successful establishment of an in vitro model of ALF. Based on the model, IL-22 dose-dependently inhibited the activation and proliferation of HSCs and progression of ALF. This was partly related to enhancing the activity of antioxidant axis Nrf2-keap1-ARE in HSCs. These results could provide an experimental and theoretical target and basis for new drug development for treatment of ALF.
The Nrf2-keap1-ARE axis is an important internal antioxidative stress reaction system. Under basal conditions, cytoplasmic Nrf2 mostly combines with its binding protein keap1 and is in an inactive state. Oxidative stress may trigger dissociation of Nrf2 from keap1 and release free Nrf2. Nrf2 rapidly translocates into the nucleus and transactivates ARE in the promoter region of many antioxidant genes, triggering transcription of downstream antioxidant proteins (e.g., GSH and heme oxygenase-1).
This study investigated proliferation and response to acetaldehyde, a metabolite of alcohol, as an in vitro model of ALF. The authors found that acetaldehyde increases HSCs’ proliferation, decreases G0/G1 phases, and increases S phase. IL-22 can return the acetaldehyde effect on the cell proliferation which can be mediated through Nrf2-keap1-ARE.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ghobadloo SM, Osna NA S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Zhang FF
| 1. | Barbero-Becerra VJ, López-Velázquez JA, Sánchez-Valle V, Uribe M, Méndez-Sánchez N. Alcohol effects on liver diseases: good or bad buddy? Ann Hepatol. 2012;11:944-948. [PubMed] |
| 2. | Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1096] [Cited by in RCA: 1363] [Article Influence: 97.4] [Reference Citation Analysis (0)] |
| 3. | Mello T, Ceni E, Surrenti C, Galli A. Alcohol induced hepatic fibrosis: role of acetaldehyde. Mol Aspects Med. 2008;29:17-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Ceni E, Mello T, Galli A. Pathogenesis of alcoholic liver disease: role of oxidative metabolism. World J Gastroenterol. 2014;20:17756-17772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 286] [Cited by in RCA: 349] [Article Influence: 31.7] [Reference Citation Analysis (5)] |
| 5. | Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl Acad Sci USA. 1994;91:9926-9930. [PubMed] |
| 6. | Niture SK, Khatri R, Jaiswal AK. Regulation of Nrf2-an update. Free Radic Biol Med. 2014;66:36-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 730] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 7. | Baird L, Dinkova-Kostova AT. The cytoprotective role of the Keap1-Nrf2 pathway. Arch Toxicol. 2011;85:241-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 766] [Cited by in RCA: 776] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 8. | Wu KC, Liu J, Klaassen CD. Role of Nrf2 in preventing ethanol-induced oxidative stress and lipid accumulation. Toxicol Appl Pharmacol. 2012;262:321-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 9. | Chen S, Zou L, Li L, Wu T. The protective effect of glycyrrhetinic acid on carbon tetrachloride-induced chronic liver fibrosis in mice via upregulation of Nrf2. PLoS One. 2013;8:e53662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 10. | Della Latta V, Cecchettini A, Del Ry S, Morales MA. Bleomycin in the setting of lung fibrosis induction: From biological mechanisms to counteractions. Pharmacol Res. 2015;97:122-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 338] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 11. | Bansal G, Das D, Hsieh CY, Wang YH, Gilmore BA, Wong CM, Suzuki YJ. IL-22 activates oxidant signaling in pulmonary vascular smooth muscle cells. Cell Signal. 2013;25:2727-2733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Ki SH, Park O, Zheng M, Morales-Ibanez O, Kolls JK, Bataller R, Gao B. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291-1300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 364] [Cited by in RCA: 365] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 13. | Xing WW, Zou MJ, Liu S, Xu T, Gao J, Wang JX, Xu DG. Hepatoprotective effects of IL-22 on fulminant hepatic failure induced by d-galactosamine and lipopolysaccharide in mice. Cytokine. 2011;56:174-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Kong X, Feng D, Mathews S, Gao B. Hepatoprotective and anti-fibrotic functions of interleukin-22: therapeutic potential for the treatment of alcoholic liver disease. J Gastroenterol Hepatol. 2013;28 Suppl 1:56-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 15. | Duddempudi AT. Immunology in alcoholic liver disease. Clin Liver Dis. 2012;16:687-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1483] [Article Influence: 105.9] [Reference Citation Analysis (0)] |
| 17. | Orman ES, Odena G, Bataller R. Alcoholic liver disease: pathogenesis, management, and novel targets for therapy. J Gastroenterol Hepatol. 2013;28 Suppl 1:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 179] [Article Influence: 14.9] [Reference Citation Analysis (2)] |
| 18. | Jotzu C, Alt E, Welte G, Li J, Hennessy BT, Devarajan E, Krishnappa S, Pinilla S, Droll L, Song YH. Adipose tissue-derived stem cells differentiate into carcinoma-associated fibroblast-like cells under the influence of tumor-derived factors. Anal Cell Pathol (Amst). 2010;33:61-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 56] [Reference Citation Analysis (0)] |
| 19. | Yang Y, Yang F, Wu X, Lv X, Li J. EPAC activation inhibits acetaldehyde-induced activation and proliferation of hepatic stellate cell via Rap1. Can J Physiol Pharmacol. 2016;94:498-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Zhao Q, Qin CY, Zhao ZH, Fan YC, Wang K. Epigenetic modifications in hepatic stellate cells contribute to liver fibrosis. Tohoku J Exp Med. 2013;229:35-43. [PubMed] |
| 21. | Wang H, Guan W, Yang W, Wang Q, Zhao H, Yang F, Lv X, Li J. Caffeine inhibits the activation of hepatic stellate cells induced by acetaldehyde via adenosine A2A receptor mediated by the cAMP/PKA/SRC/ERK1/2/P38 MAPK signal pathway. PLoS One. 2014;9:e92482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Wang Q, Dai X, Yang W, Wang H, Zhao H, Yang F, Yang Y, Li J, Lv X. Caffeine protects against alcohol-induced liver fibrosis by dampening the cAMP/PKA/CREB pathway in rat hepatic stellate cells. Int Immunopharmacol. 2015;25:340-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 23. | Yang Y, Wang H, Lv X, Wang Q, Zhao H, Yang F, Yang Y, Li J. Involvement of cAMP-PKA pathway in adenosine A1 and A2A receptor-mediated regulation of acetaldehyde-induced activation of HSCs. Biochimie. 2015;115:59-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Zhu H, Jia Z, Misra H, Li YR. Oxidative stress and redox signaling mechanisms of alcoholic liver disease: updated experimental and clinical evidence. J Dig Dis. 2012;13:133-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 124] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 25. | Leung TM, Nieto N. CYP2E1 and oxidant stress in alcoholic and non-alcoholic fatty liver disease. J Hepatol. 2013;58:395-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 384] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 26. | Xu W, Shao L, Zhou C, Wang H, Guo J. Upregulation of Nrf2 expression in non-alcoholic fatty liver and steatohepatitis. Hepatogastroenterology. 2011;58:2077-2080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Yang L, Zhang Y, Wang L, Fan F, Zhu L, Li Z, Ruan X, Huang H, Wang Z, Huang Z. Amelioration of high fat diet induced liver lipogenesis and hepatic steatosis by interleukin-22. J Hepatol. 2010;53:339-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |