Published online Jul 28, 2015. doi: 10.3748/wjg.v21.i28.8660
Peer-review started: September 11, 2014
First decision: October 14, 2014
Revised: November 21, 2014
Accepted: January 8, 2015
Article in press: January 8, 2015
Published online: July 28, 2015
Processing time: 322 Days and 14.2 Hours
AIM: To examined the efficacy and safety of treatment with boceprevir, PEGylated-interferon and ribavirin (PR) in hepatitis C virus genotype 1 (HCVGT1) PR treatment-failures in Asia.
METHODS: The Boceprevir Named-Patient Program provided boceprevir to HCVGT1 PR treatment-failures. Participating physicians were invited to contribute data from their patients: baseline characteristics, on-treatment responses, sustained virological response at week 12 (SVR12), and safety were collected and analysed. Multivariate analysis was performed to determine predictors of response.
RESULTS: 150 patients were enrolled from Australia, Malaysia, Singapore and Thailand (Asians = 86, Caucasians = 63). Overall SVR12 was 61% (Asians = 59.3%, Caucasians = 63.5%). SVR12 was higher in relapsers (78%) compared with non-responders (34%). On-treatment responses predicted SVR, with undetectable HCVRNA at week 4, 8 and 12 leading to SVR12s of 100%, 87%, and 82% respectively, and detectable HCVRNA at week 4, 8 and 12, leading to SVR12s of 58%, 22% and 6% respectively. Asian patients were similar to Caucasian patients with regards to on-treatment responses. Patients with cirrhosis (n = 69) also behaved in the same manner with regards to on-treatment responses. Those with the IL28B CC genotype (80%) had higher SVRs than those with the CT/TT (56%) genotype (P = 0.010). Multivariate analysis showed that TW8 and TW12 responses were independent predictors of SVR. Serious adverse events occurred in 18.6%: sepsis (2%), decompensation (2.7%) and blood transfusion (14%). Discontinuations occurred in 30.7%, with 18.6% fulfilling stopping rules.
CONCLUSION: Boceprevir can be used successfully in PR treatment failures with a SVR12 > 80% if they have good on-treatment responses; however, discontinuations occurred in 30% because of virological failure or adverse events.
Core tip: This is the first report of boceprevir, PEGylated interferon and ribavirin in PEGylated interferon treatment failures in the Asia-Pacific and showed an overall sustained virological response at week 12 (SVR12) of 61%. Asians were compared with Caucasians and had similar SVR12 results: Asians 59.3% and Caucasians 63.5%. On-treatment responses at week 4, 8 and 12 predicted SVR12. Multivariate analysis showed that treatment week 8 and 12 were independent predictors of SVR12. Serious adverse events occurred in 18.6%, comprising sepsis (2%), decompensation (2.7%) and blood transfusion (14%). Discontinuations occurred in 30.7%.
- Citation: Sukeepaisarnjaroen W, Pham T, Tanwandee T, Nazareth S, Galhenage S, Mollison L, Totten L, Wigg A, Altus R, Colman A, Morales B, Mason S, Jones T, Leembruggen N, Fragomelli V, Sendall C, Guan R, Sutedja D, Tan SS, Dan YY, Lee YM, Luman W, Teo EK, Than YM, Piratvisuth T, Lim SG. Boceprevir early-access for advanced-fibrosis/cirrhosis in Asia-pacific hepatitis C virus genotype 1 non-responders/relapsers. World J Gastroenterol 2015; 21(28): 8660-8669
- URL: https://www.wjgnet.com/1007-9327/full/v21/i28/8660.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i28.8660
Over 170 million people globally may be infected with hepatitis C virus (HCV)[1], many of whom live in Asia[2]. In many Asian countries, the standard-of-care still remains a combination of PEGylated-interferon and ribavirin (PR), which achieves a sustained virological response (SVR) in approximately 50% of Caucasian HCV genotype 1-infected subjects[3]. However, in Asia, the largest randomized control study showed 74% SVR with 48 wk of PR because of the prevalence of the IL28B good-responder (CC) genotype in 80% of their patients[4], the most common genotype in Asians[5]. However, many Asians have failed therapy; therefore, a substantial unmet need remains for more effective anti-HCV treatments in patients who may otherwise progress to liver cirrhosis and hepatocellular carcinoma.
Boceprevir is a protease inhibitor that is active against genotype 1 HCV. In the SPRINT-2 study[6], treatment-naïve genotype 1 patients randomized to the boceprevir and PR (BPR) response guided therapy arm achieved 67% SVR, while BPR fixed duration achieved 68% SVR and PR achieved 40%. In PR treatment failures (RESPOND-2 study)[7], BPR response guided therapy patients achieved 59% SVR, while BPR fixed duration therapy achieved 66%, and PR achieved 21% SVR. Only 36 Asian patients were included out of a total of 2095 patients[8] in the pivotal boceprevir phase 3 studies, and only 24 received BPR. Asians achieved 67% SVR compared with 34% in non-Asians with PR, and 79.2% achieved SVR in Asians and 62.3% in non-Asians with BPR. Consequently, there are very limited data on boceprevir therapy in Asians, and no information on the efficacy of boceprevir in PR treatment failures.
With the approval of boceprevir, Merck Sharpe and Dohme instituted a global early access program in those with prior treatment failures (Boceprevir Named Patient Program, BNPP), of which the CUPIC study[9] was one of the first reports. The BNPP provided an opportunity to characterize the response of Asians to boceprevir, in prior treatment failures.
In August 2011, Merck Sharpe and Dohme provided a global early access program for HCV genotype 1 patients who had previously failed therapy with PR. The inclusion criteria were: (1) the patient must be 18 years or older; (2) the patient must have HCV genotype 1 infection only (Documentation required) Note: mixed genotypes including HCV 1 were ineligible; (3) the patient had previously failed treatment with PEGinterferon alpha-2b/ribavirin or PEGinterferon alpha-2a/ribavirin or interferon-minimum of 12 wk of treatment without dose reduction or interruption; and (4) the patient must have compensated liver disease with documented bridging fibrosis or cirrhosis consistent with chronic hepatitis C and no other etiology (Documentation required-liver biopsy or non-invasive markers).
Note: patients with cirrhosis should have a liver imaging study (e.g., ultrasound, CT scan or MRI) within the preceding 6 months showing no evidence of hepatocellular carcinoma. (1) The patient meets all of the requirements and none of the contra-indications for treatment with PEGinterferon alpha-2b/ribavirin or PEGinterferon alpha-2a/ribavirin as defined in the labels for the PEGinterferon and ribavirin to be used in combination with boceprevir; and (2) the patient is able and willing to provide signed informed consent (prepared by and administered by the physician) as required by local country requirements.
The exclusion criteria were: (1) the patient has received boceprevir, narlaprevir, telaprevir, or any other HCV protease inhibitor treatment; (2) the patient has evidence of decompensated liver disease including, but not limited to, a history or presence of clinical ascites, bleeding varices, or hepatic encephalopathy; and (3) the patient meets any of the following exclusionary hematological and biochemical criteria (documentation required): (1) hemoglobin < 12 g/dL for females and < 13 g/dL for males; (2) Neutrophils < 1500 mm3 (blacks < 1200 mm3); and (3) platelets < 100000 mm3.
The patient has an organ transplant other than cornea or hair. The patient is co-infected with human immunodeficiency virus (HIV) or hepatitis B virus (HBsAg positive). The patient requires, or is anticipated to require, any of the following prohibited medications: midazolam, pimozide, amiodarone, flecainide, propafenone, quinidine, and ergot derivatives. The patient with a clinical diagnosis or evidence of substance abuse involving alcohol, intravenous drugs, inhalational (not including marijuana) psychotropics, narcotics, cocaine prescription or over-the-counter drugs. Patients receiving opiate substitution therapy monitored by a physician may be enrolled at the investigator's discretion. A patient previously showing clinically significant hypersensitivity or other contraindication to any component of the boceprevir formulation. This drug contains lactose. Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency, or glucose-galactose malabsorption should not take this medicine. Serious illness, including malignancy, active coronary artery disease or cardiac dysfunction within 24 wk prior to study entry, that in the opinion of the site investigator may preclude completion of the treatment regimen. Hemoglobinopathy (e.g., thalassemia), coagulopathy or any other cause of, or tendency to, hemolysis or bleeding.
Treatment for patients without cirrhosis: Treatment duration was 48 wk: 4 wk of PEGinterferon alpha + ribavirin, then 32 wk of triple therapy with PEGinterferon alpha + ribavirin + boceprevir, then 12 wk with PEGinterferon alpha + ribavirin.
Treatment for patients with cirrhosis and patients who were null responders: Treatment duration was 48 wk: 4 wk of therapy with PEGinterferon alpha + ribavirin, then 44 wk of triple therapy with PEGinterferon alpha + ribavirin + boceprevir. Null responders were defined as those patients who previously failed PEG-IFN and ribavirin treatment, and did not achieve > 2-log10 decline in HCV viral RNA at TW12 or who had < 0.5-log10 HCV-RNA decline in viral load at TW4 with PEGinterferon alpha and ribavirin alone.
Physician: Name, city, country.
Patient: (1) Demographics-age, gender, ethnicity, weight, height; (2) Type of prior treatment failure; (3) baseline data: subgenotype, HCV RNA titer, fibrosis stage (method used for evaluation), LFT, FBC, PT/INR; (4) on-treatment response: HCV RNA at week 4, 8, 12, 24 and 48 after starting PR; (5) dose adjustments, termination or interruption: boceprevir, pIFN or ribavirin; (6) post-treatment response: SVR12; (7) use of supportive therapy: erythropoietin, G-CSF or eltrombopag, blood transfusions; and (8) serious adverse events and adverse events.
The study was approved by each institution’s Institutional Review Board and overall approval was provided by the National Healthcare Group Domain Specific Institutional Review Board (DSRB) under waiver of consent based on anonymization of patient identifiers (DSRB number 2012/01032). Consequently, no informed consent was obtained from patients for data sharing. The presented data were anonymized and the risk of identification is low. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Asia Pacific physicians who enrolled patients into the BNPP were invited to participate in the study to evaluate the results of their patients in the program. Physicians who agreed to participate had to provide anonymized information on their patients using a data collection spreadsheet. Patients who did not have IL28B polymorphisms tested were invited to provide a blood sample for this test. A separate consent form was signed by patients for this test.
Serum HCV RNA levels were measured with the use of the Cobas TaqMan 2.0 assay (Roche Diagnostics GmbH, Mannheim, Germany) in almost all cases, which has lower limits of detection of 9.3 IU per milliliter.
HCV RNA was measured at regular intervals after starting PR therapy, week 4 (also known as Rapid Virological Response or RVR), week 8 (4 wk after starting boceprevir), and week 12 (week 8 after starting boceprevir), week 24 (week 20 of boceprevir) and week 48 (week 44 of boceprevir).
The primary endpoint of the study was SVR12. Secondary endpoints included on treatment responses, safety (including serious adverse events (SAEs), major adverse events (AEs) and discontinuations), as well as predictors of response.
The stopping rule was based on the BNPP critieria, HCV RNA > 100 IU/mL at week 12 or HCV RNA detectable at week 24.
Statistical analysis was performed using SPSS version 19. The data were analysed using intention-to-treat analysis. Descriptive statistics were used for baseline characteristics. Differences between groups, time points and ethnicity (Asian vs Caucasian) were compared using Pearson’s χ2 test. Multivariate Cox regression was performed to compare HCV RNA undetectable results between relapser and non-responder groups, and SVR12, after adjusting for baseline differences. Multiple logistic regression was performed for predictors of SVR12. The statistical methods of the study were reviewed by Dr. Y.M. Than, a biostatistician from the Dept. of Medicine, National University Health System, Singapore.
50 patients were evaluated (Asians = 86, Caucasians = 63, one patient-missing data). Compared with Asians, Caucasians had more cirrhosis, 61.3% vs 35.3% (P = 0.004), higher BMI, 28.4 vs 26.2 (P = 0.012), lower serum albumin levels, 39.6 vs 43.9 (P < 0.001), higher ALT levels, 130 U/L vs 80 U/L (P = 0.004), lower HCV RNA levels, 3.2 log × 106 IU/mL vs 5.3 log × 106 IU/mL (P = 0.037) and lower platelet counts, 159 × 109/L vs 190 × 109/L (P = 0.002) (Table 1).
| SVR12(+) (n = 91) | SVR12(-) (n = 55) | P value | Total1 (n = 146) | |
| Male gender | 69 (75.8) | 42 (76.4) | 0.941 | 114 (76.0) |
| Asians | 51 (56.0) | 31 (56.4) | 0.970 | 86 (57.3) |
| Caucasians | 40 (43.9) | 23 (41.8) | 0.800 | 63 (42.0) |
| Relapsers | 62 (68.3) | 17 (30.9) | < 0.001 | 80 (53.3) |
| Non-responders | 18 (19.8) | 32 (58.2) | < 0.001 | 53 (35.3) |
| Cirrhosis | 44 (48.4) | 23 (41.8) | 0.463 | 69 (46.0) |
| IL28B-CC genotype | 37 (40.7) | 9 (14.5) | 0.001 | 46 (30.7) |
| IL28B-Non-CC genotype | 27 (29.7) | 21 (38.2) | 0.289 | 48 (32.0) |
| Age | 52.4 (50.7-54.2) | 52.7 (48.6-56.7) | 0.636 | 52.7 (51.1-54.4) |
| BMI | 26.4 (25.0-27.7) | 24.6 (22.9-26.3) | 0.735 | 26.1 (24.9-27.4) |
| Albumin (g/L) | 44.4 (42.9-45.7) | 44.8 (42.9-45.8) | 0.142 | 44.2 (42.9-45.5) |
| Total Bilirubin (μmol/dL) | 14.2 (10.7-17.7) | 10.8 (8.4-13.1) | 0.704 | 13.3 (10.9-15.7) |
| ALT (U/L) | 86.7 (65.2-108.1) | 72.4 (47.0-97.9) | 0.061 | 81 (65.4-96.7) |
| INR | 1.03 (1.01-1.06) | 1.03 (0.99-1.07) | 0.752 | 1.04 (1.02-1.06) |
| HCVRNA(× 106 IU/mL) | 6.2 (3.9-8.4) | 4.5 (1.9-7.1) | 0.425 | 5.5 (3.8-7.1) |
| Hb (g/L) | 14.7 (14.3-15.1) | 14.4 (13.5-15.3) | 0.743 | 14.6 (14.2-14.9) |
| WBC (× 109/L) | 7.04 (6.2-7.9) | 6.9 (6.02-7.9) | 0.839 | 6.9 (6.4-7.6) |
| Neutrophil (× 109/L) | 3.8 (3.2-4.4) | 3.4 (2.9-3.9) | 0.922 | 3.7 (3.2-4.1) |
| Platelets (× 109/L) | 171.2 (149.5-192.9) | 195.3 (163.8-226.7) | 0.425 | 177.2 (159.9-194.6) |
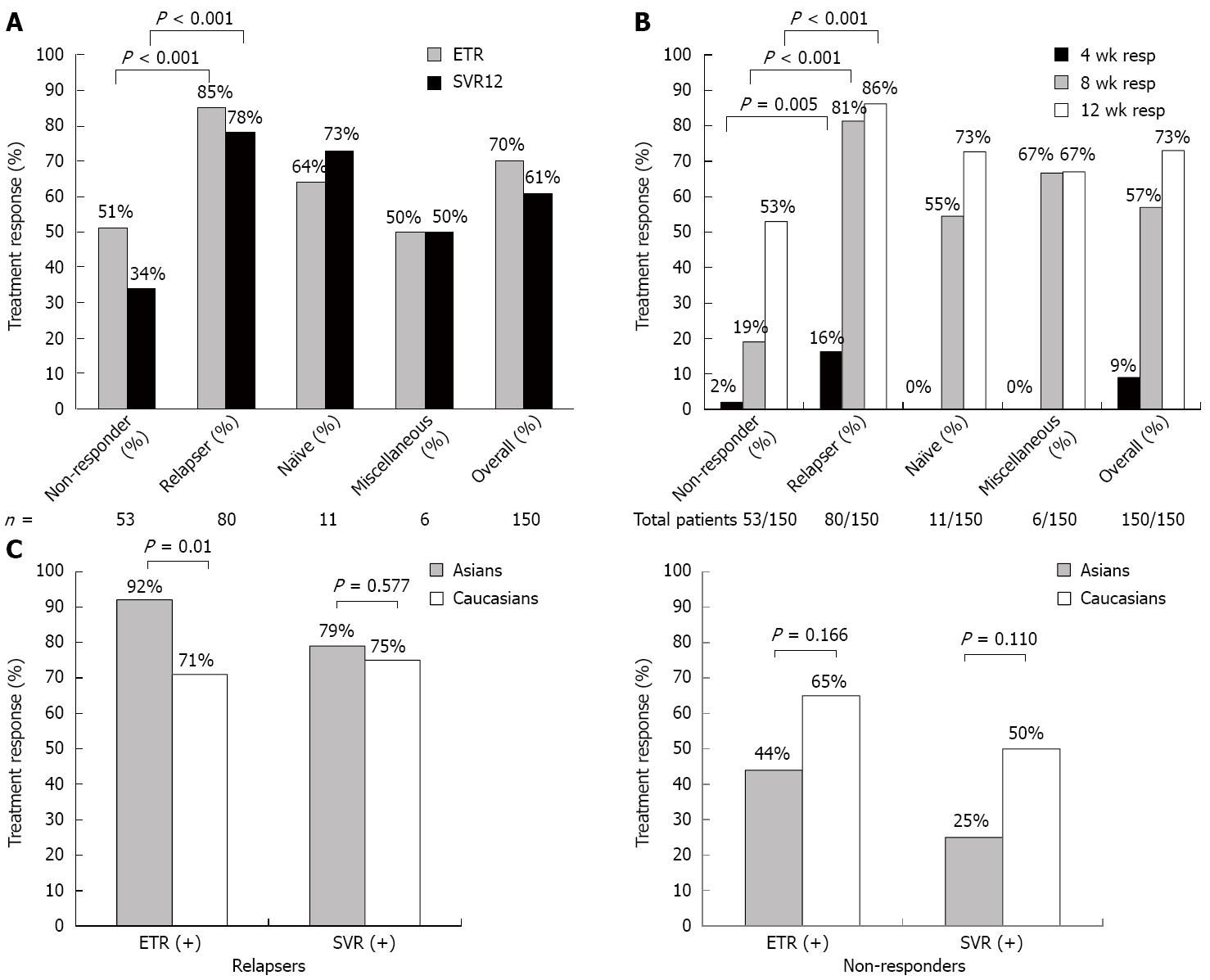
Overall results: End of treatment response (ETR) and sustained virological response. Out of 150 patients, 80 were relapsers, 53 were non-responders, 11 were treatment naïve and six were not classifiable (because of insufficient information during their initial therapy). The overall ETR rate was 70% and overall SVR rate was 61% (Figure 1A). In non-responders, the ETR was 51%, while the SVR12 was 34%, while in relapsers they were 85% and 78% (Figure 1A). A few patients were inadvertently enrolled who were treatment naïve (n = 11) and a few who were unclassifiable because the nature of their previous treatment failure was not well documented (n = 6). In these patients, the SVR12 rates were 73% and 50% respectively (Figure 1A).
On-treatment responses: The proportion of patients with undetectable HCV RNA at week 4, 8 and 12 was 9%, 57% and 73%, respectively (Figure 1B). Prior relapsers had the highest level of undetectable week 4 HCV RNA (16%) compared with non-responders (2%; P = 0.005), while almost no patients in the other groups achieved RVR, indicating that the study truly was enriched for treatment poor responders who are unlikely to have achieve RVR. Moreover, the 11 patients who were treatment naïve and six patients who had insufficient information on prior treatment failure, behaved as treatment failures, based on their week 4 response; hence, they were unlikely to affect treatment results. Treatment week 8 responses were similar to treatment week 4 responses, where 81% of relapsers had undetectable HCV RNA compared with 19% of non-responders (P < 0.001). By week 12, 86% of relapsers had undetectable HCV RNA compared with 53% of non-responders (P < 0.001) (Figure 1B).
ETR and SVR12 by prior treatment failure in Asians vs Caucasians: When comparing Asians with Caucasians in relapsers, the ETR was higher in Asians (92%) compared with Caucasians (71%) (P = 0.01); however, SVR12 was similar for both (79% vs 75%) (P = 0.577) (Figure 1C). For non-responders, the ETR and SVR12 was numerically higher in Caucasians (65% and 50%) compared with Asians (44% and 25%) (P = 0.166 and P =0.110 respectively) (Figure 1C), but both were not statistically significant.
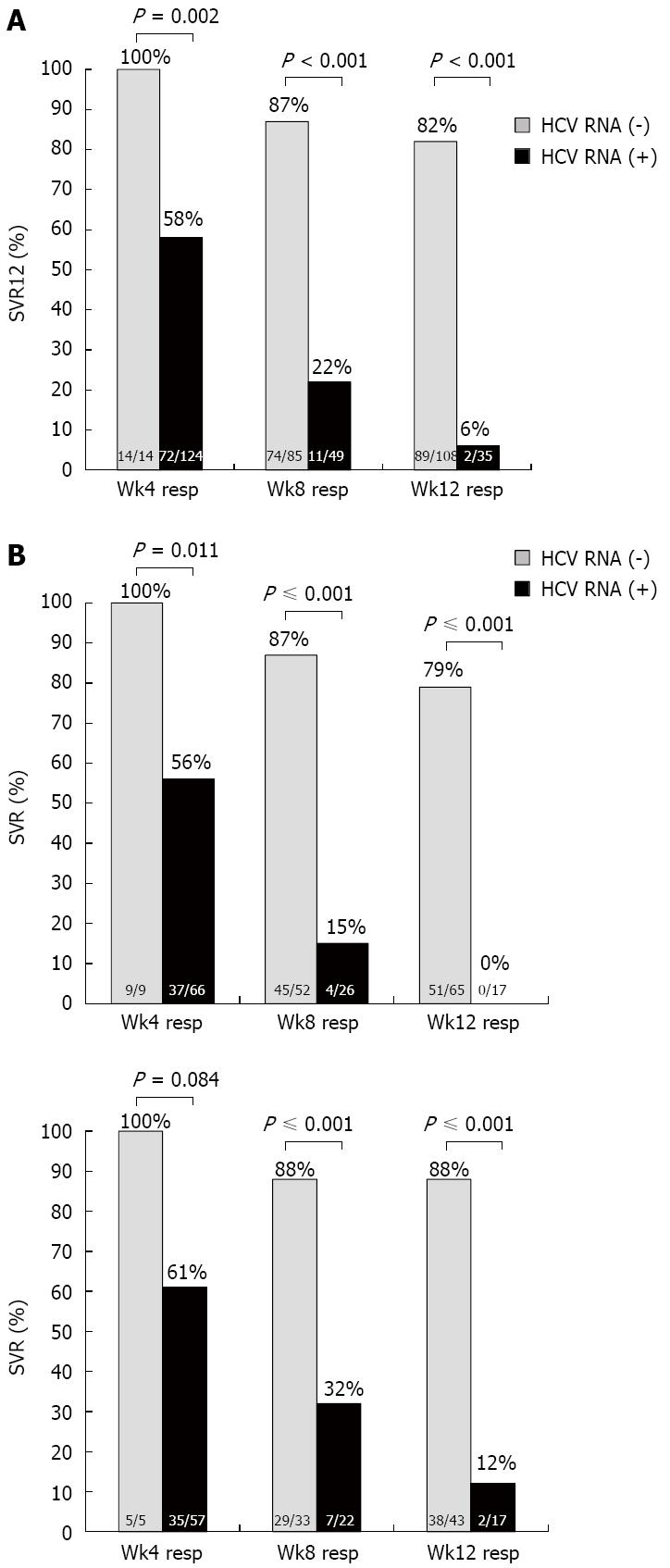
SVR12 by on-treatment responses: Those patients who were HCV RNA undetectable at treatment week 4, 8 and 12 achieved 100%, 87% and 82% SVR12 respectively, which was significantly higher than those with detectable HCV RNA at treatment week 4, 8 and 12, who achieved 58%, 22% and 6% SVR12 respectively (P = 0.002, P < 0.001, P < 0.001 respectively) (Figure 2A).
When comparing Asians and Caucasians, similar findings with regards to SVR12 rates were found in those who were HCV RNA undetectable at week 4, 8 and 12, regardless of ethnicity (Figure 2B) with SVR12 rates in Asians of 100%, 87% and 79% respectively, while Caucasians had SVR12 rates of 100%, 88% and 88% respectively. In those with detectable HCV RNA at week 4, 8 and 12, the SVR12 rates were considerably lower compared with those with undetectable HCV RNA. Consequently there was no statistical difference in SVR between Asians and Caucasians based on detectable or undetectable HCV RNA at week 4, 8 or 12.
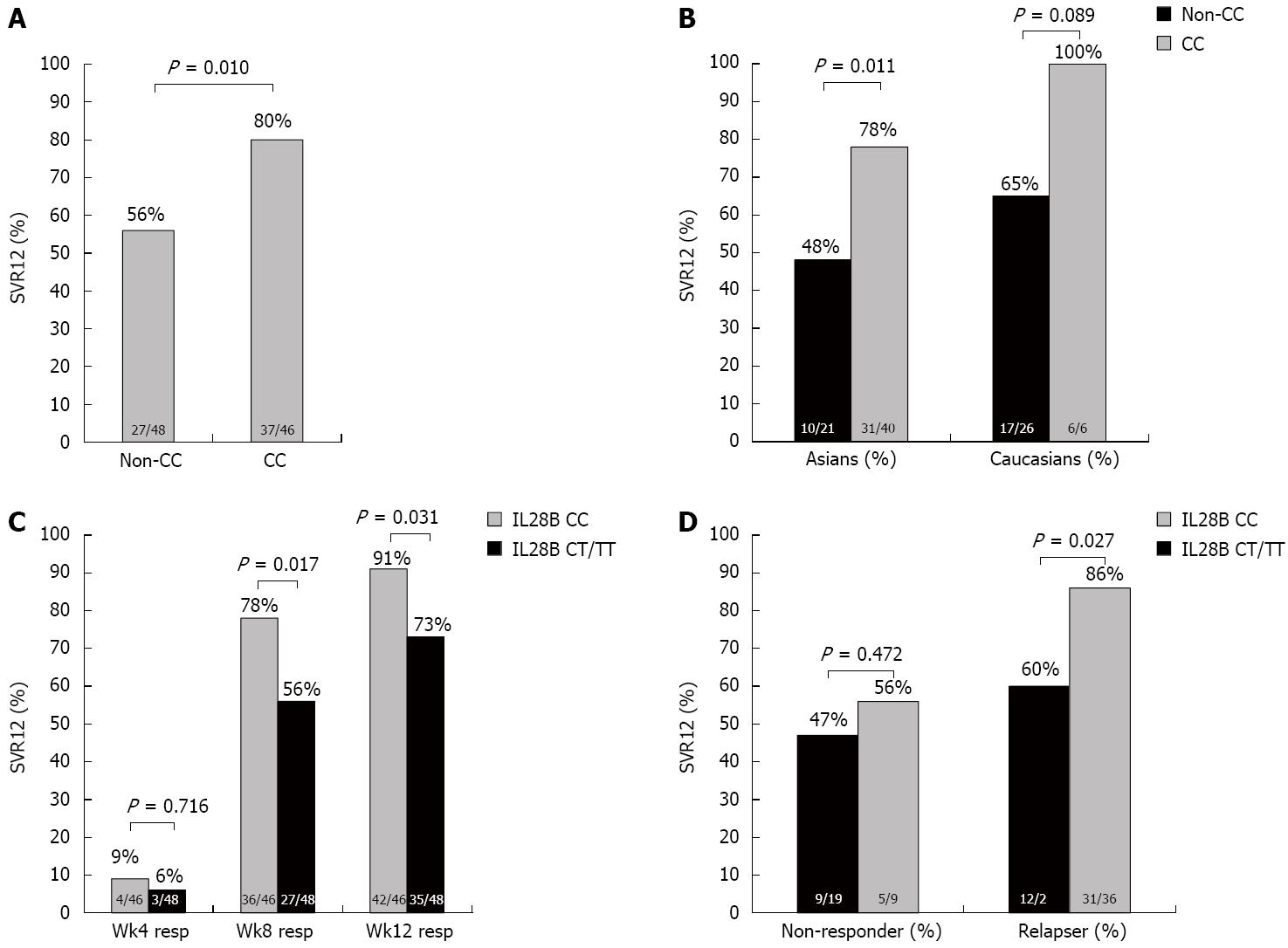
IL28B results: IL28 genotyping was performed in only 94 of 150 patients (62.6%). The distribution of IL28B CC was 48.9% and CT/TT genotype was 51.1%. In those with IL28B CC genotype, 80% achieved SVR12, while in those with IL28B CT/TT genotypes, the SVR12 was 56% (P = 0.010) (Figure 3A). SVR was higher in both Asians and Caucasians with IL28B CC genotype (78% and 100%) compared to non-CC genotypes (48% and 65% respectively) (P = 0.011 and P = 0.089 respectively) (Figure 3B). In those who had undetectable HCV RNA at week 4, 8 and 12, the proportion of IL28B CC vs CT/TT genotype was 9% vs 6%, 78% vs 56%, and 91% vs 73%, respectively (P = 0.716, P = 0.017, P = 0.031, respectively) (Figure 3C). In non-responders, the proportion with IL28B CC genotypes were similar in patients who achieved SVR12; however, in relapsers there was a higher proportion of patients with IL28B CC (86%) compared with non-CC genotypes (60%) (P = 0.027) (Figure 3D). Consequently, the IL28B CC genotype seemed to be an important factor in achieving SVR only in relapsers (Figure 3D).
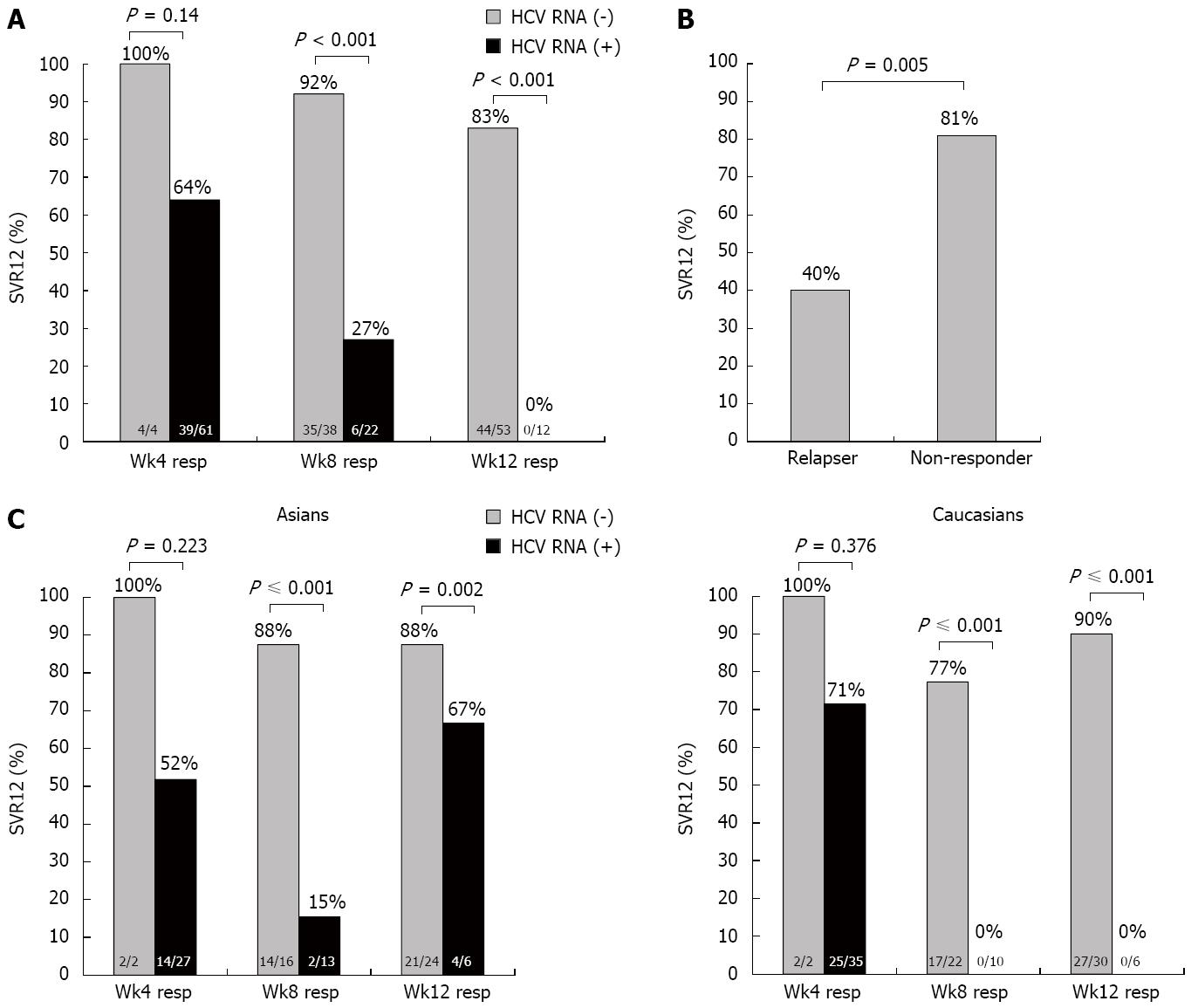
SVR12 in cirrhosis: A total of 69 patients had liver cirrhosis. Those that had undetectable HCV RNA at week 4, 8 and 12 had SVR12 rates of 100%, 92% and 83%, respectively, but if they had detectable HCV RNA at week 4, 8 and 12, the SVR12 rate was 64%, 27% and 0%, respectively (Figure 4A) (P = 0.14, P < 0.001, P < 0.001 respectively). There was a significant difference in SVR12 when comparing cirrhotic relapsers (81%) to non-responders (40%) (Figure 4B, P = 0.005). When comparing Asians with Caucasians, the results were not significantly different with regards to SVR12 rates when HCV RNA was undetectable at week 4, 8 and 12: 100% vs 100% at week 4, 86.5% vs 87.8% at week 8, and 78.4% vs 88.4% at week 12 (P = not significant for all). In those who had detectable HCV RNA at week 4, 8 and 12, the SVR was lower both in Asians and Caucasians, with no significant differences between them (Figure 4C).
Baseline and on-treatment variables were compared in patients who achieved SVR12 vs those who did not (Table 1). Univariate analysis showed that prior history of relapse, undetectable HCV RNA at week 4, 8 and 12, and IL28B CC genotype were associated with SVR12. On multivariate analysis, week 8 (RR = 0.205, 95%CI: 0.052-0.810) and 12 response (RR = 0.036, 95%CI: 0.006-0.200) were significant predictors of SVR12. Notably, IL28B CC genotype was not a significant predictor of SVR in this population of prior treatment failures.
Serious adverse events: Twenty-eight SAEs were reported (18.6%). These comprised sepsis (3/150, 2%), liver decompensation (4/150, 2.6%) and blood transfusions (21/150, 14%). Asians and Caucasians showed similar rates of development of SAEs, except for sepsis, which was seen more frequently in Caucasians (Table 2). Univariate analysis showed that age, male gender, white blood count and hemoglobin were significantly associated with SAEs. Multivariate analysis showed that only older age was a significant protective factor for SAEs (RR = 0.895, 95%CI: 0.809-0.991).
| Asian n = 86 | Caucasian n = 63 | P-value | Total1n = 150 | |
| SAEs | 15 (17.4) | 13 (20.6) | 0.613 | 28 (18.6) |
| Death | 0 (0) | 0 (0) | NA | 0 (0) |
| Sepsis | 0 (0) | 3 (4.8) | 0.038 | 3 (2) |
| Decompensation | 1 (1.2) | 3 (4.8) | 0.168 | 4 (2.6) |
| Blood transfusions | 16 (18.6) | 5 (7.9) | 0.076 | 21 (14) |
| AEs | ||||
| Anemia (< 10 g/dL) | 40 (46.5) | 38 (60.3) | 0.088 | 78 (52) |
| Anemia (< 8 g/dL) | 14 (16.3) | 11 (17.5) | 0.839 | 25 (16.7) |
| Neutropenia (< 500 cells/dL) | 1 (1.2) | 7 (11.1) | 0.008 | 8 (5.3) |
| Neutropenia (< 100 cells/dL) | 12 (13.9) | 10 (15.9) | 0.736 | 22 (14.7) |
| Thrombocytopenia (< 30000/dL) | 2 (2.3) | 5 (7.9) | 0.108 | 7 (4.7) |
| Skin Rash | 8 (9.3) | 19 (30.2) | 0.001 | 27 (18) |
| Weight loss | 25 (29.1) | 30 (47.6) | 0.003 | 55 (36.7) |
| Dysgusia | 21 (24.4) | 48 (76.2) | < 0.0001 | 69 (46) |
| Diarrhea | 1 (1.2) | 5 (7.9) | 0.037 | 6 (4) |
| Nausea | 9 (10.5) | 12 (19.0) | 0.134 | 21 (14) |
| Discontinuations | 19 (22) | 26 (41) | 0.023 | 46 (30.6) |
| Due to AEs | 4 (4.6) | 9 (14.2) | 0.052 | 13 (8.6) |
| Fulfilled stopping rules | 15 (17.4) | 12 (19) | 0.175 | 28 (18.6) |
| Others | 0 (0) | 5 (7.9) | 0.033 | 5 (3.3) |
Adverse events: Table 2 lists the most common AEs reported. By far the most common was anemia (Hb < 10 g/dL), which occurred in 52% of patients. More severe anemia (Hb < 8 g/dL) occurred in 16.7% of patients. The next most common AEs were dysgusia (46%), weight loss (36.7%) and skin rash (18%) (Table 2).
Discontinuations: Forty-six patients discontinued therapy (30.7%). Of these 28 (18.6%) fulfilled stopping rules, which was similar in Asians (17.4%) compared with Caucasians (19%). Thirteen (28.3%) developed AEs leading to therapy discontinuation, more in Caucasians (14.2%) than in Asians (4.6%), and five (10.9%) stopped for a variety of reasons, including urgent surgery, loss to follow up and withdrawal of consent (Table 2).
Differences between Asians and Caucasians: Significant differences were found in SAE, AE and discontinuations between Asians and Caucasians (Table 2). Caucasians had significantly higher levels of sepsis, neutropenia, skin rash, weight loss, dysgusia, diarrhea and higher discontinuations than Asians.
This was is the first study of boceprevir treatment in Asian patients who had failed PR therapy, a group of difficult-to-treat patients that are poorly characterized. The study had an important advantage in being able to compare responses in Asian and Caucasian patients. The overall SVR was 61%, and in general, Asians and Caucasians behaved in a similar fashion, with no significant differences between them. When examining on-treatment responses, patients who had undetectable HCV RNA at week 4, 8 or 12 had SVR12 > 80%, for both Asians and Caucasians. For those who had detectable HCV RNA at those time points, SVR12 was significantly lower. In cirrhotic patients, the on-treatment responses were similar to non-cirrhotics. Efficacy was better in those with prior relapse; however, on-treatment responses were the best guide to achieving SVR12, with undetectable HCV RNA at week 4, 8 or 12 providing high SVR12 rates > 80%. Multivariate analysis confirmed that undetectable HCV RNA at week 8 and 12 was the strongest predictor of SVR12. IL28B CC genotype was not a significant predictor of SVR in this population of prior treatment failures, suggesting that once treatment has failed, factors other than IL28B are important. Our SVR12 rates appeared to be similar to the phase 3 RESPOND 2 study[7], but less than the PROVIDE study[10], where SVR12 rates for relapsers were 93% and for partial responders were 67%. Clinical trial patients, however, are different from real life patients who have more co-morbidities and more advanced liver disease. In the final report from the CUPIC study, the overall SVR12 was 44% of 206 boceprevir treated patients, with SVR12 of 54.3% in relapsers, 38.3% in partial responders and 0% in null responders[11]. The study only included cirrhotic patients diagnosed by liver biopsy or non-invasive markers, with a mean MELD score of 8.1; most patients having a MELD ≤ 10 (82.5%)[11]. Our overall SVR12 was 61%, superior to the CUPIC study, but this may reflect the inclusion of fewer cirrhotic patients (46%) compared with CUPIC (100%). In our study, patients who had prior relapses had SVR12 rates of 78%, higher than those in the CUPIC study, while non-responders (null and partial responders) had SVR12 of 34%, which was similar to the partial responders in the CUPIC study.
However it became apparent that in real life scenarios, such as the CUPIC study[9], there were major safety concerns of severe complications in boceprevir treated patients, including an SAE rate of 32.7%, premature discontinuations of 26.3%, mortality of 0.5%, sepsis of 2.4% and hepatic decompensation of 2.9%. Low platelet counts and low serum albumin were predictors of the development of such complications. In our study, the proportion of patients with SAEs was only 18.6%, with no mortality but comparable rates of sepsis (2%) and hepatic decompensation (2.6%). Discontinuation rates were similar (30.6%) to CUPIC, with 18.6% of patients discontinuing because of stopping rules, while only 8.6% discontinued because of AEs. With regards to AEs, they were similar to those reported in CUPIC. Consequently, our study did not sound the same alarm bells as the CUPIC study, but may be a reflection of our patients, who had less advanced liver disease. However, we did find that Caucasian patients had significantly higher rates of AEs and higher discontinuations than Asians.
In conclusion, this study provided the first report of efficacy and safety in Asian HCV genotype 1 patients with prior treatment failure, and shows that boceprevir is a useful and important agent. Patients with advanced fibrosis or cirrhosis may experience liver disease progression and should be considered for therapy immediately. Response guided therapy can guide good responders, while limiting exposure for poor responders, particularly because tolerance seems to be reasonable in our patients. However, the overall SVR of 61% in prior PR treatment failures, and particularly the 34% SVR in non-responders, indicated that the second generation direct-acting antivirals are needed in Asia. In the long term, oral interferon-free regimens represent the ideal therapy, but these have yet to be be approved.
The treatment of hepatitis C virus (HCV) genotype 1 is evolving rapidly and boceprevir was only approved recently for HCV in many Asia Pacific Countries. No data on Asian patients and experiences with boceprevir have been reported, as the clinical studies had only 1% of Asian patients. In addition comparison with responses in Caucasian patients have not been performed, an important issue because the distribution of IL28B genotypes is different. Finally, few reports of rescue therapy in Asian patients who failed PEGylated interferon and ribavirin have been reported.
This study contributes significantly to the evolution of HCV genotype 1 treatment and provides strong evidence for rescue therapy in prior treatment failures to PEGylated interferon and ribavirin.
Boceprevir, PEGylated interferon and ribavirin are suitable rescue therapies for Asians who failed PEGylated interferon and ribavirin, particularly in relapsers. We found that a response-guided strategy could optimize the management of such patients, leading to a high, sustained virological response (SVR), even in those with cirrhosis.
Boceprevir is a suitable rescue therapy for PEGylated interferon and ribavirin treatment failures, particularly in the absence of the new direct acting antiviral therapies.
This study highlights the very important issue of non-responder and relapsers of PEGylated-interferon ribavirin therapy. These groups of patients need special treatment to achieve SVR and BOCEPREVIR was tested as an option for these groups.
P- Reviewer: Abenavoli L, Afzal MS, Lens S, Virlogeux V S- Editor: Qi Y L- Editor: Stewart G E- Editor: Zhang DN
| 1. | Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17:107-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 919] [Cited by in F6Publishing: 929] [Article Influence: 71.5] [Reference Citation Analysis (2)] |
| 2. | Yu ML, Chuang WL. Treatment of chronic hepatitis C in Asia: when East meets West. J Gastroenterol Hepatol. 2009;24:336-345. [PubMed] [Cited in This Article: ] |
| 3. | Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Häussinger D, Diago M, Carosi G, Dhumeaux D. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-982. [PubMed] [Cited in This Article: ] |
| 4. | Liu CH, Liang CC, Liu CJ, Tseng TC, Lin CL, Yang SS, Su TH, Hsu SJ, Lin JW, Chen JH. Interleukin 28B genetic polymorphisms and viral factors help identify HCV genotype-1 patients who benefit from 24-week pegylated interferon plus ribavirin therapy. Antivir Ther. 2012;17:477-484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Thomas DL, Thio CL, Martin MP, Qi Y, Ge D, O’Huigin C, Kidd J, Kidd K, Khakoo SI, Alexander G. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798-801. [PubMed] [Cited in This Article: ] |
| 6. | Poordad F, McCone J, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195-1206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1948] [Cited by in F6Publishing: 1951] [Article Influence: 150.1] [Reference Citation Analysis (0)] |
| 7. | Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, Poordad F, Goodman ZD, Sings HL, Boparai N. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207-1217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1287] [Cited by in F6Publishing: 1288] [Article Influence: 99.1] [Reference Citation Analysis (0)] |
| 8. | Hu KQ, Alvarez Bognar F, Thompson S, Pedicone L, Wahl J, LiM SG. Boceprevir plus Peginterferon Alfa-2b/Ribavirin in the Treatment of Chronic Hepatitis C Virus Genotype-1 Infected Asian Patients in the Sprint-1, SPRINT-2, and RESPOND-2 Trials. Taipei: Asia Pacific Association for Study of Liver Taiwan 2012; . [Cited in This Article: ] |
| 9. | Hézode C, Fontaine H, Dorival C, Larrey D, Zoulim F, Canva V, de Ledinghen V, Poynard T, Samuel D, Bourlière M. Triple therapy in treatment-experienced patients with HCV-cirrhosis in a multicentre cohort of the French Early Access Programme (ANRS CO20-CUPIC) - NCT01514890. J Hepatol. 2013;59:434-441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 356] [Cited by in F6Publishing: 370] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 10. | Vierling JM, Davis M, Flamm S, Gordon SC, Lawitz E, Yoshida EM, Galati J, Luketic V, McCone J, Jacobson I. Boceprevir for chronic HCV genotype 1 infection in patients with prior treatment failure to peginterferon/ribavirin, including prior null response. J Hepatol. 2014;60:748-756. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Hézode C, Fontaine H, Dorival C, Zoulim F, Larrey D, Canva V, De Ledinghen V, Poynard T, Samuel D, Bourliere M. Effectiveness of telaprevir or boceprevir in treatment-experienced patients with HCV genotype 1 infection and cirrhosis. Gastroenterology. 2014;147:132-142.e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 212] [Article Influence: 21.2] [Reference Citation Analysis (0)] |