Published online Dec 14, 2016. doi: 10.3748/wjg.v22.i46.10232
Peer-review started: August 22, 2016
First decision: September 12, 2016
Revised: September 27, 2016
Accepted: October 27, 2016
Article in press: October 27, 2016
Published online: December 14, 2016
Processing time: 115 Days and 5.7 Hours
To analyse the long-term prognostic impact of circulating tumour cells (CTCs) in gastric cancer patients who underwent surgery.
A 7.5-mL peripheral vein blood sample was obtained from each patient with treatment-negative gastric adenocarcinoma before surgery. OBP-401, a telomerase-specific, replication-selective, oncolytic adenoviral agent carrying the green fluorescent protein gene, was used to label CTCs. Correlations between the number of CTCs and clinical end points were evaluated.
The median follow-up period of the surviving patients with gastric cancer was 60 mo. The CTC number tended to increase concomitantly with disease progression. The overall survival of patients with more than five CTCs in 7.5-mL of peripheral blood was lower than that of patients with five or less CTCs, although the difference was not significant (P = 0.183). A significant difference in relapse-free survival was found between patients with more than five and those with five or less CTCs (P = 0.034).
A lower number of CTCs was correlated with higher relapse-free survival rates in patients. Detection of CTCs using OBP-401 may be useful for predicting prognosis in gastric cancer.
Core tip: We show the long-term prognostic impact of circulating tumour cells (CTCs) in 65 patients with gastric cancer in this report. OBP-401, a telomerase-specific, replication-selective, oncolytic adenoviral agent carrying the green fluorescent protein gene, was used to label CTCs. A lower number of CTCs was correlated with higher relapse-free survival rates in patients with gastric cancer.
- Citation: Ito H, Sato J, Tsujino Y, Yamaguchi N, Kimura S, Gohda K, Murakami K, Onimaru M, Ohmori T, Ishikawa F, Inoue H. Long-term prognostic impact of circulating tumour cells in gastric cancer patients. World J Gastroenterol 2016; 22(46): 10232-10241
- URL: https://www.wjgnet.com/1007-9327/full/v22/i46/10232.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i46.10232
The presence of circulating tumour cells (CTCs) in peripheral blood indicates a systemic disease stage in various malignancies, as CTCs are thought to be the source of haematogenous metastasis[1]. Detection of CTCs in peripheral blood is useful for prognosis, monitoring of disease progression, and evaluation of treatment efficacy in breast[2], lung[3], prostate[4], skin[5], colon[6], gastric[7], and esophageal cancer[8,9]. Although various methods have been developed to detect CTCs, the most commonly used techniques for their enrichment and characterisation are density gradient separation[10], immunomagnetic separation[11], flow cytometry[12], direct enrichment by filtration[13], and microchip technology[14]. The CellSearch System (Veridex, LLC, Raritan, NJ, United States), which is based on immunomagnetic cell enrichment, is one of the most widely used techniques for automated enrichment and detection of CTCs[15,16]. The advantage of immunomagnetic cell separation is that CTCs can be directly visualised under a microscope. In the CellSearch assay, cells detected with antibodies against epithelial markers (e.g., epithelial cell adhesion molecules, or EpCAMs) are classified as CTCs. During the epithelial-mesenchymal transition (EMT), an important process that occurs in CTCs[17], expression of epithelial surface markers is reduced. Thus, systems that rely on epithelial markers may fail to detect CTCs undergoing EMT[18]. Methodologies based on direct enrichment by filtration may circumvent this issue to some extent, although cells detected in this manner often lack tumourigenicity.
Increased telomerase activity is a common characteristic of malignant tumours, and telomerase plays important roles in carcinogenesis and disease progression[19]. OBP-401 (TelomeScan, Oncolys BioPharma, Tokyo, Japan) is a telomerase-specific, replication-selective modified viral agent in which the human telomerase reverse transcriptase (TERT) gene promoter is inserted into the E1 region, and the green fluorescent protein (GFP) gene is placed under the control of the cytomegalovirus promoter in the E3 region as a marker of viral replication[20]. Thus, OBP-401 only proliferates in viable cells with high telomerase activity and provides a fluorescent label that allows tumour cells to be labelled, regardless of their epithelial marker expression profiles. We previously used OBP-401 to detect cells with high telomerase activity in blood samples of healthy and treatment-negative gastric cancer patients before surgery. We took 7.5-mL peripheral blood samples from cancer patients before surgery and healthy volunteers. We detected viable GFP-positive CTCs in the blood samples after incubation with OBP-401. This revealed that in patients with gastric cancer, a greater proportion of “high telomerase activity” cells was associated with a significantly poorer prognosis[21]. In this report, we describe the final long-term results (median follow-up time of five years) of this initial study, which demonstrate that the OBP-401-dependent CTC assay has clinical utility in patients with gastric cancer.
This report was the final analysis of our prospective preliminary study on CTCs from 65 patients with treatment-negative gastric adenocarcinoma who underwent surgery at the Digestive Disease Center of the Showa University Northern Yokohama Hospital between April 2010 and May 2011, and from whom we extracted peripheral blood samples before treatment. The inclusion criteria were: (1) histologically proven adenocarcinoma of the stomach by endoscopic biopsy; (2) clinical solitary tumour; (3) no prior endoscopic resection, chemotherapy, or radiotherapy; (4) aged 20-80 years; (5) Eastern Cooperative Oncology Group performance status (Oken et al[22], 1982) of 0 or 1; (6) sufficient organ function; and (7) written informed consent. The exclusion criteria were: (1) synchronous or metachronous malignancy; (2) pregnant or breast-feeding women; (3) active or chronic viral hepatitis; (4) active bacterial or fungal infection; (5) diabetes mellitus; (6) systemic administration of corticosteroids; and (7) unstable hypertension. The pathologic stage of the disease was determined according to the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer TNM classification system[23]. The depth of the tumour invasion in four patients without gastrectomy and the regional lymph node status of seven patients without sufficient lymphadenectomy were surgically diagnosed.
All of the patients were checked regularly in our hospital every 3 mo for the first 3 years post-operation, and every 6 mo for the following two post-operative years. The patients also underwent endoscopy and computed tomography at least once a year, according to their disease stage and course. Healthy volunteers were also recruited as controls. All healthy volunteers were employees of Sysmex Corporation, which included seven men (mean age, 31.4 years; range, 24-39 years) and three women (mean age, 33.7 years; range, 26-48 years). All volunteers underwent medical check-ups upon employment and annually; check-ups included medical interviews, auscultation, chest radiography, and blood and urine analyses. In addition, individual interviews were conducted before sample collection; any volunteer who was currently receiving medical treatment, pregnant, or breast-feeding or who had donated blood within the past month was excluded.
OBP-401, a telomerase-specific, replication-selective adenoviral agent in which the TERT promoter element drives the expression of the EIA and EIB genes and into which the GFP gene is integrated, was used. The sensitivity and specificity of the assay using OBP-401 have been reported previously[24]. Viral samples were stored at -80 °C.
Details of sample preparation and assay were described in our previous report[21]. A 7.5-mL peripheral vein blood sample was obtained from each patient before surgery and from each healthy volunteer. The samples were drawn into tubes containing citric acid, phosphoric acid, and dextrose, and stored at 4 °C. The assay was started within 48 h of sample collection. The samples were centrifuged for 5 min at 540 ×g, and the plasma phase was removed. The cells were then washed four times with phosphate-buffered saline (PBS) and twice with Roswell Park Memorial Institute medium. The samples were infected with 4 × 108 plaque-forming units of OBP-401 virus by incubation in the medium for 24 h at 37 °C. Dead cells were stained with the red-fluorescent reactive dye L23102 (Life Technologies, Carlsbad, CA, United States), OBP-401 was inactivated, and the cells were fixed with 2% paraformaldehyde for 20 min at room temperature. The samples were treated with a surface-active agent (Emalgen 2025G; Kao Chemicals, Tokyo, Japan) for 10 min at 40 °C to degrade red blood cells. Phycoerythrin-labelled anti-human CD45 antibody (BioLegend, San Diego, CA, United States) was diluted 1:5, and Pacific Blue-labelled anti-human CD326 (EpCAM) antibody (BioLegend) was diluted 1:10 in PBS containing 2% foetal bovine serum. Cells were incubated with the diluted antibodies for 30 min at 25 °C. After being washed with PBS containing 2% foetal bovine serum, the cells were mounted on two glass slides for microscopic analysis.
Approximately 30000 cultured cells were added into 7.5-mL blood samples from healthy volunteers, which were mixed with various cancer cell lines: A549 (lung carcinoma), HepG2 (hepatocellular carcinoma), HEC-1 (endometrial carcinoma), KATO-III (gastric carcinoma), SBC-3 (small cell lung carcinoma), LNCaP (prostate adenocarcinoma), MDA-MB-468 (breast carcinoma), and OVCAR-3 (ovarian carcinoma). The cell lines were cultured according to the vendor’s specifications.
The threshold for GFP fluorescence intensity and cell size (diameter) were set based on the values from samples of healthy volunteers and the patients with gastric cancer by using receiver operating characteristic (ROC) analysis. The blood samples were subjected to the CTC detection assay, and GFP-positive cells were scored by fluorescence microscopy.
All detectable GFP-positive cells on the two slides were analysed under a computer-controlled fluorescence microscope (IX71, Olympus, Tokyo, Japan); the observer was blinded to the sample details. Cells with fluorescence intensities and diameters exceeding the threshold were scored as GFP-positive. Both EpCAM-positive and EpCAM-negative subpopulations were found in these cells, consistent with the finding that tumour cells undergoing EMT can be EpCAM-negative[18].
The study was approved by the Institutional Review Board of the Showa University, Northern Yokohama Hospital (No. 0903-03). This study was registered with the University Hospital Medical Information Network in Japan, UMIN000004026.
The study protocol was explained to the patients and volunteers before written informed consent was obtained.
All statistical analysis was performed using JMP Pro 12.2.0 (SAS Institute, Cary, NC, United States). Non-parametric comparisons were performed using the Wilcoxon signed-rank test, with a normal approximation. ROC analysis was performed to examine the difference between GFP fluorescence intensity and cell size in the blood samples of patients versus those in healthy volunteers. Cox proportional hazards analysis was used to investigate risk factors for survival, and to calculate overall and relapse-free survival rates. P ≤ 0.05 was considered statistically significant.
The clinicopathological characteristics of 65 patients (46 men and 19 women; mean age 60.7 years; range 33-76 years) are summarised in Table 1. The median follow-up period for the surviving patients was 60 mo. Fifty-seven of the 65 patients underwent pathological curative surgery, and of these patients, 10 experienced disease recurrence. Fifteen patients died. Twenty-nine patients had distal gastrectomy, 32 had total gastrectomy, and four had exploratory laparotomy. Twenty of the 57 patients that underwent curative surgery also received adjuvant chemotherapy, and nine of these 20 patients received therapeutic chemotherapy after disease recurrence.
| Variable | Number of patients | |
| Sex | Male | 46 |
| Female | 19 | |
| Age (yr; mean, range) | 58.8; 33-76 | |
| Gastrectomy | Distal | 29 |
| Total | 32 | |
| None | 4 | |
| Surgical curability | R0 | 57 |
| R1 | 0 | |
| R2 | 8 | |
| Clinical course | Survival without relapse | 47 |
| Survival after relapse | 2 | |
| Survival after non-curative surgery | 1 | |
| Decease | 15 | |
| Recurrence site (including overlap) | None | 47 |
| Remnant stomach | 1 | |
| Hematogenous | 5 | |
| Lymphatic | 4 | |
| Peritoneal dissemination | 5 | |
| Non-curative surgery | 8 | |
| Postoperative chemotherapy | None | 37 |
| Adjuvant chemotherapy | 11 | |
| Adjuvant and therapeutic chemotherapy | 9 | |
| Therapeutic chemotherapy after non-curative surgery | 8 | |
| TNM stage | I | 40 |
| II | 6 | |
| III | 10 | |
| IV | 9 | |
| Depth of tumour invasion | T1 | 36 |
| T2 | 8 | |
| T3 | 9 | |
| T4 | 12 | |
| Lymph node metastasis | N0 | 39 |
| N1 | 5 | |
| N2 | 6 | |
| N3 | 15 | |
| Distant metastasis | M0 | 56 |
| M1 | 9 | |
| Main histological type | Differentiated | 25 |
| Undifferentiated | 40 | |
| Lymphatic invasion | L0 | 35 |
| L1 | 26 | |
| LX | 4 | |
| Venous invasion | V0 | 35 |
| V1-2 | 26 | |
| VX | 4 | |
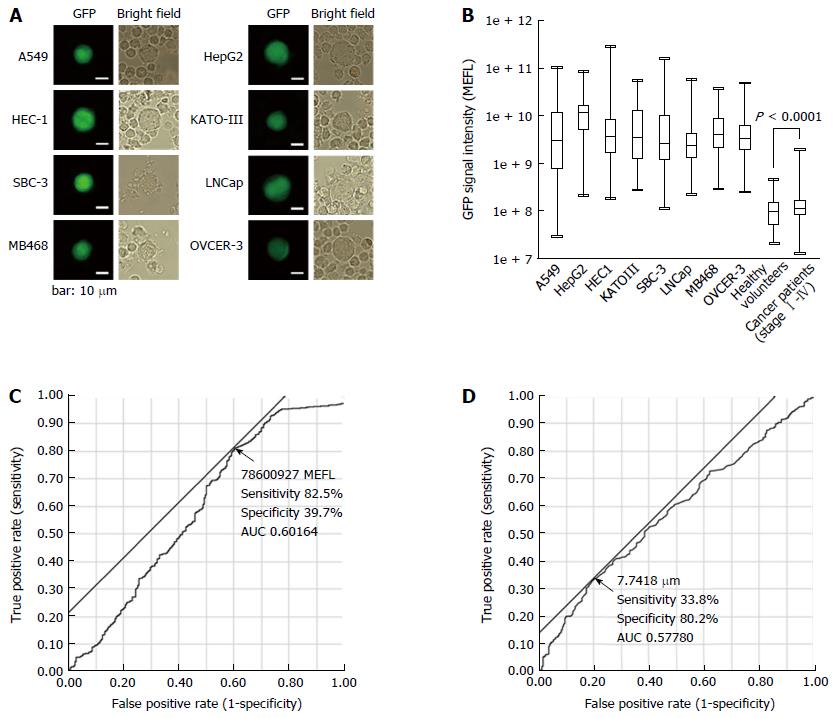
After OBP-401 infection, GFP-positive cancer cell lines were detected (Figure 1A).
The GFP fluorescence intensity [mean equivalent fluorochrome (MEFL)] of the cell lines and the GFP-positive cells detected in the peripheral blood samples are shown in Figure 1B. MEFL was higher in cell lines than in the GFP-positive cells in the peripheral blood samples from either healthy volunteers or patients with gastric cancer. In turn, MEFL was higher in GFP-positive cells from patients with gastric cancer than in the corresponding cells from healthy volunteers.
The GFP fluorescence intensity and diameter of cells isolated from the peripheral blood samples are shown in Figure 1C and D. Based on ROC analyses, we defined cells with 78600927 MEFL or higher GFP fluorescence intensity and 7.7418 μm or larger diameter as the CTCs.
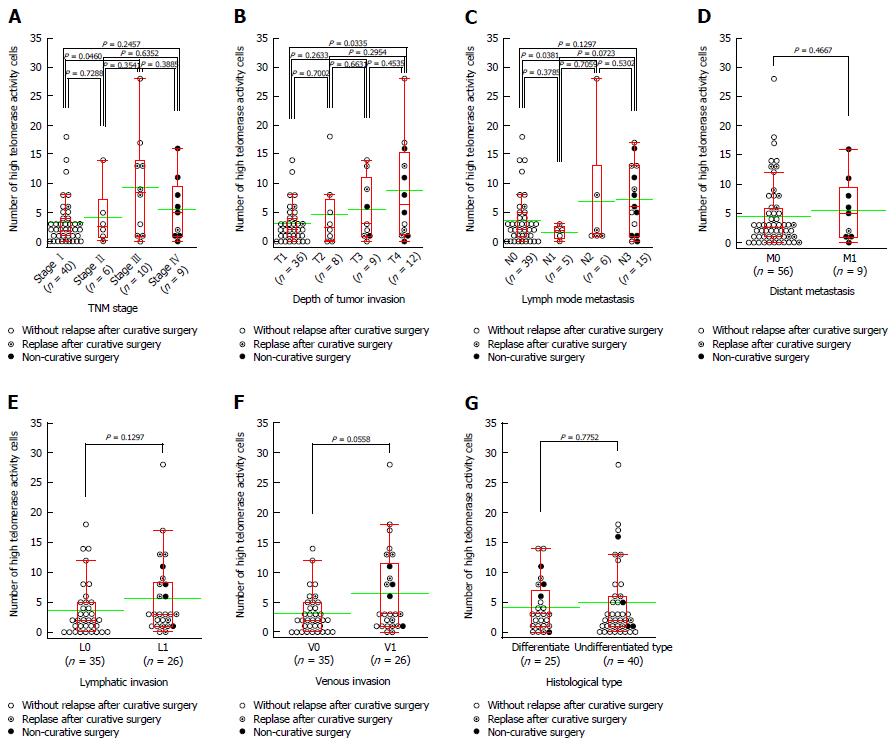
An increased number of CTCs was associated with disease progression. There was statistically significant difference in the number of CTCs between samples from patients with Stage I and those from patients with Stage III disease (P = 0.0460, Figure 2A). The number of CTCs also tended to increase concomitantly with progression of the primary tumour, as there was a statistically significant difference in the number of CTCs between samples from patients with T1 and those from patients with T4 tumours (P = 0.0335, Figure 2B). There was also a statistically significant difference in the number of CTCs between samples from patients with N0 and those with N2 lymph node spread status (P = 0.0381, Figure 2C). However, there was no significant difference in the number of CTCs between samples from patients with distant metastases and those in which distant metastasis was absent (P = 0.4667, Figure 2D). The number of CTCs was also higher in samples from patients with lymphatic invasion, although there was no significant difference compared to patients without this clinical feature (P = 0.1297, Figure 2E). Similarly, although the number of CTCs in samples from the patients with venous invasion was higher than those in samples without this complication, the difference was not significant (P = 0.0558, Figure 2F). Finally, we observed no significant difference in the number of CTCs in samples from patients with differentiated tumours when compared to those with undifferentiated malignancies (P = 0.7752, Figure 2G).
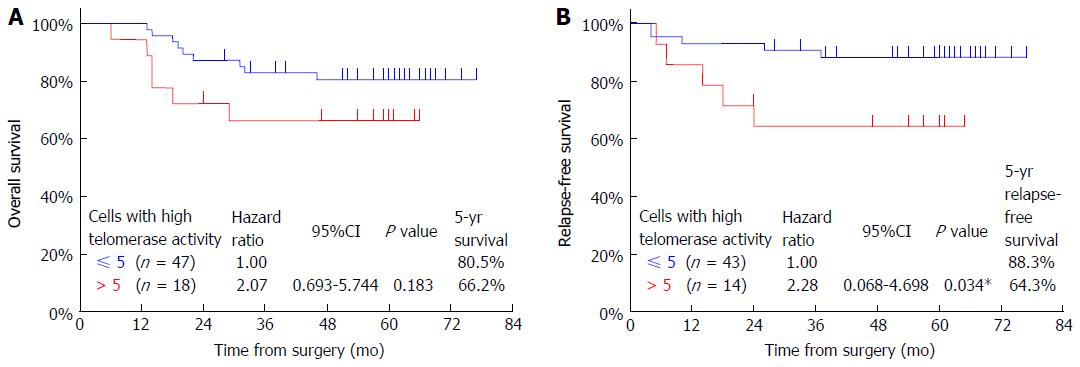
The overall survival rate of patients who had more than five CTCs (66.2%) was lower than that of patients who had five or less CTCs (80.5%); however, this difference was not significant (P = 0.183, Figure 3A). The relapse-free survival rate of patients who had more than five CTCs (64.3%) was significantly lower than that of patients who had five or less CTCs (88.3%) (P = 0.034, Figure 3B).
We investigated prognostic factors related to patient survival by using Cox proportional hazards analysis. Univariate analysis showed that fStage was, in some cases, a significant factor (fStage II, P = 0.196; fStage III, P = 0.0003; fStage IV, P < 0.0001). In contrast, the presence of more than five CTCs was not a significant factor (P = 0.183). Multivariate analysis including these two factors showed fStage to be the only significant factor (fStage II, P = 0.182; fStage III, P = 0.0004; fStage IV, P < 0.0001), and the number of CTCs (more than five) to be non-significant (P = 0.847) (Table 2).
| Variable | Univariate analysis | Multivariate analysis | ||||
| Hazard ratio | 95%CI | P value | Hazard ratio | 95%CI | P value | |
| Number of high telomerase activity cells | ||||||
| ≤ 5 | 1.0 | 1.0 | ||||
| > 5 | 2.069 | 0.693-5.744 | 0.183 | 0.900 | 0.294-2.591 | 0.847 |
| fStage | ||||||
| fStage I | 1.0 | 1.0 | ||||
| fStage II | 7.097 | 0.281-179.3 | 0.196 | 7.106 | 0.281-179.6 | 0.182 |
| fStage III | 25.18 | 4.053-482.6 | 0.0003b | 26.17 | 4.017-510.6 | 0.0004b |
| fStage IV | 83.57 | 14.86-1567 | < 0.0001b | 85.76 | 14.91-1623 | < 0.0001b |
We also investigated factors for increased risk of relapse by Cox proportional hazards analysis. Univariate analysis showed that certain fStages were significant risk factors (fStage II, P = 0.337; fStage III, P = 0.0001; fStage IV, P = 0.005). However, the presence of more than 5 CTCs had no significant influence on relapse rates (P = 0.052). Multivariate analysis including these two factors showed fStage to be the only significant factor (fStage II, P = 0.343; fStage III, P = 0.001; fStage IV, P = 0.004), whereas the number of CTCs was non-significant (P = 0.350, Table 3).
| Variable | Univariate analysis | Multivariate analysis | ||||
| Hazard ratio | 95%CI | P value | Hazard ratio | 95%CI | P value | |
| Number of high telomerase activity cells | ||||||
| ≤ 5 | 1.0 | 1.0 | ||||
| > 5 | 3.566 | 0.988-12.88 | 0.052 | 1.971 | 0.471-8.861 | 0.350 |
| fStage | ||||||
| fStage I | 1.0 | 1.0 | ||||
| fStage II | 3.635 | 0.169-37.96 | 0.337 | 3.576 | 0.166-37.35 | 0.343 |
| fStage III | 17.78 | 4.065-121.8 | 0.0001b | 13.75 | 2.806-100.7 | 0.001b |
| fStage IV | 239.6 | 7.943-7785 | 0.005b | 289.4 | 9.331-9733 | 0.004b |
Here, we used a telomerase-specific adenoviral agent to detect CTCs to avoid relying on the heterogeneous expression of epithelial markers in CTCs undergoing EMT. The enumeration of CTCs is particularly important in gastric cancer, which is the second leading cause of cancer-related death worldwide. Our current data indicate that detection of CTCs may indeed be a useful prognostic indicator for use in patients with gastric cancer, and are consistent with previous reports[25,26].
Our previous preliminary study showed that the number of CTCs isolated from cancer patients was related to surgical and pathological disease progression. Specifically, there were more CTCs in samples from patients with Stage III than in those with Stage I disease. The CTC count was also higher in patients with tumour depth T4 than in those with T1, and in individuals with lymph node metastasis status N2 versus those with N0. In addition, we found that the number of CTCs was associated with disease stage and relapse after curative surgery in gastric cancer patients. In the current study, the relapse-free survival rate of patients who had more than five CTCs was significantly lower than that of patients who had five or less. The overall survival rate of patients with more than five CTCs tended to be lower than that of the patients with five or less; in this case, however, the difference was not statistically significant. The number of CTCs was not an independent risk factor for either overall or relapse-free survival. However, we suggest this may be due to the relatively small sample size we studied, and that examination of larger cohorts might reveal a more significant impact of CTC number on these clinical parameters. Cancer stem cells (CSCs) in the blood of cancer patients are increasingly viewed as important determinants of cancer metastasis[27,28] and prognosis[29]. Given that CSCs have high telomerase activity, and share many of the molecular hallmarks of EMT[30], we suggest that our CTC assay could be used to detect both CSCs and CTCs during EMT.
One limitation of our study is that we could not achieve maximal sensitivity and specificity with regard to CTC detection. The definition of CTCs in this study was based on the threshold of GFP fluorescence intensity and cell diameter. However, these criteria resulted in a significant overlap between the data of healthy volunteers and those of cancer patients. More studies that compare healthy individuals with a larger population of patients with different cancer types are needed to clarify the suitability of CTC detection for clinical use. Another limitation of our study was that we did not determine the metastatic potential of the CTCs that we detected. Ideally, the functions of CTCs should be analysed after cell sorting, and CTCs with metastatic potential could be identified using additional tools such as DNA ploidy analysis[31,32]. Furthermore, gene expression profiling of CTCs, primary tumours, and metastatic tumours will also provide important insight into the mechanisms responsible for cancer metastasis. In summary, CTCs are useful predictors of disease progression in gastric cancer patients, but they do not constitute an independent prognostic factor.
In conclusions, CTC number tended to increase with surgical and pathological disease progression. Although not an independent risk factor, a higher number of CTCs was significantly correlated with disease relapse in gastric cancer after curative surgery. However, our study analysed only a small number of participants, and whether all the CTCs we detected have true metastatic potential was not determined. Further studies with a larger number of participants are therefore required to confirm the findings of this study.
We are grateful to all of the patients and volunteers who donated blood for this study. We would like to thank Professor Toshiyoshi Fujiwara (Okayama University Graduate School of Medicine, Okayama, Japan) for helpful comments and suggestions, Dr. Yasuo Urata (Oncolys BioPharma, Tokyo, Japan) for supplying OBP-401, Dr. Toshiyuki Ozawa and Dr. Akinori Masago (Sysmex Corporation, Kobe, Japan) for their valuable support, and the clinical staff involved in this project.
Detection of circulating tumour cells (CTCs) in peripheral blood is useful for prognosis, monitoring disease progression, and evaluation of treatment efficacy in malignancies, and various methods have been developed to detect CTCs.
Most CTC detection systems that rely on epithelial markers may fail to detect CTCs undergoing the epithelial-mesenchymal transition.
Because OBP-401 is a telomerase-specific adenoviral agent, the OBP-401 assay does not depend on the expression of surface epithelial markers.
In this study, viable CTCs with high telomerase activity were detected using OBP-401 in blood samples from patients with gastric carcinoma. The authors showed that a lower number of CTCs correlated with higher relapse-free survival rates in patients with gastric cancer.
The authors believe that the OBP-401 assay for detection of CTCs is clinically useful for patients with gastric carcinoma.
The authors presented a novel technique for detection of CTCs that does not depend on surface epithelial markers. The authors showed that a lower number of CTCs was correlated with higher relapse-free survival rates in patients with gastric cancer.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Fiorentini G, Fiorentini HX, Tarnawski AS S- Editor: Yu J L- Editor: A E- Editor: Zhang FF
| 1. | Liotta LA, Kleinerman J, Saidel GM. Quantitative relationships of intravascular tumor cells, tumor vessels, and pulmonary metastases following tumor implantation. Cancer Res. 1974;34:997-1004. [PubMed] |
| 2. | Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3360] [Cited by in RCA: 3382] [Article Influence: 161.0] [Reference Citation Analysis (0)] |
| 3. | Krebs MG, Sloane R, Priest L, Lancashire L, Hou JM, Greystoke A, Ward TH, Ferraldeschi R, Hughes A, Clack G. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol. 2011;29:1556-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 689] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 4. | Moreno JG, Miller MC, Gross S, Allard WJ, Gomella LG, Terstappen LW. Circulating tumor cells predict survival in patients with metastatic prostate cancer. Urology. 2005;65:713-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 173] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 5. | Mocellin S, Del Fiore P, Guarnieri L, Scalerta R, Foletto M, Chiarion V, Pilati P, Nitti D, Lise M, Rossi CR. Molecular detection of circulating tumor cells is an independent prognostic factor in patients with high-risk cutaneous melanoma. Int J Cancer. 2004;111:741-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:3213-3221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1354] [Cited by in RCA: 1410] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 7. | Inoue M, Otsuka K, Shibata H. Circulating tumor cell count as a biomarker of a specific gastric cancer subgroup characterized by bone metastasis and/or disseminated intravascular coagulation - an early indicator of chemotherapeutic response. Oncol Lett. 2016;11:1294-1298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Ito H, Kanda T, Nishimaki T, Sato H, Nakagawa S, Hatakeyama K. Detection and quantification of circulating tumor cells in patients with esophageal cancer by real-time polymerase chain reaction. J Exp Clin Cancer Res. 2004;23:455-464. [PubMed] |
| 9. | Honma H, Kanda T, Ito H, Wakai T, Nakagawa S, Ohashi M, Koyama Y, Valera VA, Akazawa K, Hatakeyama K. Squamous cell carcinoma-antigen messenger RNA level in peripheral blood predicts recurrence after resection in patients with esophageal squamous cell carcinoma. Surgery. 2006;139:678-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Gertler R, Rosenberg R, Fuehrer K, Dahm M, Nekarda H, Siewert JR. Detection of circulating tumor cells in blood using an optimized density gradient centrifugation. Recent Results Cancer Res. 2003;162:149-155. [PubMed] |
| 11. | Talasaz AH, Powell AA, Huber DE, Berbee JG, Roh KH, Yu W, Xiao W, Davis MM, Pease RF, Mindrinos MN. Isolating highly enriched populations of circulating epithelial cells and other rare cells from blood using a magnetic sweeper device. Proc Natl Acad Sci USA. 2009;106:3970-3975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 378] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 12. | He W, Wang H, Hartmann LC, Cheng JX, Low PS. In vivo quantitation of rare circulating tumor cells by multiphoton intravital flow cytometry. Proc Natl Acad Sci USA. 2007;104:11760-11765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 217] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 13. | Vona G, Sabile A, Louha M, Sitruk V, Romana S, Schütze K, Capron F, Franco D, Pazzagli M, Vekemans M. Isolation by size of epithelial tumor cells: a new method for the immunomorphological and molecular characterization of circulatingtumor cells. Am J Pathol. 2000;156:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 827] [Cited by in RCA: 801] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 14. | Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky A. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235-1239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3036] [Cited by in RCA: 2575] [Article Influence: 143.1] [Reference Citation Analysis (0)] |
| 15. | Okabe H, Tsunoda S, Hosogi H, Hisamori S, Tanaka E, Tanaka S, Sakai Y. Circulating Tumor Cells as an Independent Predictor of Survival in Advanced Gastric Cancer. Ann Surg Oncol. 2015;22:3954-3961. [PubMed] |
| 16. | Shimazu K, Fukuda K, Yoshida T, Inoue M, Shibata H. High circulating tumor cell concentrations in a specific subtype of gastric cancer with diffuse bone metastasis at diagnosis. World J Gastroenterol. 2016;22:6083-6088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Książkiewicz M, Markiewicz A, Zaczek AJ. Epithelial-mesenchymal transition: a hallmark in metastasis formation linking circulating tumor cells and cancer stem cells. Pathobiology. 2012;79:195-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 18. | Gorges TM, Tinhofer I, Drosch M, Röse L, Zollner TM, Krahn T, von Ahsen O. Circulating tumour cells escape from EpCAM-based detection due to epithelial-to-mesenchymal transition. BMC Cancer. 2012;12:178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 393] [Cited by in RCA: 426] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 19. | Blackburn EH. Telomere states and cell fates. Nature. 2000;408:53-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 986] [Cited by in RCA: 964] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 20. | Fujiwara T, Kagawa S, Kishimoto H, Endo Y, Hioki M, Ikeda Y, Sakai R, Urata Y, Tanaka N, Fujiwara T. Enhanced antitumor efficacy of telomerase-selective oncolytic adenoviral agent OBP-401 with docetaxel: preclinical evaluation of chemovirotherapy. Int J Cancer. 2006;119:432-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Ito H, Inoue H, Sando N, Kimura S, Gohda K, Sato J, Murakami K, Ito S, Odaka N, Satodate H. Prognostic impact of detecting viable circulating tumour cells in gastric cancer patients using a telomerase-specific viral agent: a prospective study. BMC Cancer. 2012;12:346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7038] [Cited by in RCA: 7978] [Article Influence: 190.0] [Reference Citation Analysis (0)] |
| 23. | Sobin LH, Gospodarowicz MK, Wittekind C. International Union Against Cancer: TNM classification of malignant tumours. 7th ed. Chichester, West Sussex, UK; Hoboken, NJ: Wiley-Blackwell, 2010. . |
| 24. | Kim SJ, Masago A, Tamaki Y, Akazawa K, Tsukamoto F, Sato J, Ozawa T, Tsujino Y, Noguchi S. A novel approach using telomerase-specific replication-selective adenovirus for detection of circulating tumor cells in breast cancer patients. Breast Cancer Res Treat. 2011;128:765-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Beeharry MK, Liu WT, Yan M, Zhu ZG. New blood markers detection technology: A leap in the diagnosis of gastric cancer. World J Gastroenterol. 2016;22:1202-1212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Wang HY, Wei J, Zou ZY, Qian XP, Liu BR. Circulating tumour cells predict survival in gastric cancer patients: a meta-analysis. Contemp Oncol (Pozn). 2015;19:451-457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Wicha MS. Cancer stem cells and metastasis: lethal seeds. Clin Cancer Res. 2006;12:5606-5607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Zhong J, Chen Y, Wang LJ. Emerging molecular basis of hematogenous metastasis in gastric cancer. World J Gastroenterol. 2016;22:2434-2440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Pilati P, Mocellin S, Bertazza L, Galdi F, Briarava M, Mammano E, Tessari E, Zavagno G, Nitti D. Prognostic value of putative circulating cancer stem cells in patients undergoing hepatic resection for colorectal liver metastasis. Ann Surg Oncol. 2012;19:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 30. | Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2555] [Cited by in RCA: 2437] [Article Influence: 135.4] [Reference Citation Analysis (0)] |
| 31. | Bonsing BA, Beerman H, Kuipers-Dijkshoorn N, Fleuren GJ, Cornelisse CJ. High levels of DNA index heterogeneity in advanced breast carcinomas. Evidence for DNA ploidy differences between lymphatic and hematogenous metastases. Cancer. 1993;71:382-391. [PubMed] |
| 32. | Kolostova K, Matkowski R, Gürlich R, Grabowski K, Soter K, Lischke R, Schützner J, Bobek V. Detection and cultivation of circulating tumor cells in gastric cancer. Cytotechnology. 2016;68:1095-1102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |