Published online Apr 28, 2015. doi: 10.3748/wjg.v21.i16.4840
Peer-review started: August 5, 2014
First decision: September 15, 2014
Revised: October 17, 2014
Accepted: December 19, 2014
Article in press: December 22, 2014
Published online: April 28, 2015
Processing time: 266 Days and 5.1 Hours
AIM: To investigate the effect of hydrogen sulfide (H2S) on smooth muscle motility in the gastric fundus.
METHODS: The expression of cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) in cultured smooth muscle cells from the gastric fundus was examined by the immunocytochemistry technique. The tension of the gastric fundus smooth muscle was recorded by an isometric force transducer under the condition of isometric contraction with each end of the smooth muscle strip tied with a silk thread. Intracellular recording was used to identify whether hydrogen sulfide affects the resting membrane potential of the gastric fundus in vitro. Cells were freshly separated from the gastric fundus of mice using a variety of enzyme digestion methods and whole-cell patch-clamp technique was used to find the effects of hydrogen sulfide on voltage-dependent potassium channel and calcium channel. Calcium imaging with fura-3AM loading was used to investigate the mechanism by which hydrogen sulfide regulates gastric fundus motility in cultured smooth muscle cells.
RESULTS: We found that both CBS and CSE were expressed in the cultured smooth muscle cells from the gastric fundus and that H2S increased the smooth muscle tension of the gastric fundus in mice at low concentrations. In addition, nicardipine and aminooxyacetic acid (AOAA), a CBS inhibitor, reduced the tension, whereas Nω-nitro-L-arginine methyl ester, a nonspecific nitric oxide synthase, increased the tension. The AOAA-induced relaxation was significantly recovered by H2S, and the NaHS-induced increase in tonic contraction was blocked by 5 mmol/L 4-aminopyridine and 1 μmol/L nicardipine. NaHS significantly depolarized the membrane potential and inhibited the voltage-dependent potassium currents. Moreover, NaHS increased L-type Ca2+ currents and caused an elevation in intracellular calcium ([Ca2+]i).
CONCLUSION: These findings suggest that H2S may be an excitatory modulator in the gastric fundus in mice. The excitatory effect is mediated by voltage-dependent potassium and L-type calcium channels.
Core tip: The results demonstrated that the cystathionine β-synthase and cystathionine γ-lyase were both expressed in cultured smooth muscle of the gastric fundus. Hydrogen sulfide (H2S) increased the tension of the gastric fundus and depolarized the resting membrane potential. H2S decreased the current of voltage dependent potassium channel and calcium channel and then increased the intracellular calcium.
- Citation: Meng XM, Huang X, Zhang CM, Liu DH, Lu HL, Kim YC, Xu WX. Hydrogen sulfide-induced enhancement of gastric fundus smooth muscle tone is mediated by voltage-dependent potassium and calcium channels in mice. World J Gastroenterol 2015; 21(16): 4840-4851
- URL: https://www.wjgnet.com/1007-9327/full/v21/i16/4840.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i16.4840
Hydrogen sulfide (H2S) has been proved to be a novel gasotransmitter in addition to nitric oxide (NO) and carbon monoxide (CO) in recent years[1,2]. The endogenous production of H2S in the gastrointestinal tract has been demonstrated in tissue homogenates[3,4]. Two pyridoxal-dependent enzymes, cystathionine β-synthase (CBS) and cystathionine g-lyase (CSE), are mainly responsible for H2S synthesis. CBS and CSE have been found throughout the entire gastrointestinal tract[4] and are detected in several cell types, including smooth muscle cells, enteric neurons, interstitial cells of Cajal (ICC) and epithelial cells, varying between species and regions of the gastrointestinal tract[4-8].
Recently, many reports have demonstrated the role of endogenous and exogenous H2S in gastrointestinal motility. The first work on the role of H2S in gastrointestinal smooth muscle involved the guinea pig ileum smooth muscle, in which cyanide and nitroprusside augmented its relaxation but H2S reversed the relaxation caused by nitric oxide[9]. The ATP-sensitive potassium (KATP) channel has been demonstrated to contribute to intestinal smooth muscle relaxation in the rat jejunum[10], the human, rat and mouse jejunum and colon[11]. Nevertheless, in the urinary bladder, H2S increased the bladder contraction mediated by capsaicin-sensitive nerves[12].
The gastric fundus is mainly responsible for gastric receptive relaxation; for example, after swallowing, gastric accommodation of the meal involves fundic relaxation via activation of the vagal inhibitory pathway. Then, the stored fundic contents are gradually delivered to the caudad stomach via peristaltic contractions, modified by the vagal excitatory pathway[13]. This basic theory suggests that the basic tone of the gastric fundus smooth muscle is very important to gastric receptive relaxation. However, few studies have investigated the effect of H2S on gastric fundus motility. The present study aimed to investigate the effect of H2S on gastric fundus motility and its ion channel-based mechanism.
This study was carried out in strict accordance to the recommendation in the Guide for the Care and Use of Laboratory Animals of the Science and Technology Commission of P.R.C. (STCC Publication No. 2, revised 1988). The protocol was approved by the Committee on the Ethics of Animal Experiments of Shanghai Jiaotong University School of Medicine (Permit Number: Hu 686-2009).
Adult male ICR mice aged 5 wk (20-35 g) were provided by the Experimental Animal Center of the Chinese Academy of Sciences, Shanghai, China. The mice were housed at a constant temperature (20-25 °C) under a 12 h light/dark cycle with free access to water and food.
The mice were killed by cervical dislocation, and the stomach was removed quickly, usually in 2 min, and placed in aerated (95% O2 and 5% CO2) Krebs solution containing the following (in mmol/L): NaCl 121.9, NaHCO3 15.5, KCl 5.9, MgSO4 1.2, KH2PO4 1.2, glucose 11.5, and CaCl2 2.5. The stomach was cut along the lesser curvature, washed with iced Krebs, pinned to the base of a Sylgard dish with the mucosa facing upward, and the mucosa and submucosa were removed. Full-thickness muscle strips (2 mm × 8 mm) of the fundus were obtained along the circular axis. A silk thread (USP 5/0) was attached to both ends of the strips, and the strips were hung along the circular axis in 8-mL organ baths perfused with warm (37 °C) oxygenated Krebs solution. Mechanical activity was recorded by an isometric force transducer (RM6240C, Chengdu Instrument Factory, China) connected to an amplifier. The strip was equilibrated for 30 min with 0.3-0.5 g of the basal tension before addition of the experimental drugs.
Mouse gastric smooth muscle was isolated as described above, with a few modifications. After washing three times in phosphate-buffered saline (PBS) with 1% antibiotic/antimycotic (Gibco, Grand Island, NY, United States), the muscle was planted in six-well plates immersed in Dulbecco’s Modified Eagle Medium (DMEM) (Gibco, Grand Island, NY, United States), supplemented with 10% fetal bovine serum and 1% antibiotic/antimycotic (Gibco, Grand, NY, United States). The culture medium was changed every 48 h, and the cells were subcultured for 4-6 d.
A double-labeling immunocytochemical study was used to examine the expression of CBS and CSE. Cells grown on polylysine-coated sterile glass cover-slips were washed three times with 0.1 mol/L PBS and fixed with 4% paraformaldehyde for 20 min at 4 °C. The cells were washed in PBS for 10 min and incubated in PBS containing 10% normal goat serum for 30 min on ice, followed closely by being incubated with either rabbit anti-CBS (1:100, Abcam Ltd., Hong Kong) or rabbit anti-CSE polyclonal antibody (1:100, Proteintech Group, Ltd., United States) mixed with mouse monoclonal anti-smooth muscle α-actin (1:100, Santa Cruz Ltd., United States) at 4 °C overnight. After washing, the cells were incubated at room temperature with Alexa Fluor 488-conjugated goat anti-mouse IgG (1:100, Jackson Immuno Research, West Grove, PA, United States) mixed with Dylight 594-conjugated goat anti-rabbit IgG (1:100, ImmunoReagents Inc, Raleigh, NC, United States) for 30 min. Nuclei were stained with 4’,6-diamidino-2-phenylindole for 5 min. The controls used the same procedure but omitted the primary antibodies. The cells were observed under a fluorescence microscope (BX3, Olympus, Tokyo, Japan).
The strips (8 mm × 10 mm) were pinned in a chamber with a piece of Sylgard in the bottom with the circular muscle side up and perfused with Krebs solution. A 2-h equilibration is necessary before performing the recording. Nicardipine is present to lessen the movement of the strips. We used a glass microelectrode filled with 3 mol/L KCl (30-60 MΩ of resistance) to impale the cells. Membrane potentials were recorded with a standard electrometer (Duo 773, WPI Inc., Sarasota, FL, United States). The 3% KCl-agar bridge between the bath solution and the Ag-AgCl reference electrode was used to stabilize the electrode potentials.
Smooth muscle cells were prepared from the fundus as described above. The strip was incubated in a Ca2+-free solution containing the following (in mmol/L): NaCl 135, KCl 5, glucose 10, Hepes 10, and MgCl2 1.2, adjusted to pH 7.4 with Tris. The strip was cut into pieces and incubated in 1 mL of digestive medium (Ca2+-free solution) containing 2 mg of collagenase I (Sigma-Aldrich, St. Louis, MO, United States), 500 μg of papain (Sigma-Aldrich, St. Louis, MO, United States), 2 mg of bovine serum albumin (Sigma-Aldrich, St. Louis, MO, United States) and 1.5 mg of DTT at 37 °C for 10 min. After digestion, the tissue fragment was reserved and washed with modified Kraft-Bruhe (KB) solution containing the following (in mmol/L): glutamic acid 50, taurine 20, EGTA 0.5, Hepes 10, MgCl2 3, KCl 50, KH2PO4 20, and glucose 10, adjusted to pH 7.4 with KOH. Then, the solution was triturated with a glass pipette and kept in modified Kraft-Bruhe (KB) solution. The suspension was transferred to a perfusion chamber on the stage of an inverted microscope, and the cells were recorded after being allowed to settle for 30 min. The cells were perfused in physiologic saline solution (PSS) containing the following (in mmol/L): NaCl 135, KCl 5, CaCl2 2.5, glucose 10, Hepes 10, and MgCl2 1.2, adjusted to pH 7.4 with Tris. A single 4-channel perfusion system (BPS-4, ALA Inc., Westbury, NY, United States) was used to change the perfusate. The whole-cell patch-clamp technique was used to record the transient outward potassium current and L-type Ca2+ current with an EPC-10 amplifier (HEKA Elektronik, Lambrecht, Germany). The pipette resistance was 2-4 MΩ. For recording the transient outward potassium current, the pipette was filled with a solution comprising the following (in mmol/L): KCl 20, potassium-aspartic acid 110, di-tris-creatine phosphate 2.5, Mg-ATP 5, Hepes 5, MgCl2 1.0 and EGTA 10, adjusted to pH 7.3 with Tris. For recording the L-type Ca2+ channel current, the pipette was filled was a solution comprising the following (in mmol/L): CsCl 130, MgCl2 1, Na2ATP 5, Na2GTP 0.5, EGTA 11 and HEPES 10, adjusted to pH 7.3 with CsOH.
The cells were obtained as previously described and placed on polylysine-coated slides. They were cultivated in a carbon dioxide incubator at 37 °C. [Ca2+]i was measured in cells loaded with 1 μmol/L fura-3 acetoxymethyl ester (fura-3AM) (Sigma-Aldrich, St. Louis, MO, United States) dissolved in PSS containing 1 μmol/L F127 in a carbon dioxide incubator for 1 h. After fura-3AM loading, the cells were washed three times in PSS and placed under a fluorescence microscope (BX3, Olympus, Tokyo, Japan). The cells were perfused in a flowing PSS perfusion solution at room temperature.
Sodium hydrogen sulfide (NaHS), 4-aminopyridine (4-AP), nicardipine, aminooxyacetic acid (AOAA), DL-propargylglycine (PAG), Nω-nitro-L-arginine methyl ester (L-NAME) were all purchased from Sigma (Sigma-Aldrich, St. Louis, MO, United States). All were dissolved in distilled water except nicardipine, which was distilled in DMSO (dimethyl sulfoxide).
The data were analyzed using Origin 7.5 software and are expressed as mean ± SE. Data from multiple groups were evaluated using one-way analysis of variance followed by a post-hoc Bonferroni test, whereas Student’s paired t-test was used to evaluate paired data sets. A P value < 0.05 was considered statistically significant.
To determine whether H2S can be generated from the gastric fundus smooth muscle, CBS and CSE protein immunoreactivity (IR) was examined by double immunofluorescence labeling of cultured fundus smooth muscle cells. We found that both CBS and CSE were expressed in α-actin-positive cells (Figure 1), which suggests that H2S can be endogenously generated in gastric fundus smooth muscle cells.
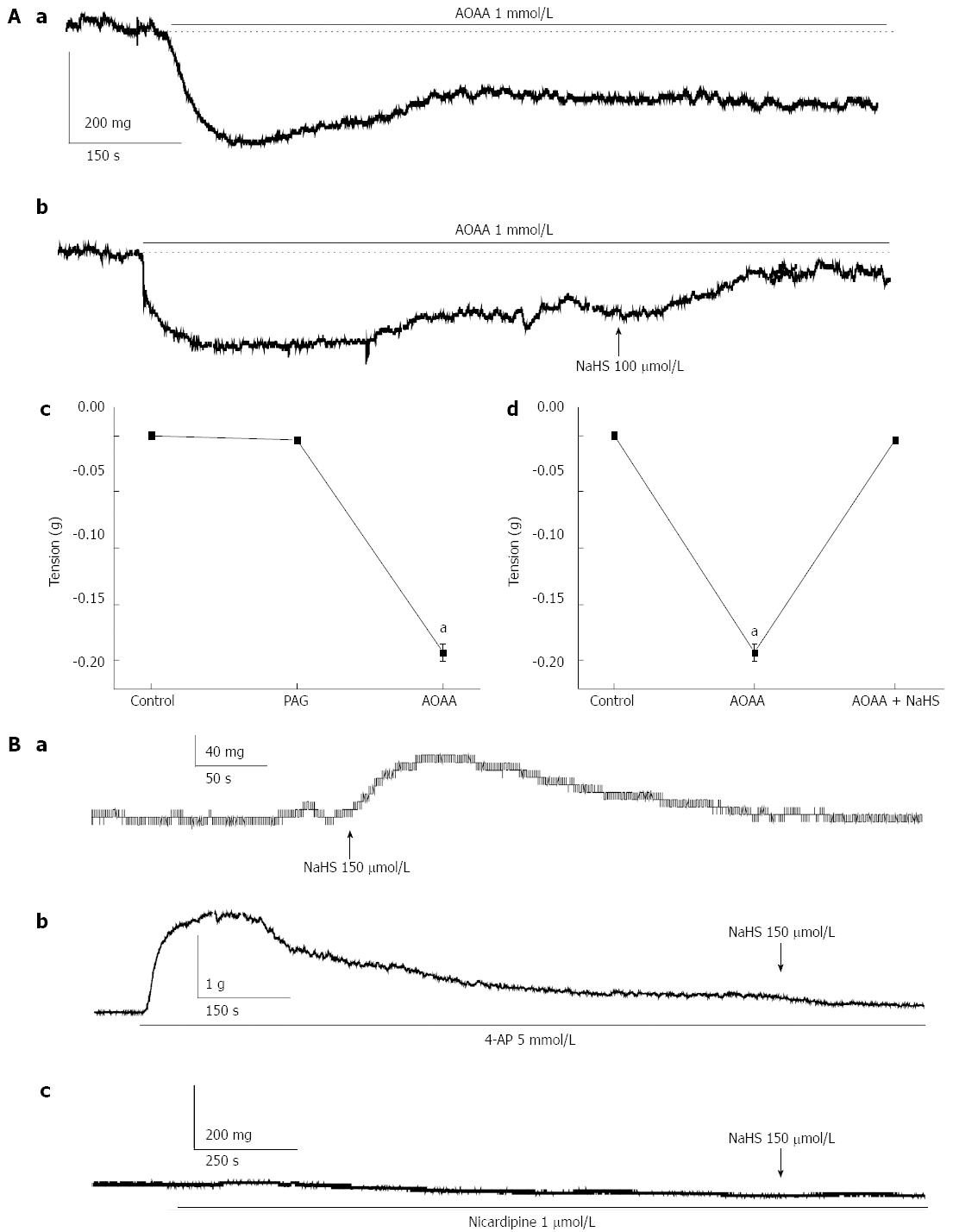
We observed the effect of H2S on gastric fundus smooth muscle tonic contraction. We observed that NaHS, an H2S donor, significantly enhanced the tension of the fundus smooth muscle at lower concentrations. The basal tension was increased from 0 mg in the control to 6.71 ± 2.11, 16.86 ± 5.67, 33.57 ± 10.32, 52.86 ± 13.06, 72.00 ± 9.62, 84.43 ± 6.56, and 52.57 ± 5.99 mg in force in cells treated with NaHS at 60, 90, 120, 150, 180, 210, and 240 μmol/L, respectively (P < 0.05, n = 10; Figure 2Aa, Ab). L-NAME (100 μmol/L), a non-specific inhibitor of NOS, increased the tension of fundus smooth muscle strips from 0 mg in the control to 52.40 ± 16.47 mg in force (P < 0.05, n = 10), whereas the CBS inhibitor AOAA (1 mmol/L) decreased the tension from 0 mg in force in the control to -241.30 ± 28.57 mg in force (P < 0.05, n = 10, Figure 2Ba, Bb). The AOAA-induced decrease in the tension was reversed by NaHS (Figure 3Aa, Ab, Ad; P < 0.05, n = 10). Interestingly, the CSE inhibitor, PAG (1 mmol/L), did not significantly affect the tension (from 0 mg in force by the control to -4.25 ± 2.81 mg in force, Figure 3Ac, P > 0.05, n = 10), which indicates that CBS may predominate over the gastric fundus in modulating smooth muscle contraction. Consequently, we investigated whether potassium and calcium channels are involved in the NaHS-induced excitatory effect on fundus smooth muscle tonic contraction. 4-AP (5 mmol/L), a voltage-dependent potassium channel blocker, elicited strong tonic contraction and completely blocked the NaHS-induced enhancement of fundus smooth muscle tone (Figure 3Ba, Bb; n = 8). We then tested the effect of nicardipine, an L-type calcium channel blocker, on NaHS-induced fundus smooth muscle tonic contraction. As shown in Figure 3Ba and Bc (n = 8), nicardipine (1 μmol/L) completely blocked the excitatory effect of NaHS (150 μmol/L) on fundus smooth muscle tonic contraction. These results suggest that the excitatory effect of NaHS may be mediated via the voltage-dependent potassium channels and L-type calcium channels, resulting in the depolarization of membrane potential and Ca2+ influx.
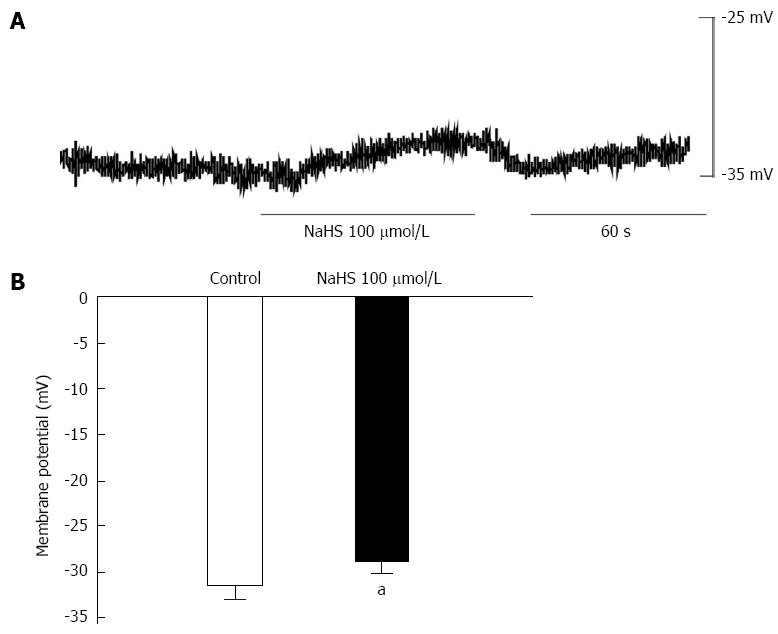
To further understand the above results, we observed the effect of NaHS (100 μmol/L) on membrane potential by intracellular recording. We found that NaHS depolarized the membrane potential from -31.82 ± 1.36 mV in the control to -25.44 ± 1.13 mV (Figure 4A, B; P < 0.05, n = 6). The result indicates that the NaHS-induced excitatory effect may be related to depolarization of the membrane potential.
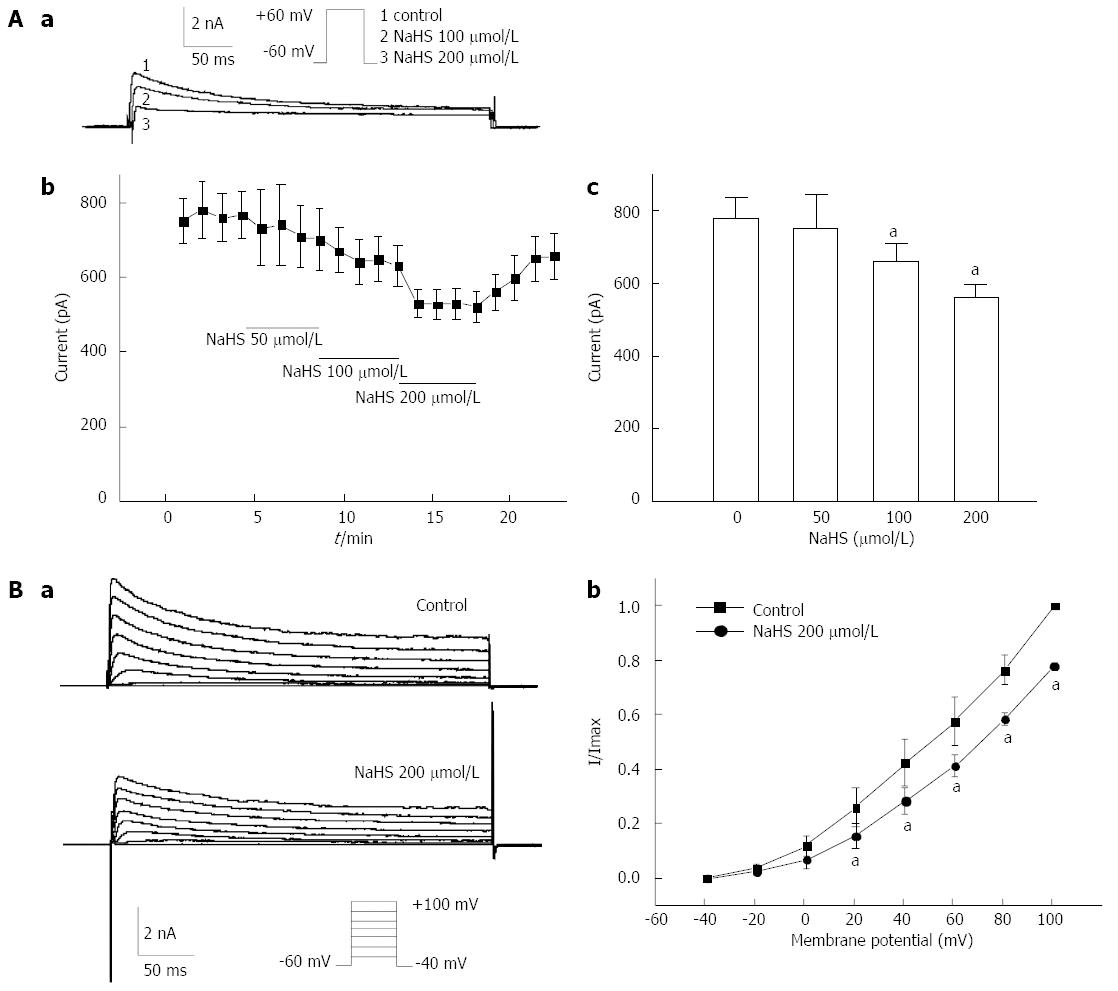
To determine the mechanism of NaHS-induced membrane potential depolarization, we further examined the effect of NaHS on the voltage-dependent potassium current (IKv). Initially, IKv was elicited by a single depolarizing step pulse (in which the membrane potential was held at -60 mV and depolarized to +60 mV in 10-s intervals) for 440 ms using the whole-cell patch-clamp technique in freshly dispersed fundus smooth muscle cells. The mean peak current was increased from 777.26 ± 59.78 pA in the control to 753.89 ± 89.70 pA, 659.86 ± 48.04 pA, and 559.06 ± 36.02 pA by 50 μmol/L, 100 μmol/L, and 200 μmol/L NaHS, respectively (Figure 5Aa, Ab, Ac; P < 0.05, n = 6). To further determine the effect of NaHS on the current-voltage (I-V) relationship of IKv, IKv was elicited by a step voltage command pulse from -40 mV to +100 mV for 400 ms with a 20-mV increment in 10-s intervals. NaHS significantly decreased IKv at every membrane potential level from +20 mV to +100 mV in the I-V curve (Figure 5Ba). The IKv at +60 mV was decreased by 16.18% ± 4.96% (Figure 5Bb, P < 0.05, n = 6) with the application of 200 μmol/L NaHS. These results suggest that IKv may contribute to NaHS-induced membrane potential depolarization in mouse gastric fundus smooth muscle.
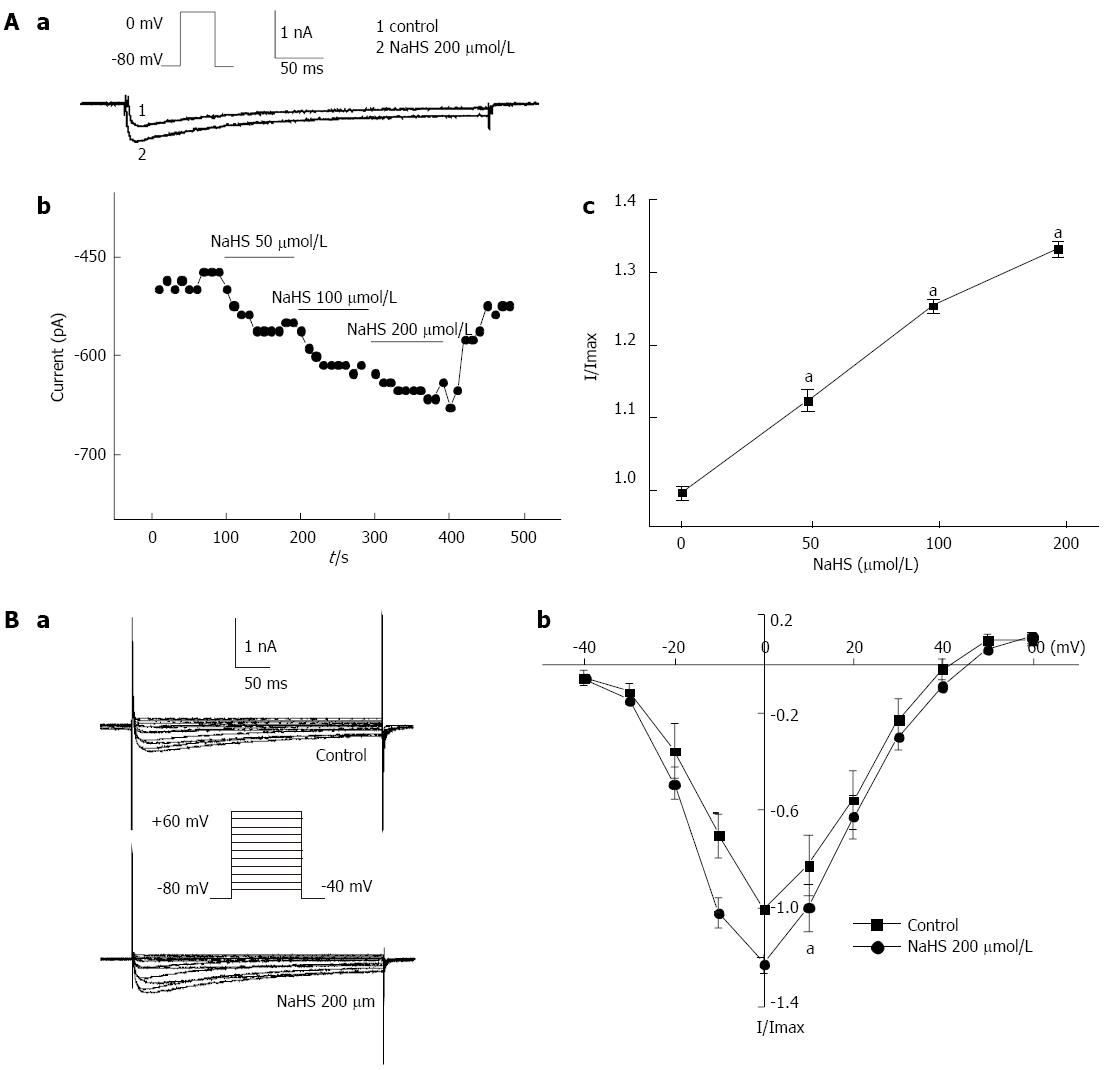
The L-type calcium current (ICa) was activated by a single depolarizing step pulse (in which the membrane potential was held at -80 mV and depolarized to 0 mV in 10-s intervals) for 440 ms first by using the whole-cell patch-clamp technique. The inward calcium current was increased with the application of a succession of NaHS. The peak relative currents were increased from 1 in the control to 1.13 ± 0.13, 1.26 ± 0.05, and 1.34 ± 0.08, by 50 μmol/L, 100 μmol/L, and 200 μmol/L NaHS, respectively (Figure 6Aa, Ab, Ac; P < 0.05, n = 6). The effect of NaHS on the I-V relationship of ICa is shown in Figure 6. The bath application of 200 μmol/L NaHS showed augmentation of the peak current on the I-V curve. NaHS significantly increased ICa at membrane potentials from -10 mV to +10 mV in the I-V curve (Figure 6Bb; P < 0.05, n = 6). The ICa at 0 mV was increased by 22.10% ± 3.90% (Figure 6Bb; P < 0.05, n = 6) with the application of 200 μmol/L NaHS. These results suggest that the L-type calcium channel is involved in the excitatory effect of NaHS on fundus smooth muscle contraction.
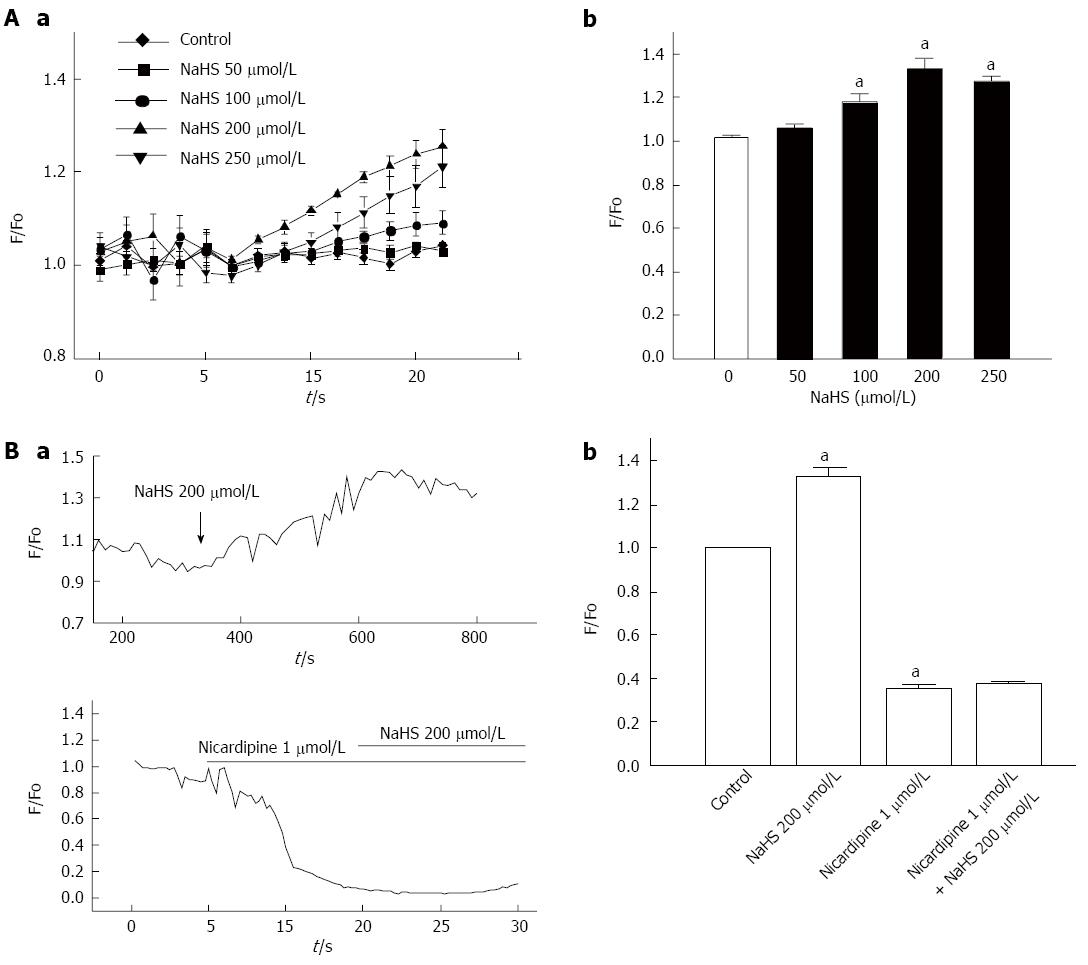
The above results suggest that NaHS-induced depolarization activates the L-type calcium channel via the inhibition of voltage-dependent potassium currents. Thus, we directly observed the effect of NaHS on changes in intracellular calcium levels. NaHS has been shown to elicit an increase in intracellular Ca2+ in cultured fundus smooth muscles cells (Figure 7Aa and Ab). The F/Fo response to NaHS varied in magnitude from 1.02 ± 0.004 in the control to 1.06 ± 0.015, 1.18 ± 0.037, 1.33 ± 0.047, and 1.27 ± 0.023 when treated with 50 μmol/L, 100 μmol/L, 200 μmol/L, and 250 μmol/L NaHS, respectively, with the maximum at 200 μmol/L (Figure 7Ab; P < 0.05, n = 6).
Because extracellular Ca2+ entry can result from the opening of L-type calcium channels, we observed whether the change in intracellular Ca2+ is associated with L-type calcium channels. When perfused with PSS containing 200 μmol/L NaHS, the F/Fo was markedly increased (Figure 7Ba), but pretreatment with 1 μmol/L nicardipine caused the NaHS-induced increase in F/Fo to be almost completely abolished (Figure 7Ba). Then, we perfused the sample with PSS containing 1 μmol/L nicardipine and 200 μmol/L NaHS, which attenuated the increase in intracellular Ca2+ induced by 200 μmol/L NaHS (Figure 7Bb). These results indicate that NaHS increases the intracellular Ca2+ levels via L-type calcium channels.
Gasotransmitters are gas molecules endogenously synthesized in a regulated manner, causing well-defined physiological and/or pathophysiological effects, acting at specific cellular and molecular targets and employing specific mechanisms of inactivation[2,14,15]. H2S is of particular interest in the gastrointestinal tract as it is both produced both by gastrointestinal tissues and generated in large quantities by the bacterial flora in the lumen of the gut[3,4,15-18]. In the gastrointestinal tract, both excitatory and inhibitory effects on smooth muscle have been reported. For example, NaHS concentration-dependently relaxed prostaglandin F2a-contracted circular muscle strips of mouse fundus and distal colon[19,20]. NaHS also exerted relaxant effects on guinea pig, rabbit and rat ileum and jejunum preparations[3,10,19,21,22]. Furthermore, NaHS inhibits peristaltic activity in the mouse small intestine and colon[11]. Our previous studies indicated dual effects of H2S on the spontaneous contraction of gastric antral smooth muscle; for example, a low concentration of NaHS increased tonic contraction, whereas a high concentration reduced the amplitude and tone of gastric smooth muscle spontaneous contraction in guinea pigs[23]. We have explored that NO and H2S play opposite roles in regulating the tension of gastric antrum before, and they share no common pathways[24]. These studies suggest that the role of H2S in the regulation of gastrointestinal motility displays a regional variation. The stomach gastric fundus is involved in receptive contraction concerned with gastric accommodation, and the gastric antrum is involved in gastric emptying concerned with the pyloric pump; in contrast, the jejunum and colon are involved in migrating the motility complex aimed at absorption. Therefore, the effects of H2S in the stomach might differ from those in the jejunum and colon.
In the present study, we found that both CBS and CSE, which catalyze the generation of H2S, were expressed in primary cultured smooth muscle cells (Figure 1). We deduce that H2S can be generated endogenously and continuously in gastric smooth muscle cells and influences physiological processes. Meanwhile, we observed that NaHS at lower concentrations increased the basal tension of smooth muscles in the gastric fundus (Figure 2). AOAA, an inhibitor of CBS, decreased the basal tension, and PAG, an inhibitor of CSE, did not affect the tension significantly (Figure 3). All these results demonstrate that CBS may be the predominant enzyme in the gastric fundus. Although AOAA is widely used as an inhibitor of several pyridoxal phosphate-dependent enzymes, including aspartate transaminase, 4-aminobutyrate and dopa-decarboxylase[25], it may also inhibit NADH shuttles[26]. In contrast, Martin et al[4] have demonstrated that AOAA reduces H2S generation. In our study, we also observed that the decrease in the tension induced by AOAA was significantly reversed by NaHS (Figure 3), suggesting that the AOAA-induced inhibitory effect on gastric fundus smooth muscles was partially mediated by inhibition of CBS to generate endogenous H2S. NO is a well-known relaxation agent for smooth muscle as a contrary experiment control, L-NAME, a nonspecific inhibitor of NOS, significantly enhanced the tension of fundus smooth muscle. As shown in our results, the effect of AOAA on gastric fundus smooth muscle is the opposite to that of L-NAME, which indicates that H2S may be an excitatory gaseous transmitter in the gastric fundus under physiological conditions.
It is undeniable that membrane potential is important for electric-contraction coupling. Furthermore, distinct from other parts of the gastrointestinal tract, gastric fundus smooth muscle cells are electrically quiescent or, in some occasions, generate the discharge of membrane noises[27]. We observed the membrane potential of gastric fundus smooth muscles using the intracellular recording technique and found that 100 μmol/L NaHS significantly depolarized the membrane potential (Figure 4). Because voltage-dependent potassium channels are the most important regulators of maintaining the resting membrane potential, we observed the effect of 4-AP, an inhibitor of IKV, on the NaHS-induced excitatory effect in succession and found that the NaHS-induced tonic contraction of fundus smooth muscle was completely blocked by 4-AP. Gastrointestinal smooth muscle cells express voltage-dependent calcium channels[28,29], and these channels are the backbone of electric-contraction coupling in the gut[30]. Therefore, we also used nicardipine to block the L-type calcium channels and found that the NaHS-induced tonic contraction was completely abolished (Figure 3). These results suggest that H2S may be activated by L-type calcium channels through the inhibition of IKV and depolarization of the membrane potential.
However, H2S inhibited L-type calcium channels, resulting in the inhibition of intracellular calcium concentrations in rat cardiomyocytes[31]. Moreover, H2S raised the intracellular calcium concentrations in endothelial cells via KATP channels and the Na+-Ca2+ exchanger[32]. These studies indicated that the function of H2S may be complicated in different tissues. In the present study, to further explore the ion channel mechanism involved in the NaHS-induced excitatory effect on fundus smooth muscles, we observed the effect of H2S on IKV, L-type calcium current and intracellular calcium concentration using the whole-cell patch-clamp and calcium imaging techniques. We found that H2S inhibited IKV (Figure 5) but increased the L-type calcium current (Figure 6) and intracellular calcium levels (Figure 7). The H2S-induced increase in intracellular calcium was significantly blocked by nicardipine (Figure 7). We conclude that the excitatory effect of NaHS on the fundus smooth muscle was mediated by intracellular calcium due to the activation of L-type calcium channels via inhibition of the IKV-induced depolarization of the membrane potential. The present study demonstrates that H2S is an excitatory gaseous molecule in the gastric fundus in mice, but its exact mechanism still needs further investigation.
In summary, we showed that the H2S-producing enzymes CBS and CSE are expressed in the gastric fundus in mice. H2S at physiological concentrations may excite the fundus smooth muscles and induce tonic contraction. CBS may be more important for the excitatory effect of endogenous H2S on gastric motility. Endogenous H2S induces the depolarization of membrane potential via the inhibition of voltage-dependent potassium channels. In succession, NaHS-induced depolarization activates L-type calcium channels, raises the intracellular calcium level and finally induces fundus smooth muscle tonic contraction. Under physiological conditions, endogenous H2S and NO levels might maintain a relative balance to ensure the basic physiological tone of fundus smooth muscle.
Hydrogen sulfide is considered a gaseous signal molecular for its wide effects in pathophysiology process. Numerous studies have shown that hydrogen sulfide serves as a vasodilator. The gastric fundus is responsible for receptive relaxation and little is known on the effect of hydrogen sulfide on the fundus.
Hydrogen sulfide is mainly catalyzed by cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE). The authors previous studies have shown that hydrogen sulfide enhanced the tension of gastric antrum via voltage dependent potassium channels and ATP sensitive potassium channels. In this study, the authors demonstrate that hydrogen sulfide-induced enhancement of gastric fundus smooth muscle tone is mediated by voltage-dependent potassium and calcium channels in mice.
Recent reports have highlighted the effects of hydrogen sulfide on gastrointestinal muscle and enteric nervous system. This is the first study to report that hydrogen sulfide enhances the tension of gastric fundus smooth muscle via raising intracellular calcium.
This study may represent a future strategy for therapeutic intervention in disorders of gastrointestinal motility by understanding the mechanism of action of hydrogen sulfide on gastric fundus tension.
Hydrogen sulfide is mainly catalyzed by CBS and CSE. In the gastrointestinal tract, hydrogen sulfide is involved in gastrointestinal motility, absorption and secretion.
The authors of the manuscript entitled “Hydrogen sulfide-induced enhancement of gastric fundus smooth muscle tone mediated by voltage-dependent potassium and calcium channels in mice” present a very nice work of basic science physiology on gastric smith muscles.
P- Reviewer: Larentzakis A S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Zhang DN
| 1. | Ali MY, Ping CY, Mok YY, Ling L, Whiteman M, Bhatia M, Moore PK. Regulation of vascular nitric oxide in vitro and in vivo; a new role for endogenous hydrogen sulphide? Br J Pharmacol. 2006;149:625-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Wang R. Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16:1792-1798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1408] [Cited by in RCA: 1406] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 3. | Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun. 1997;237:527-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 952] [Cited by in RCA: 961] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 4. | Martin GR, McKnight GW, Dicay MS, Coffin CS, Ferraz JG, Wallace JL. Hydrogen sulphide synthesis in the rat and mouse gastrointestinal tract. Dig Liver Dis. 2010;42:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Schicho R, Krueger D, Zeller F, Von Weyhern CW, Frieling T, Kimura H, Ishii I, De Giorgio R, Campi B, Schemann M. Hydrogen sulfide is a novel prosecretory neuromodulator in the Guinea-pig and human colon. Gastroenterology. 2006;131:1542-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 173] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 6. | Linden DR, Sha L, Mazzone A, Stoltz GJ, Bernard CE, Furne JK, Levitt MD, Farrugia G, Szurszewski JH. Production of the gaseous signal molecule hydrogen sulfide in mouse tissues. J Neurochem. 2008;106:1577-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Hennig B, Diener M. Actions of hydrogen sulphide on ion transport across rat distal colon. Br J Pharmacol. 2009;158:1263-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Gil V, Gallego D, Jiménez M. Effects of inhibitors of hydrogen sulphide synthesis on rat colonic motility. Br J Pharmacol. 2011;164:485-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Kruszyna H, Kruszyna R, Smith RP. Cyanide and sulfide interact with nitrogenous compounds to influence the relaxation of various smooth muscles. Proc Soc Exp Biol Med. 1985;179:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Nagao M, Duenes JA, Sarr MG. Role of hydrogen sulfide as a gasotransmitter in modulating contractile activity of circular muscle of rat jejunum. J Gastrointest Surg. 2012;16:334-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Gallego D, Clavé P, Donovan J, Rahmati R, Grundy D, Jiménez M, Beyak MJ. The gaseous mediator, hydrogen sulphide, inhibits in vitro motor patterns in the human, rat and mouse colon and jejunum. Neurogastroenterol Motil. 2008;20:1306-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Patacchini R, Santicioli P, Giuliani S, Maggi CA. Hydrogen sulfide (H2S) stimulates capsaicin-sensitive primary afferent neurons in the rat urinary bladder. Br J Pharmacol. 2004;142:31-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Langley JN. On Inhibitory Fibres in the Vagus for the end of the OEsophagus and the Stomach. J Physiol. 1898;23:407-414. [PubMed] |
| 14. | Li L, Moore PK. An overview of the biological significance of endogenous gases: new roles for old molecules. Biochem Soc Trans. 2007;35:1138-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Linden DR, Levitt MD, Farrugia G, Szurszewski JH. Endogenous production of H2S in the gastrointestinal tract: still in search of a physiologic function. Antioxid Redox Signal. 2010;12:1135-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 16. | Blachier F, Davila AM, Mimoun S, Benetti PH, Atanasiu C, Andriamihaja M, Benamouzig R, Bouillaud F, Tomé D. Luminal sulfide and large intestine mucosa: friend or foe? Amino Acids. 2010;39:335-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 165] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 17. | Teague B, Asiedu S, Moore PK. The smooth muscle relaxant effect of hydrogen sulphide in vitro: evidence for a physiological role to control intestinal contractility. Br J Pharmacol. 2002;137:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Wallace JL. Physiological and pathophysiological roles of hydrogen sulfide in the gastrointestinal tract. Antioxid Redox Signal. 2010;12:1125-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 19. | Dhaese I, Lefebvre RA. Myosin light chain phosphatase activation is involved in the hydrogen sulfide-induced relaxation in mouse gastric fundus. Eur J Pharmacol. 2009;606:180-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Dhaese I, Van Colen I, Lefebvre RA. Mechanisms of action of hydrogen sulfide in relaxation of mouse distal colonic smooth muscle. Eur J Pharmacol. 2010;628:179-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Nagao M, Linden DR, Duenes JA, Sarr MG. Mechanisms of action of the gasotransmitter hydrogen sulfide in modulating contractile activity of longitudinal muscle of rat ileum. J Gastrointest Surg. 2011;15:12-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Kasparek MS, Linden DR, Farrugia G, Sarr MG. Hydrogen sulfide modulates contractile function in rat jejunum. J Surg Res. 2012;175:234-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Zhao P, Huang X, Wang ZY, Qiu ZX, Han YF, Lu HL, Kim YC, Xu WX. Dual effect of exogenous hydrogen sulfide on the spontaneous contraction of gastric smooth muscle in guinea-pig. Eur J Pharmacol. 2009;616:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Huang X, Meng XM, Liu DH, Wu YS, Guo X, Lu HL, Zhuang XY, Kim YC, Xu WX. Different regulatory effects of hydrogen sulfide and nitric oxide on gastric motility in mice. Eur J Pharmacol. 2013;720:276-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | John RA, Charteris A. The reaction of amino-oxyacetate with pyridoxal phosphate-dependent enzymes. Biochem J. 1978;171:771-779. [PubMed] |
| 26. | Casimir M, Rubi B, Frigerio F, Chaffard G, Maechler P. Silencing of the mitochondrial NADH shuttle component aspartate-glutamate carrier AGC1/Aralar1 in INS-1E cells and rat islets. Biochem J. 2009;424:459-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Kito Y. The functional role of intramuscular interstitial cells of Cajal in the stomach. J Smooth Muscle Res. 2011;47:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Kovac JR, Preiksaitis HG, Sims SM. Functional and molecular analysis of L-type calcium channels in human esophagus and lower esophageal sphincter smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2005;289:G998-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Rich A, Kenyon JL, Hume JR, Overturf K, Horowitz B, Sanders KM. Dihydropyridine-sensitive calcium channels expressed in canine colonic smooth muscle cells. Am J Physiol. 1993;264:C745-C754. [PubMed] |
| 30. | Sanders KM. Regulation of smooth muscle excitation and contraction. Neurogastroenterol Motil. 2008;20 Suppl 1:39-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Sun YG, Cao YX, Wang WW, Ma SF, Yao T, Zhu YC. Hydrogen sulphide is an inhibitor of L-type calcium channels and mechanical contraction in rat cardiomyocytes. Cardiovasc Res. 2008;79:632-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 132] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 32. | Moccia F, Bertoni G, Pla AF, Dragoni S, Pupo E, Merlino A, Mancardi D, Munaron L, Tanzi F. Hydrogen sulfide regulates intracellular Ca2+ concentration in endothelial cells from excised rat aorta. Curr Pharm Biotechnol. 2011;12:1416-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |