Copyright
©The Author(s) 2015.
World J Gastroenterol. Jul 28, 2015; 21(28): 8660-8669
Published online Jul 28, 2015. doi: 10.3748/wjg.v21.i28.8660
Published online Jul 28, 2015. doi: 10.3748/wjg.v21.i28.8660
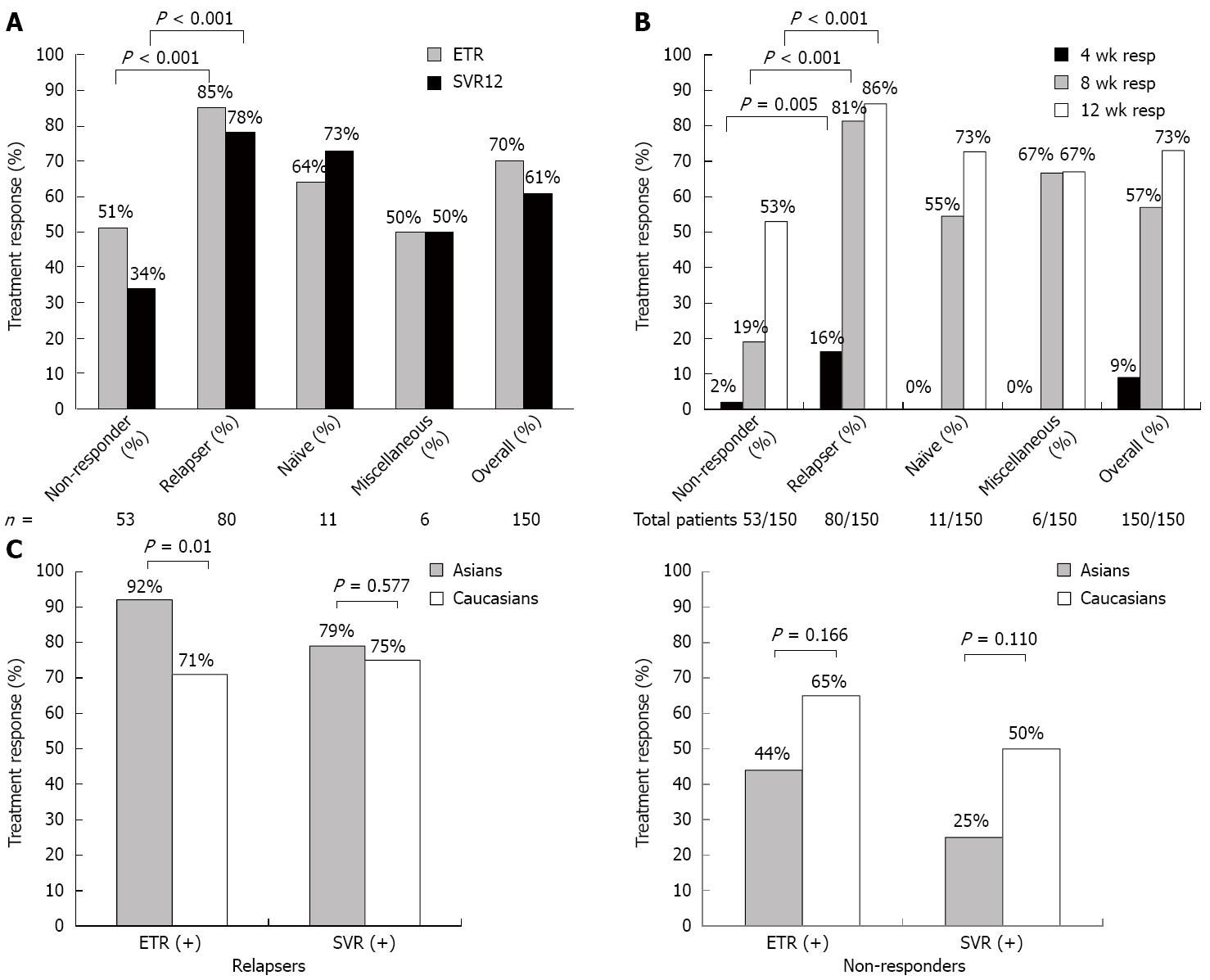
Figure 1 Treatment responses.
A: End of treatment (ETR) and sustained virological response at week 12 (SVR12) in prior treatment failures (non-responders [partial and null responders] and relapsers or miscellaneous), and in all patients; B: Undetectable HCV RNA at week 4, 8 and 12 in non-responders, relapsers, treatment naïve and miscellaneous and overall patients groups; C: ETR and SVR rates in Asians vs Caucasians in prior relapsers (Asian = 51; Caucasians = 28) and non-responders (Asian = 32; Caucasians = 20). Only significant differences are shown.
- Citation: Sukeepaisarnjaroen W, Pham T, Tanwandee T, Nazareth S, Galhenage S, Mollison L, Totten L, Wigg A, Altus R, Colman A, Morales B, Mason S, Jones T, Leembruggen N, Fragomelli V, Sendall C, Guan R, Sutedja D, Tan SS, Dan YY, Lee YM, Luman W, Teo EK, Than YM, Piratvisuth T, Lim SG. Boceprevir early-access for advanced-fibrosis/cirrhosis in Asia-pacific hepatitis C virus genotype 1 non-responders/relapsers. World J Gastroenterol 2015; 21(28): 8660-8669
- URL: https://www.wjgnet.com/1007-9327/full/v21/i28/8660.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i28.8660