Published online Aug 28, 2016. doi: 10.3748/wjg.v22.i32.7332
Peer-review started: June 17, 2016
First decision: July 12, 2016
Revised: July 26, 2016
Accepted: August 8, 2016
Article in press: August 8, 2016
Published online: August 28, 2016
To explore the effect of sleeve gastrectomy (SG) with jejuno-jejunal or jejuno-ileal loop on glycolipid metabolism in diabetic rats.
Diabetic rats, which were induced by high-fat diet (HFD), nicotinamide and low-dose streptozotocin, underwent sham operations, SG, SG with jejuno-ileal loop (SG-JI) and SG with jejuno-jejunal loop (SG-JJ) followed by postoperative HFD. Then, at the time points of baseline and 2, 12 and 24 wk postoperatively, we determined and compared several variables, including the area under the curve for the results of oral glucose tolerance test (AUCOGTT), serum levels of triglyceride, cholesterol and ghrelin in fasting state, homeostasis model assessment of insulin resistance (HOMA-IR), body weight, calorie intake, glucagon-like peptide (GLP)-1 and insulin secretions after glucose gavage at dose of 1 g/kg.
At 2 wk postoperatively, rats that underwent SG, SG-JJ and SG-JI, compared with sham-operated (SHAM) rats, demonstrated lower body weight, calorie intake and ghrelin (P < 0.05 vs SHAM), enhanced secretion of insulin and GLP-1 after glucose gavage (P < 0.05 vs SHAM), improved AUCOGTT, HOMA-IR, fasting serum triglyceride and cholesterol (AUCOGTT: 1616.9 ± 83.2, 837.4 ± 83.7, 874.9 ± 97.2 and 812.6 ± 81.9, P < 0.05 vs SHAM; HOMA-IR: 4.31 ± 0.54, 2.94 ± 0.22, 3.17 ± 0.37 and 3.41 ± 0.22, P < 0.05 vs SHAM; Triglyceride: 2.35 ± 0.17, 1.87 ± 0.23, 1.98 ± 0.30 and 2.04 ± 0.21 mmol/L, P < 0.05 vs SHAM; Cholesterol: 1.84 ± 0.21, 1.53 ± 0.20, 1.52 ± 0.20 and 1.46 ± 0.23 mmol/L). At 12 wk postoperatively, rats receiving SG-JJ and SG-JI had lower body weight, reduced levels of triglyceride and cholesterol and elevated level of GLP-1 compared to those receiving SG (P < 0.05 vs SG). At 24 wk after surgery, compared with SG, the advantage of SG-JJ and SG-JI for glucolipid metabolism was still evident (P < 0.05 vs SG). SG-JI had a better performance in lipid metabolism and GLP-1 secretion of rats than did SG-JJ.
SG combined with intestinal loop induces better glycolipid metabolism than simple SG, with the lipid metabolism being more improved with SG-JI compared to SG-JJ.
Core tip: To improve the effect of sleeve gastrectomy (SG), surgeons sporadically use different intestinal loops; however, these innovative surgical procedures lack a theoretical foundation. We explored the effect of SG with jejuno-jejunal loop (SG-JJ) or jejuno-ileal loop (SG-JI) on glycolipid metabolism in diabetic rats. We discovered that SG-JJ and SG-JI were superior to SG in improving glycolipid metabolism. Compared with SG-JJ, the improvement in lipid metabolism after SG-JI was more apparent. These findings might help surgeons select procedures for individual patients.
- Citation: Zhong MW, Liu SZ, Zhang GY, Zhang X, Hu SY. Effects of sleeve gastrectomy with jejuno-jejunal or jejuno-ileal loop on glycolipid metabolism in diabetic rats. World J Gastroenterol 2016; 22(32): 7332-7341
- URL: https://www.wjgnet.com/1007-9327/full/v22/i32/7332.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i32.7332
Recently, international diabetes organizations made a joint statement in Diabetes Care[1]. This statement indicated that patients with type 2 diabetes mellitus (T2DM) with class III [body mass index (BMI) ≥ 40 kg/m2] and class II (BMI 35.0-39.9 kg/m2) obesity as well as those with class I obesity (BMI 30.0-34.9 kg/m2) should consider bariatric/metabolic surgery, if T2DM is inadequately controlled despite optimal treatment with oral or injectable medication. During the past decade, bariatric surgery, especially sleeve gastrectomy (SG), has been increasingly performed in patients. Currently, the most commonly performed procedure in patients of the United States, Canada and the Asia/Pacific region is SG, which outnumbers Roux-en-Y gastric bypass (RYGB)[2]. As a novel metabolic surgery, SG is characterized by a lower complication rate, faster operation, fewer technical requirements, and fewer postoperative nutritional problems[3], and some researchers have reported that SG and RYGB have equal efficacy for diabetes[4,5]. However, long-term randomized controlled comparison of the effects of SG and RYGB in patients with T2DM is surprisingly limited. Other researchers doubt the long-term effect of SG on diabetes patients[3]. In addition, SG has been reported as an independent predictor for the recurrence of diabetes[6].
Surgeons have designed many additional surgical procedures for SG to improve excessive body weight loss and diabetes control, such as SG with duodeno-jejunal bypass[7], loop gastroileostomy[8] jejuno-ileal bypass[9] and duodeno-ileal bypass[10]. Although these surgical procedures showed some improvement with regard to excessive body weight loss and diabetes control, they were performed with resection of different segments of small intestine or different intestinal loops. The procedures were not compared in randomized controlled trials (RCTs) and were limited to clinical retrospective studies. In the present study, we conducted a RCT in a rat model to compare the effect of SG with different intestinal loops on diabetes control.
Several factors of the diabetes postoperative recurrence have been confirmed, including preoperative BMI, patient age, T2DM course and severity, excessive weight loss, body weight regain, and postoperative diet and lifestyle[6,11,12]. Dietary control seems more important for patients with SG[13], and in our previous study, we demonstrated that the improvement in glucose metabolism after metabolic surgery can be reversed by postoperative high-fat diet (HFD) in rats[14]. In this present study, we used rats with postoperative HFD to simulate patients with an undesirable diet. Diabetic rats were treated with sham operation (SHAM), SG, SG with jejuno-jejunal loop (SG-JJ) or SG with jejuno-ileal loop (SG-JI). At the stated time points, we determined and compared the glucose and lipid metabolic profiles, serum parameters including insulin, glucagon-like peptide (GLP)-1 and ghrelin.
Under the conditions of constant temperature at 24 to 26 °C, humidity at 50% to 60% and light/dark alternation at 12 h interval, 70 8-wk-old Wistar rats, provided by Laboratory Animal Center of Shandong University, were separately housed in independently ventilated cages. All the rats received 1-wk adaptive feeding, followed by 4-wk HFD (Huafukang Biotech Company, China), which contains 40% fat as calories, to induce insulin resistance. A 12-h fasting period was succeeded by a single intraperitoneal injection with nicotinamide at the dose of 170 mg/kg. After 15 min, streptozotocin (Sigma-Aldrich, St Louis, MO, United States), at the dose of 65 mg/kg, was administrated to the rats by injection, so that they could reach a diabetic state[15]. Two weeks later, 39 rats met the criteria for the diabetic state, which included fasting blood glucose level of more than or equal to 7.1 mmol/L or the 2-h blood glucose level of more than or equal to 11.1 mmol/L during oral glucose tolerance test (OGTT). We excluded the rats with extreme hyperglycemia (blood glucose level of more than 16.7 mmol/L)[16]. All animal experimental procedures involved in our study had been approved by the Animal Care and Utilization Committee of Qilu Hospital of Shandong University, Jinan, China.
We fed the diabetic rats with low-residue diet for 48 h, then performed SHAM (SHAM group, n = 10), SG (SG group, n = 10), SG-JJ (SG-JJ group, n = 10) and SG-JI (SG-JI group, n = 9) on them under anesthesia with 10% chloral hydrate solution. All rats were continuously provided with HFD, followed by 72 h low-residue diet feeding post-operation.
At the time points of baseline, 2, 12 and 24 wk postoperatively, we measured several variables including the results of OGTT, homeostasis model assessment of insulin resistance (HOMA-IR), levels of triglyceride, cholesterol and ghrelin in fasting serum, body weight, calorie intake and secretion of GLP-1 and insulin after gavage (1 g/kg).
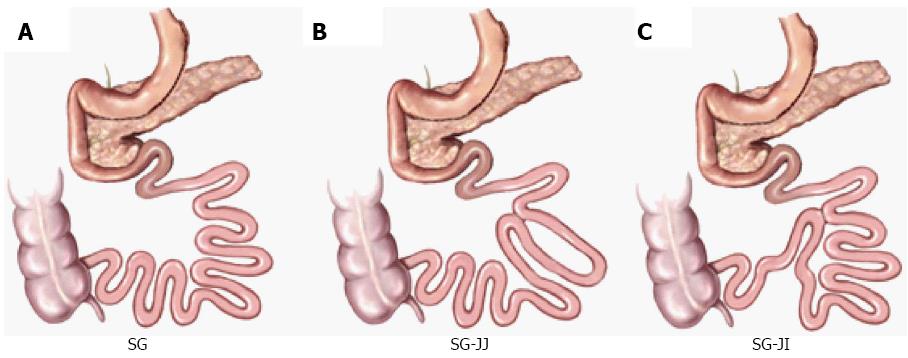
The surgical procedures were performed as described in our and other previous studies[17,18] (Figure 1).
SG surgery: We made a 4-cm vertical midline abdominal incision along with abdominal midline from the xiphoid process. After locating the greater curvature, we freed it from the gastric cardium to the pylorus with ligating and transecting relative gastric vessels. The glandular stomach and most of the gastric body were removed (70% of total stomach). Residual stomach was closed using 7-0 silk sutures, and re-placed in the abdominal cavity. Then, a 5-0 silk suture (Ningbo Medical Needle) was applied for the closure of the incision.
SG-JJ surgery: On the basis of SG surgery, we located jejunum 15 cm and 35 cm distal to the ligament of Treitz respectively and connected them to form a jejuno-jejunal side-by-side anastomosis.
SG-JI surgery: On the basis of SG surgery, we divided jejunum 15 cm distal to the ligament of Treitz, and connected it to the distal ileum 20 cm proximal to the ileocecal valve to form a jejuno-ileal side-by-side anastomosis.
SHAM surgery: The incision and procedure were similar to the performance of SG except that we kept the glandular stomach and most of the gastric body. Same durations of operation were maintained to ensure similar stress from the surgery and anesthesia.
Upon completion of 8-h fasting, all rats received 1 g/kg glucose by oral gavage. Then, we estimated the levels of blood glucose at five time points (baseline, 10, 30, 60 and 120 min after gavage) respectively. The glucometer used was the Roche One Touch Ultra (Lifescan, Milpitas, CA, United States).
With rats under anesthesia by diethyl ether, we respectively gathered blood samples from retrobulbar venous plexus at time points of baseline, 15, 30, 60 and 120 min after gavage with glucose, similar to OGTT. Serum was collected by centrifugation (1006 ×g, 4 °C, 15 min) and stored at -80 °C for further measurement. Levels of triglyceride and cholesterol in fasting serum were detected by the Hitachi automatic biochemical analyzer (Japan). Concentrations of insulin, GLP-1 and ghrelin in serum were tested by enzyme-linked immunosorbent assay (ELISA) kits (insulin: Millipore, Billerica, MA, United States; GLP-1 and ghrelin: Uscn Life Science, Wuhan, China).
We conducted the calculations of HOMA-IR, according to the levels of insulin and blood glucose in fasting serum, by the following formula: HOMA-IR = fasting insulin (mIU/L) × fasting blood glucose (mmol/L)/22.5[19].
All quantitative data are presented as mean ± SD. By the use of trapezoidal integration, we calculated the area under the curves for OGTT (AUCOGTT). We analyzed data of different groups, including the AUCOGTT, HOMA-IR values, triglyceride, cholesterol and ghrelin serum levels, by means of one-way analysis of variance (ANOVA) followed by Bonferroni post hoc comparison. A mixed model ANOVA followed by Bonferroni post hoc comparison analysis was used in insulin and GLP-1 secretions in glucose-gavage rats. All statistical calculations were processed with the use of SPSS version 19.0 (IBM, Armonk, NY, United States), at an alpha level of 0.05.
Ten, nine, eight and nine rats survived in the SHAM, SG, SG-JJ and SG-JI groups, respectively. Three rats died of residual stomach leakage.
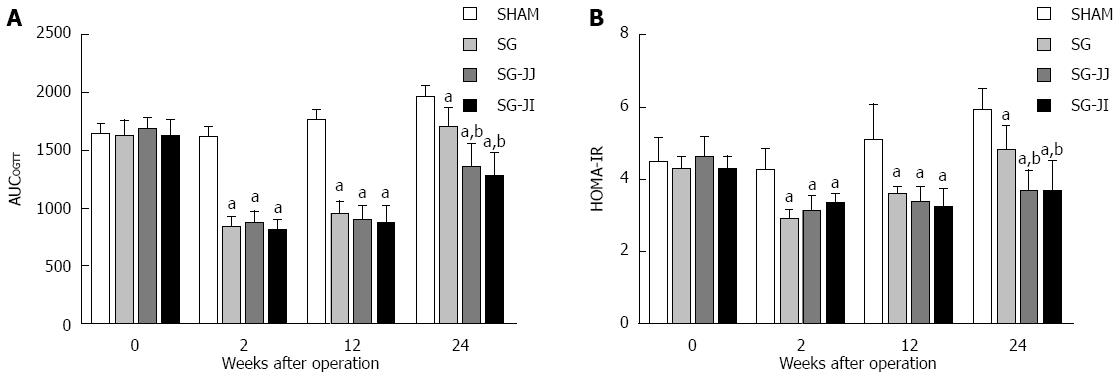
As shown in Figure 2A, in the early postoperative period (2 wk after surgery), rats in the SG (837.4 ± 83.7), SG-JJ (874.9 ± 97.2) and SG-JI (812.6 ± 81.9) groups showed lower AUCOGTT than the SHAM group (1616.9 ± 83.2, P < 0.05), and the lower AUCOGTT was sustained until the end of the study (SG: 1696.6 ± 155.5; SG-JJ: 1343.9 ± 217.3; SG-JI: 1275.9 ± 194.3; SHAM: 1965.9 ± 81.6; P < 0.05). At 24 wk after surgery, the AUCOGTT in the SG-JJ and SG-JI groups was lower than that in the SG group (P < 0.05), and no difference in AUCOGTT was observed between the SG-JJ and SG-JI groups at any time point during this study.
Rats that underwent SHAM had higher HOMA-IR than rats of other metabolic surgery types at every postoperative time point (P < 0.05). At 24 wk postoperatively, the HOMA-IR in the SG-JJ (3.72 ± 0.54) and SG-JI (3.73 ± 0.79) groups was comparable, and it was lower than that in the SG group (4.86 ± 0.62, P < 0.05) (Figure 2B).
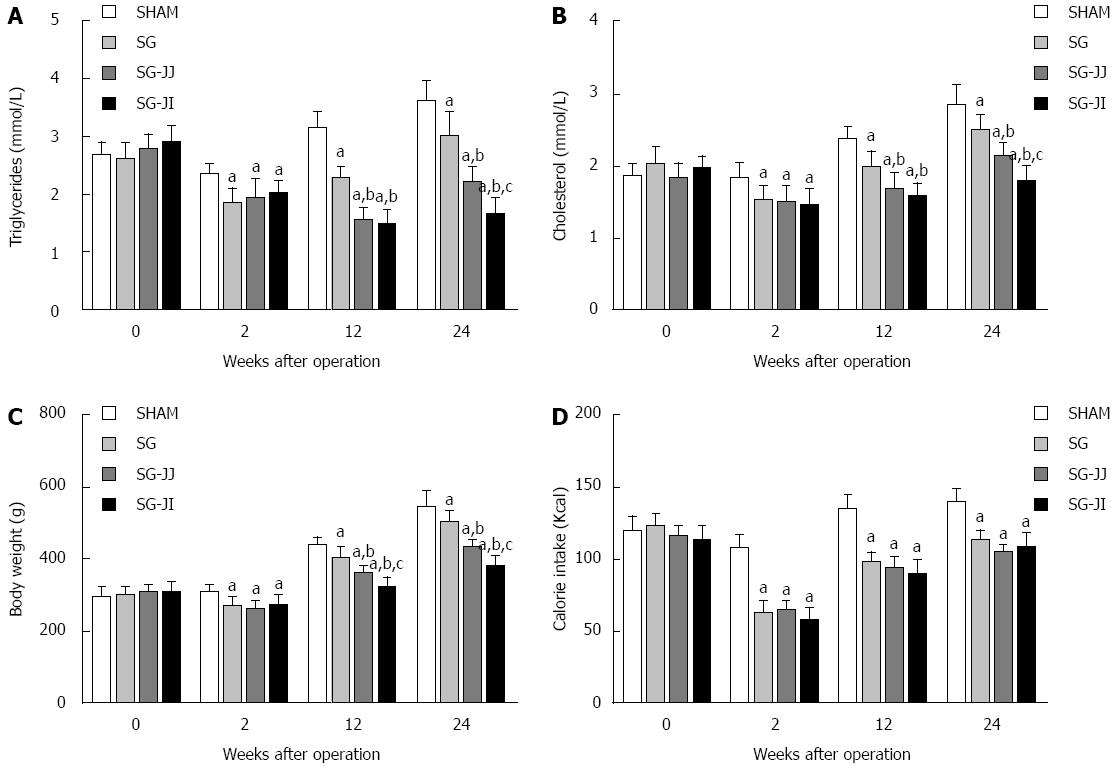
Lipid profiles showed a similar trend to AUCOGTT. At 12 wk postoperatively, rats of the SG group had significantly higher fasting triglyceride levels than those of the SG-JJ (2.27 ± 0.21 mmol/L vs 1.59 ± 0.17 mmol/L) and SG-JI (2.27 ± 0.21 mmol/L vs 1.49 ± 0.25 mmol/L, P < 0.05) groups (Figure 3A). The same trend was also observed in fasting cholesterol levels among the SG (1.98 ± 0.22 mmol/L), SG-JJ (1.70 ± 0.20 mmol/L) and SG-JI (1.58 ± 0.18 mmol/L, P < 0.05) groups (Figure 3B). Fasting triglyceride levels of the SG-JI group, at 24 wk postoperatively, were lower than in the SG-JJ group (1.68 ± 0.24 mmol/L vs 2.22 ± 0.25 mmol/L) (Figure 3A), which showed the same trend in fasting cholesterol levels of the SG-JI group compared with the SG-JJ group (1.81 ± 0.19 mmol/L vs 2.14 ± 0.17 mmol/L, P < 0.05) (Figure 3B).
At every postoperative time point, compared with the SHAM group, the rats that underwent SG, SG-JJ and SG-JI had lower body weight (Figure 3C) and calorie intake (Figure 3D) (P < 0.05). The differences of 12-wk postoperative body weight were statistically significant in the SG group, compared with the SG-JJ group (403.8 ± 31.0 g vs 366.1 ± 14.2 g, P < 0.05) and with the SG-JI group (403.8 ± 31.0 g vs 326.2 ± 24.8 g, P < 0.05), and the differences were also observed at 24 wk after surgery. No differences, however, were seen for calorie intake among the SG, SG-JJ and SG-JI groups.
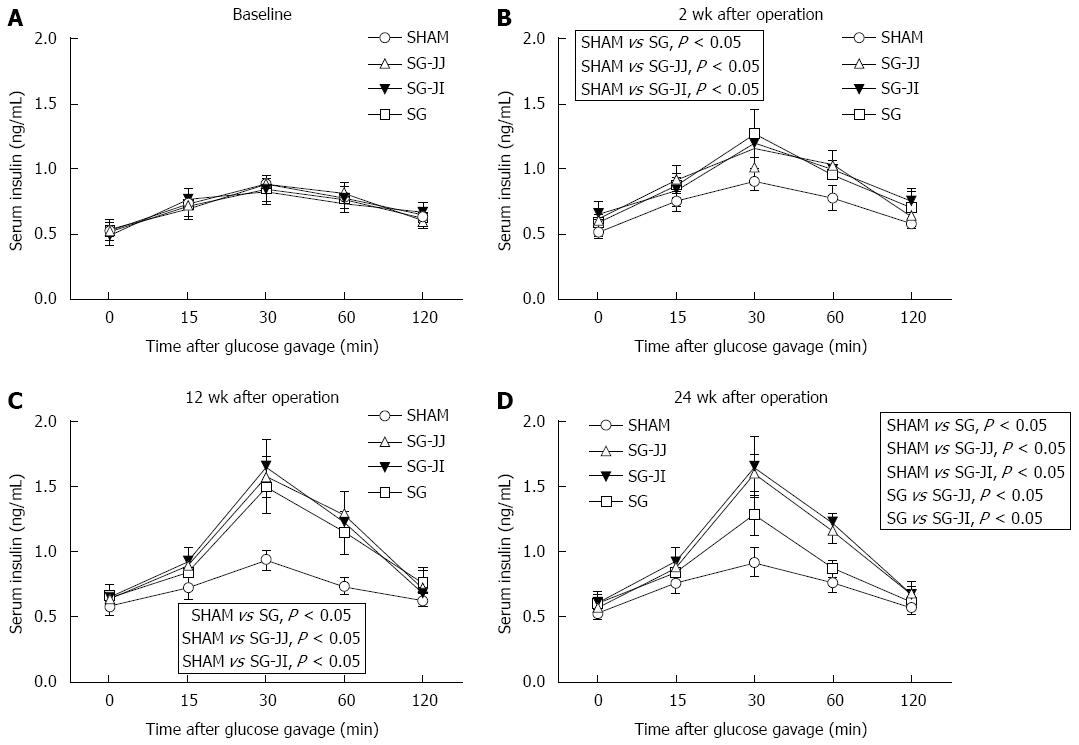
Insulin secretion after gavage at baseline, and 2, 12 and 24 wk after surgery is described in Figure 4A-D, respectively. Secretion of insulin after gavage in the SG, SG-JJ and SG-JI groups was higher than that in the SHAM group at any time postoperatively (P < 0.05). At 24 wk after surgery, compared with the SG group, rats had higher insulin secretion in the SG-JJ (P < 0.05) and SG-JI groups (P < 0.05). No statistical difference was seen for insulin secretion between the SG-JJ and SG-JI groups.
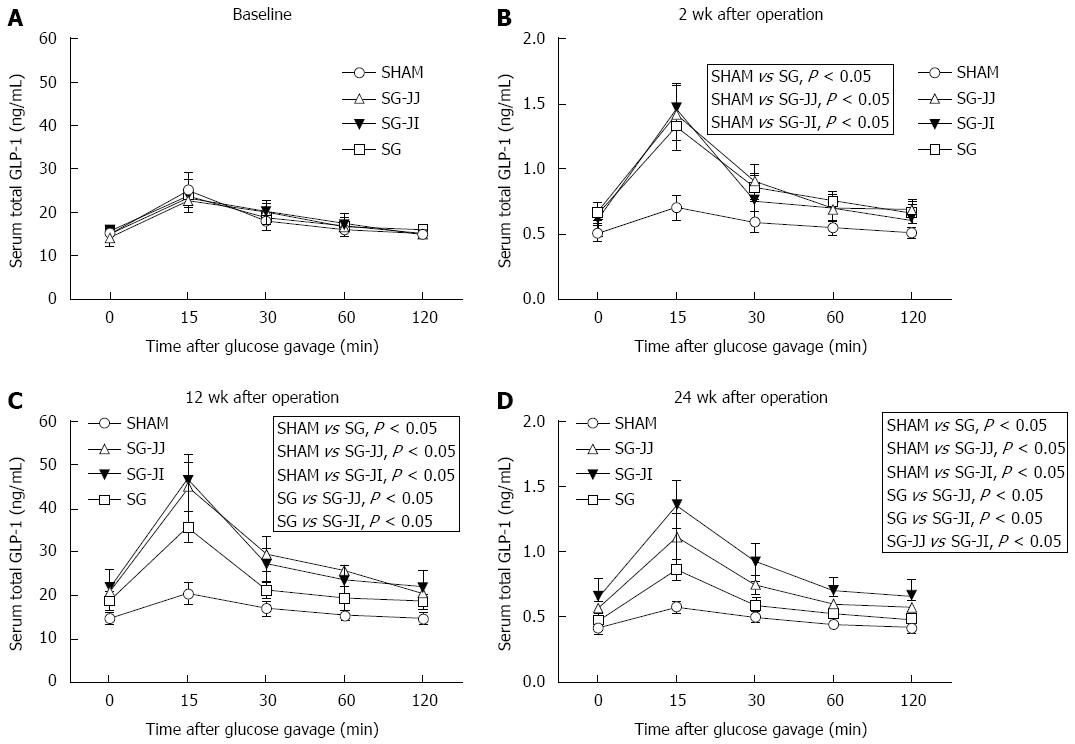
Serum GLP-1 at baseline, and 2, 12 and 24 wk after surgery is shown in Figure 5A-D, respectively. There was no difference in GLP-1 secretion at baseline among the four groups. At baseline, rats that underwent different surgeries had similar GLP-1 secretions, which were higher in the SG (P < 0.05), SG-JJ (P < 0.05) and SG-JI (P < 0.05) groups compared with the SHAM group at 2 wk postoperatively. Then, at 12 wk postoperatively, rats in the SG group secreted less GLP-1 than those in the SG-JJ (P < 0.05) and SG-JI groups (P < 0.05). Rats in the SG-JJ group had lower GLP-1 secretion than those in the SG-JI group (P < 0.05) at 24 wk postoperatively.
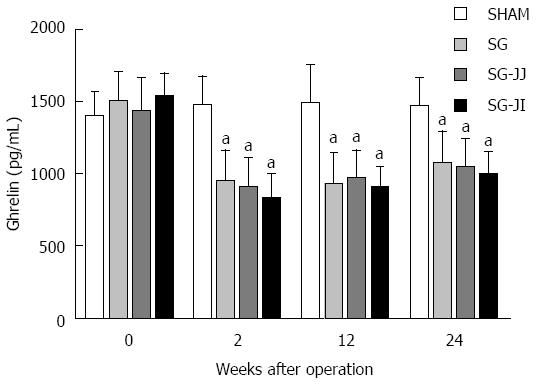
No significant differences were seen for fasting serum ghrelin at baseline in any of the rats (Figure 6) or for any other time points for the SG, SG-JJ and SG-JI groups. The SG, SG-JJ and SG-JI groups showed lower fasting serum ghrelin than those in the SHAM group at all postoperative times (P < 0.05).
Diabetes commonly threatens human health, with an estimated 422 million cases worldwide, according to the data reported by the World Health Organization in 2014; moreover, the prevalence of diabetes shows an increasing growth rate in countries with low-middle incomes[20].
Metabolic surgery has become increasingly common worldwide because of its efficacy in improving management of diabetes, especially for cases inadequately controlled by optimal treatment[1]. Metabolic surgery is classified as restrictive, malabsorptive and mixed operation. SG used to be considered as a pure restrictive operation, but in present thought is more than that. Compared with other restrictive techniques (e.g., adjustable gastric banding), performance of SG can achieve more excellent efficacy, producing more efficient gastric emptying and intestinal transit as well as higher GLP-1 levels and lower ghrelin levels, similar to RYGB[21]. Hence, SG is the most popular method of metabolic surgery in the United States/Canada and Asia/Pacific regions, in addition to its advantages of faster operation, fewer technical requirements, lower complication rate, and fewer postoperative nutritional problems[2,3]. SG, as a novel surgery approach, has unclear long-term effect on diabetes, with a reported inadequate remission rate compared with RYGB[6]. In previous clinical study, single-anastomosis duodeno-jejunal bypass with SG[7], gastroileostomy loop with SG[8], jejuno-ileal bypass with SG[9] and duodeno-ileal bypass with SG[10] were performed, with the aim to achieve improvement in controlling diabetes of SG. These studies demonstrated that SG with different intestinal bypass showed excellent antidiabetic effects. However, there have been limited prospective RCTs. In this study, we performed a RCT to compare the effect of SG, SG-JJ and SG-JI on diabetes control, and showed that all of the three surgeries have excellent antidiabetic effects shortly after surgery. What’s more, this study demonstrated that SG-JJ and SG-JI could provide better glycolipid metabolism than SG, despite a maintained HFD chow administered until 24 wk after surgery.
Excellent postoperative management contributes much to the antidiabetic effect of metabolic surgery, suggesting that good postoperative diet control is necessary for SG[22]. In this study, we used postoperative HFD to simulate undesirable dietary control. Although antidiabetic activity decreased over time, the rats with SG still manifested better diabetes control than the SHAM rats. Compared with SG, SG-JJ and SG-JI did not show an improved antidiabetic effect at 12 wk after surgery. Nevertheless, an improved antidiabetic effect of SG-JJ and SG-JI was observed at 24 wk after surgery. That finding demonstrates that SG-JJ and SG-JI can enhance improvement in glucose metabolism after SG. We did not observe any difference in AUCOGTT between the SG-JJ and SG-JI groups.
Lipid metabolism demonstrated a similar trend to glucose metabolism. SG-JI and SG-JJ rats showed lower fasting triglyceride and cholesterol level than SG rats. This may partly be because some chyme entered the distal intestine through the jejuno-jejunal or jejuno-ileal anastomotic stoma. At 24 wk after surgery, the rats with SG-JI demonstrated lower fasting triglyceride and cholesterol level than the SG-JJ group. We suggest that SG can improve glucose and lipid metabolism, SG-JI and SG-JJ can enhance these improvements, and SG-JI improves lipid metabolism more effectively than SG-JJ does.
At 12 wk postoperatively, SG-JI and SG-JJ were observed to achieve better weight control. Calorie intake, however, was not statistically different among the three groups. Similarly, since 12 wk after surgery, rats that underwent SG-JI had better weight control than those that underwent SG-JJ, but with comparable calorie intake. We speculate that body weight is affected by digestion or absorption. We regret that the caloric content in feces was not measured. It has been determined that weight loss can lead to increased insulin level, and improved glucose homeostasis and inflammation[23]. Our study showed that SG-JJ and SG-JI could lead to a higher insulin secretion level and lower HOMA-IR (an index for insulin resistance assessment) than SG, which can contribute to better diabetes control by the greater weight reduction of SG-JJ and SG-JI compared with SG.
Besides weight loss, other independent mechanisms of metabolic surgery have been explored, such as the foregut hypothesis, which demonstrates an independent beneficial effect, irrelevant to caloric intake, body weight or delivery of nutrients to the hindgut, in controlling type 2 diabetes through exclusion of the proximal small intestine[24], and the hindgut hypothesis which proposes an improvement of gastric bypass surgery in delivering nutrients and the elevated postprandial levels of gut hormones (i.e. GLP-1 and peptide YY)[25]. Our previous study compared different portions of small intestine, performing duodeno-jejunal bypass, ileal and sub-ileal interposition, and duodeno-jejunal bypass with ileal interposition, and concluded that small intestine contributes to enhancing glucose homeostasis[26].
Outcomes in the present research partly conformed to the hindgut hypothesis. We discovered that both rats with SG-JJ and SG-JI showed higher GLP-1 secretion than SG rats showed, and GLP-1 secretion in the SG-JI group was the highest one among the three metabolic surgery groups. The higher GLP-1 secretion observed in the SG-JJ and SG-JI groups may have been because of undigested chyme arriving at the hindgut through the intestinal loops. Compared with the SG-JJ group, the undigested chyme was delivered to hindgut more quickly in the SG-JI group, so the latter group demonstrated higher GLP-1 secretion. GLP-1 can regulate glucose homeostasis by stimulating insulin secretion, suppressing glucagon secretion, and promoting proliferation and inhibiting apoptosis of β cells[27]. Exenatide is a GLP-1 analogue that has been used clinically as an antidiabetic drug[28]. In this study, GLP-1 secretion showed the opposite trend to AUCOGTT, except at 24 wk after surgery. At 24 wk after surgery, GLP-1 secretion in the SG-JI group was higher than that in the SG-JJ group; however, there was no significant difference in AUCOGTT between the two groups.
Researchers have verified that serum ghrelin levels are reduced remarkably after SG, as shown in multiple clinical studies[29,30], and they believe that body weight loss and diabetes control after SG are associated with the reduced serum ghrelin levels. However, Chambers et al[31] performed SG on both ghrelin-deficient and wild-type mice, and did not find any difference in diabetes control between the two types of mice. We suggest that this finding demonstrates that the effect of SG on diabetes control is ghrelin independent, but it does not prove that ghrelin makes no contribution to diabetes control. Our research group has demonstrated that rats from which only the glandular stomach has been removed secrete ghrelin and show significant improvement of diabetes[32]. In our study, rats in the SG, SG-JJ and SG-JI groups showed lower fasting ghrelin levels than rats in the SHAM group. Nevertheless, there was no difference among the three metabolic surgery groups, indicating that ghrelin plays an important role in diabetes control after SG, SG-JJ and SG-JI, but it did not enhance diabetes control of SG after addition of jejuno-jejunal or jejuno-ileal loop.
There are some limitations in this study. First, the calorie content in feces was not measured, so the calorie absorption from food could not be calculated. Second, we demonstrated that SG-JJ and SG-JI enhanced diabetes control and lipid metabolism compared with SG alone. However, the results of animal experiments cannot be transferred into humans directly. We suggest that further clinical studies should be performed, to explore the optimal procedures (SG, SG-JJ, or SG-JI) for individual patients, after controlling for differences in BMI, age, duration and severity of T2DM, serum lipid, compliance with postoperative administration and so forth.
In conclusion, SG-JJ and SG-JI demonstrate better glycolipid metabolism than SG, which may result from better body weight control, enhanced insulin and GLP-1 secretion, and improved insulin resistance. Compared with SG-JJ, the improvement in lipid metabolism after SG-JI is more apparent and SG-JI induces further enhancement of GLP-1 secretion.
Metabolic surgery, a novel algorithm for treatment of type 2 diabetes mellitus, has been increasingly performed worldwide, the majority of which is sleeve gastrectomy (SG) with its long-term effect, however, being controversial. Moreover, SG has been reported as an independent predictor for the recurrence of diabetes, and SG seems to require urgent postoperative dietary control.
To improve the effect of SG on diabetes control, SG with duodeno-jejunal bypass, loop gastro-ileostomy, jejuno-ileal bypass, and duodeno-ileal bypass has been performed sporadically by surgeons. These additional surgical procedures to SG were not clearly elaborated and lacked a theoretical foundation.
The authors used postoperative high-fat diet to simulate undesirable dietary control and performed SG, jejuno-jejunal loop (SG-JJ) and jejuno-ileal loop (SG-JI) on diabetic rats. SG-JJ and SG-JI were superior to SG in improving glycolipid metabolism. Compared with SG-JJ, the improvement in lipid metabolism after SG-JI was more apparent.
The findings in this study suggest that if a patient is thought to have undesirable postoperative dietary control, SG-JJ and SG-JI may be considered as preferential surgical procedures. SG-JI may be selected for patients with hyperlipidemia. These discoveries might help surgeons to select surgical procedures for individual patients.
SG, a novel procedure of metabolic surgery to remove 70%-80% of the stomach from the greater curvature, has demonstrated short- and medium-term roles in improving glycolipid metabolism in obese patients with/without type 2 diabetes mellitus, but its long-term beneficial effect remains controversial. In SG-JJ and SG-JI surgery, a jejuno-jejunal or jejuno-ileal side-by-side anastomosis is made based on SG surgery.
The study is very interesting. Regarding new guidelines, there is a need for the surgical methods dedicated for diabetic patients. The authors explored the effect of SG with jejuno-jejunal or jejuno-ileal loop on glycolipid metabolism in diabetic rats.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Janik MR, Matsuda T, Rege RV, Yamaoka Y S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Ma S
| 1. | Rubino F, Nathan DM, Eckel RH, Schauer PR, Alberti KG, Zimmet PZ, Del Prato S, Ji L, Sadikot SM, Herman WH. Metabolic Surgery in the Treatment Algorithm for Type 2 Diabetes: A Joint Statement by International Diabetes Organizations. Diabetes Care. 2016;39:861-877. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 603] [Cited by in F6Publishing: 539] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 2. | Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, Scopinaro N. Bariatric Surgery Worldwide 2013. Obes Surg. 2015;25:1822-1832. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1090] [Cited by in F6Publishing: 1053] [Article Influence: 117.0] [Reference Citation Analysis (1)] |
| 3. | Gagner M. Effect of sleeve gastrectomy on type 2 diabetes as an alternative to Roux-en-Y gastric bypass: a better long-term strategy. Surg Obes Relat Dis. 2015;11:1280-1281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Cutolo PP, Nosso G, Vitolo G, Brancato V, Capaldo B, Angrisani L. Clinical efficacy of laparoscopic sleeve gastrectomy vs laparoscopic gastric bypass in obese type 2 diabetic patients: a retrospective comparison. Obes Surg. 2012;22:1535-1539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Cho JM, Kim HJ, Lo Menzo E, Park S, Szomstein S, Rosenthal RJ. Effect of sleeve gastrectomy on type 2 diabetes as an alternative treatment modality to Roux-en-Y gastric bypass: systemic review and meta-analysis. Surg Obes Relat Dis. 2015;11:1273-1280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 6. | Jiménez A, Casamitjana R, Flores L, Viaplana J, Corcelles R, Lacy A, Vidal J. Long-term effects of sleeve gastrectomy and Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus in morbidly obese subjects. Ann Surg. 2012;256:1023-1029. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 179] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 7. | Lee WJ, Lee KT, Kasama K, Seiki Y, Ser KH, Chun SC, Chen JC, Lee YC. Laparoscopic single-anastomosis duodenal-jejunal bypass with sleeve gastrectomy (SADJB-SG): short-term result and comparison with gastric bypass. Obes Surg. 2014;24:109-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Mui WL, Lee DW, Lam KK. Laparoscopic sleeve gastrectomy with loop bipartition: A novel metabolic operation in treating obese type II diabetes mellitus. Int J Surg Case Rep. 2014;5:56-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Hassn A, Luhmann A, Rahmani S, Morris-Stiff G. Medium-Term Results of Combined Laparoscopic Sleeve Gastrectomy and Modified Jejuno-Ileal Bypass in Bariatric Surgery. Obes Surg. 2016; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Sánchez-Pernaute A, Herrera MA, Pérez-Aguirre ME, Talavera P, Cabrerizo L, Matía P, Díez-Valladares L, Barabash A, Martín-Antona E, García-Botella A. Single anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S). One to three-year follow-up. Obes Surg. 2010;20:1720-1726. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 11. | Chikunguwo SM, Wolfe LG, Dodson P, Meador JG, Baugh N, Clore JN, Kellum JM, Maher JW. Analysis of factors associated with durable remission of diabetes after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2010;6:254-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 166] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 12. | Kashyap SR, Schauer P. Clinical considerations for the management of residual diabetes following bariatric surgery. Diabetes Obes Metab. 2012;14:773-779. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840-846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3146] [Cited by in F6Publishing: 3347] [Article Influence: 196.9] [Reference Citation Analysis (0)] |
| 14. | Liu SZ, Sun D, Zhang GY, Wang L, Liu T, Sun Y, Li MX, Hu SY. A high-fat diet reverses improvement in glucose tolerance induced by duodenal-jejunal bypass in type 2 diabetic rats. Chin Med J (Engl). 2012;125:912-919. [PubMed] [Cited in This Article: ] |
| 15. | Samuel VT, Beddow SA, Iwasaki T, Zhang XM, Chu X, Still CD, Gerhard GS, Shulman GI. Fasting hyperglycemia is not associated with increased expression of PEPCK or G6Pc in patients with Type 2 Diabetes. Proc Natl Acad Sci USA. 2009;106:12121-12126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 16. | Duca FA, Côté CD, Rasmussen BA, Zadeh-Tahmasebi M, Rutter GA, Filippi BM, Lam TK. Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats. Nat Med. 2015;21:506-511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 254] [Cited by in F6Publishing: 287] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 17. | Sun D, Liu S, Zhang G, Chen W, Yan Z, Hu S. Type 2 diabetes control in a nonobese rat model using sleeve gastrectomy with duodenal-jejunal bypass (SGDJB). Obes Surg. 2012;22:1865-1873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Bruinsma BG, Uygun K, Yarmush ML, Saeidi N. Surgical models of Roux-en-Y gastric bypass surgery and sleeve gastrectomy in rats and mice. Nat Protoc. 2015;10:495-507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 19. | Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412-419. [PubMed] [Cited in This Article: ] |
| 20. | World Health Organization. Global report on diabetes. Geneva: World Health Organization 2016; . [Cited in This Article: ] |
| 21. | Benaiges D, Más-Lorenzo A, Goday A, Ramon JM, Chillarón JJ, Pedro-Botet J, Flores-Le Roux JA. Laparoscopic sleeve gastrectomy: More than a restrictive bariatric surgery procedure? World J Gastroenterol. 2015;21:11804-11814. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 140] [Cited by in F6Publishing: 129] [Article Influence: 14.3] [Reference Citation Analysis (2)] |
| 22. | Weiner RA, Theodoridou S, Weiner S. Failure of laparoscopic sleeve gastrectomy--further procedure? Obes Facts. 2011;4 Suppl 1:42-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Tvarijonaviciute A, Ceron JJ, Holden SL, Morris PJ, Biourge V, German AJ. Effects of weight loss in obese cats on biochemical analytes related to inflammation and glucose homeostasis. Domest Anim Endocrinol. 2012;42:129-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Rubino F, Forgione A, Cummings DE, Vix M, Gnuli D, Mingrone G, Castagneto M, Marescaux J. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244:741-749. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 659] [Cited by in F6Publishing: 608] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 25. | Dirksen C, Jørgensen NB, Bojsen-Møller KN, Jacobsen SH, Hansen DL, Worm D, Holst JJ, Madsbad S. Mechanisms of improved glycaemic control after Roux-en-Y gastric bypass. Diabetologia. 2012;55:1890-1901. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 165] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 26. | Liu S, Zhang G, Wang L, Sun D, Chen W, Yan Z, Sun Y, Hu S. The entire small intestine mediates the changes in glucose homeostasis after intestinal surgery in Goto-Kakizaki rats. Ann Surg. 2012;256:1049-1058. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Drucker DJ. The role of gut hormones in glucose homeostasis. J Clin Invest. 2007;117:24-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 439] [Cited by in F6Publishing: 424] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 28. | Syed YY, McCormack PL. Exenatide Extended-Release: An Updated Review of Its Use in Type 2 Diabetes Mellitus. Drugs. 2015;75:1141-1152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Vigneshwaran B, Wahal A, Aggarwal S, Priyadarshini P, Bhattacharjee H, Khadgawat R, Yadav R. Impact of Sleeve Gastrectomy on Type 2 Diabetes Mellitus, Gastric Emptying Time, Glucagon-Like Peptide 1 (GLP-1), Ghrelin and Leptin in Non-morbidly Obese Subjects with BMI 30-35.0 kg/m(2): a Prospective Study. Obes Surg. 2016; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 30. | Nosso G, Griffo E, Cotugno M, Saldalamacchia G, Lupoli R, Pacini G, Riccardi G, Angrisani L, Capaldo B. Comparative Effects of Roux-en-Y Gastric Bypass and Sleeve Gastrectomy on Glucose Homeostasis and Incretin Hormones in Obese Type 2 Diabetic Patients: A One-Year Prospective Study. Horm Metab Res. 2016;48:312-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 31. | Chambers AP, Kirchner H, Wilson-Perez HE, Willency JA, Hale JE, Gaylinn BD, Thorner MO, Pfluger PT, Gutierrez JA, Tschöp MH. The effects of vertical sleeve gastrectomy in rodents are ghrelin independent. Gastroenterology. 2013;144:50-52.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 32. | Sun D, Liu S, Zhang G, Colonne P, Hu C, Han H, Li M, Hu S. Sub-sleeve gastrectomy achieves good diabetes control without weight loss in a non-obese diabetic rat model. Surg Endosc. 2014;28:1010-1018. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |