Published online Dec 7, 2014. doi: 10.3748/wjg.v20.i45.17190
Revised: April 29, 2014
Accepted: July 24, 2014
Published online: December 7, 2014
AIM: To investigate the clinical response of gastro-esophageal reflux disease (GERD) symptoms to exclusion diets based on food intolerance tests.
METHODS: A double blind, randomized, controlled pilot trial was performed in 38 GERD patients partially or completely non-responders to proton pump inhibitors (PPI) treatment. Fasting blood samples from each patients were obtained; leukocytotoxic test was performed by incubating the blood with a panel of 60 food items to be tested. The reaction of leukocytes (rounding, vacuolization, lack of movement, flattening, fragmentation or disintegration of cell wall) was then evaluated by optical microscopy and rated as follows: level 0 = negative, level 1 = slightly positive, level 2 = moderately positive, and level 3 = highly positive. A “true” diet excluding food items inducing moderate-severe reactions, and a “control” diet including them was developed for each patient. Then, twenty patients received the “true” diet and 18 the “control” diet; after one month (T1) symptoms severity was scored by the GERD impact scale (GIS). Hence, patients in the “control” group were switched to the “true” diet, and symptom severity was re-assessed after three months (T2).
RESULTS: At baseline (T0) the mean GIS global score was 6.68 (range: 5-12) with no difference between “true” and control group (6.6 ± 1.19 vs 6.7 ± 1.7). All patients reacted moderately/severely to at least 1 food (range: 5-19), with a significantly greater number of food substances inducing reaction in controls compared with the “true” diet group (11.6 vs 7.0, P < 0.001). Food items more frequently involved were milk, lettuce, brewer’s yeast, pork, coffee, rice, sole asparagus, and tuna, followed by eggs, tomato, grain, shrimps, and chemical yeast. At T1 both groups displayed a reduction of GIS score (“true” group 3.3 ± 1.7, -50%, P = 0.001; control group 4.9 ± 2.8, -26.9%, P = 0.02), although the GIS score was significantly lower in “true”vs“control” group (P = 0.04). At T2, after the diet switch, the “control” group showed a further reduction in GIS score (2.7 ± 1.9, -44.9%, P = 0.01), while the “true” group did not (2.6 ± 1.8, -21.3%, P = 0.19), so that the GIS scores didn’t differ between the two groups.
CONCLUSION: Our results suggest that food intolerance may play a role in GERD symptoms development, and leucocytotoxic test-based exclusion diets may be a possible therapeutic approach when PPI are not effective or indicated.
Core tip: We previously observed that gastro-esophageal reflux disease (GERD) symptoms respond to exclusion diets based on food intolerance tests. In this randomized controlled pilot trial we found that patient partially/completely non responders to proton pump inhibitors experience a significant improvement in symptoms, compared with controls, after one month of exclusion diet based on leukocytotoxic test’s results. These results indicate a possible role of food intolerance in the etiopathogenesis of GERD symptoms, and a possible therapeutic application of exclusion diets when proton pump inhibitor are not effective/indicated.
- Citation: Caselli M, Zuliani G, Cassol F, Fusetti N, Zeni E, Lo Cascio N, Soavi C, Gullini S. Test-based exclusion diets in gastro-esophageal reflux disease patients: A randomized controlled pilot trial. World J Gastroenterol 2014; 20(45): 17190-17195
- URL: https://www.wjgnet.com/1007-9327/full/v20/i45/17190.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i45.17190
Gastro-esophageal reflux disease (GERD) is an extremely common condition characterized by symptoms of heartburn and acid regurgitation, presenting a chronic relapsing disease course. It is more common in Western populations, with high impact on patients’ social and working life[1,2]. The etiopathogenesis of GERD remains largely unknown even though up to 20% of the adult population in Western Countries is affected by reflux symptoms on a week basis[1]. Notably, at least 50% of GERD patients remain on continuous medical therapy[3]. This condition is known to predispose to an increased risk of cancer[4,5]. Although the pathogenesis of this backward displacement of gastrointestinal contents remains elusive, it is know that abnormal contact of gastrointestinal contents with esophageal mucosa, insufficient clearance of the esophageal body, lower esophageal sphincter incompetence, and delayed gastric emptying have a putative role in GERD pathogenesis. Despite several examinations (e.g., endoscopy, 24-h pH monitoring, barium esophageal radiography, proton pump inhibitor (PPI) test, gastro-esophageal scintigraphy) are available for the diagnosis of GERD, none of these tests is diagnostic, and current guidelines recommend to treat patients younger than 50 years on the basis of their symptoms[6,7]. Consequently, symptoms evaluation and score are fundamental in diagnosis and therapy of GERD patients, and several validated questionnaires are currently used in the clinical practice[8].
The possible role of food intolerance in the etiopathogenesis of GERD has been suggested by our group in a recent retrospective report[9] in which we evaluated the results of a leukocytotoxic test for food intolerance using a panel of 60 food items. This test is based on the optical evaluation of leukocytes in a blood sample that has come into direct contact with specific food substances. On the basis of leukocytes observation, degrees of reaction to the food extracts are rated according to the following scale: level 0 = negative, level 1 = slightly positive, level 2 = moderately positive, and level 3 = highly positive. These levels correspond to (1) the state of the leukocytes, which react by swelling, then developing vacuoles, and finally deteriorate; and (2) the total number of leukocytes that react. Currently, the dedicated task forces consider these tests to have a poor clinical specificity and sensitivity[10] we hypothesized that the most important reason might be the very high frequency of mild positive results[9]. Therefore, we decided to exclude level 1 of the scale, directly starting from level 2. In this way, we could demonstrate a strong difference between GERD patients and controls, and the test-based exclusion diet resulted effective in all patients[9]. On this basis, we carried out this prospective randomized controlled pilot trial with the following aims: (1) to evaluate the prevalence of moderate-severe leukocytotoxic reactions in patients with GERD symptoms; and (2) to evaluate the clinical response to a test-based exclusion diet compared to a “control” diet.
Outpatients attending the Department of Gastroenterology of Ferrara Hospital because of typical GERD symptoms (heartburn and regurgitation) were considered for recruitment. The severity of symptoms was scored by the gastro-esophageal reflux disease impact scale (GIS) questionnaire[11]. A score equal or major to 5, concerning only the first four clinical questions (pain in the chest/behind the breastbone, regurgitation/acid taste in one’s mouth, burning sensation in the chest/behind the breastbone, and sore throat/hoarseness related to heartburn or acid reflux) was used as cut-off value for inclusion into the study. Among these patients, only non-responders to PPI therapy or partially responders (i.e., patients experiencing only slight reduction of symptoms after 4 wk of treatment or having an initial response to PPI treatment but with relapses of symptoms during the 4 wk of PPI treatment) were recruited. Patients with alarm symptoms/signs (dysphagia, unintentional weight loss, anorexia, recurrent vomiting, upper abdominal mass, anaemia, haematemesis or melena) and patients over 50 years with family history of gastric/oesophageal carcinoma were excluded. Patients with a clinical history of celiac disease or lactose intolerance were also excluded. The study protocol was approved by the local ethics committee and all patients provided written informed consent.
The study used a double blind, randomized, controlled parallel design in which patients were randomized to either a “true” or a “control” test-based diet group. In both groups the diets were test-based, but while the “true” diets excluded all the foods presenting a moderate-severe reaction at the leukocytotoxic test, the “control” diets excluded foods provoking no reactions. In this way we avoided the possibility that a standardized not test-based control diet might exclude by chance a food provoking a reaction in that patient. At the time of enrolment, a blood sample was collected from each patient and sent to the laboratory of the local Center Study Association on Food Intolerance and Nutrition, where the leukocytotoxic tests were performed. For these tests a panel of 60 food extracts was used (Antigenia s.r.l., Bologna, Italy) according to the manufacturer’s instructions. The buffy coat obtained by centrifuging blood was suspended in a mixture of sterile distilled water and serum, and then placed on a siliconized microscope slide previously coated with the dried extract of the food to be tested. A staff member evaluated by optical microscopy (40 × zoom) the unstained leukocytes at varying intervals, up to two hours. Any lack of movement, rounding, vacuolization, flattening, fragmentation, or disintegration of cells was considered, and rated as follows: level 0 = negative, level 1 = slightly positive, level 2 = moderately positive, and level 3 = highly positive, as previously said. According to our previous study[9], we excluded level 1 response, and considered only moderate-severe reactions. In this way we improved the specificity of the test (whose major criticism is related to the extremely frequent positive reactions) and excluded the fewest foods as possible thus improving the patients’ compliance to the diet. Both “true” and “control” diets were arranged on the basis of the test results. The “true” diets were produced excluding all food associated with level 2-3 reactions, while “control” diets were produced by eliminating only food that provoked no reactions. In this way we excluded the possibility that a food item inducing moderate-severe reaction might be casually excluded from the “control” diet. The proposed diets did not differ for caloric content. Patients were allocated to one of the two diet sheets based on a randomization schedule developed by using a random computer number generator; both patients and clinical/dietician staff were blinded to the group assignment of each patient.
Symptoms were assessed at the time of recruitment (T0), and after one (T1) and three months (T2). At T1, “control” diets were substituted by “true” diets, while patients assigned to “true” diet at T0 continued with the prescribed diet. At T1 and T2 each patient fulfilled the GIS questionnaire, was visited by a gastroenterologist, and was evaluated by a dietician. Both the global GIS score, and the score for every single item (P for pain in the chest/behind the breastbone, R for regurgitation/acid taste in one’s mouth, B for burning sensation in the chest/behind the breastbone, and S for sore throat/hoarseness related to heartburn or acid reflux) were considered. For each item the score was assigned based on the frequency of that specific symptom as follows: never = 0 points, sometimes = 1 point, often = 2 points, and daily = 3 points.
Continuous variables were expressed as mean ± SD or median (interquartile range) when necessary. Means (between groups) were compared by one-way ANOVA, while medians were compared by the Mann-Whitney test. Means (within group) were compared by paired-samples t test. Correlations between continuous variables were tested by Pearson’s correlation. Proportions were compared by the χ2 test. All analyses were performed by SPSS for Windows statistical package, version 13.0 (SPSS, Inc., Chicago, IL, United States).
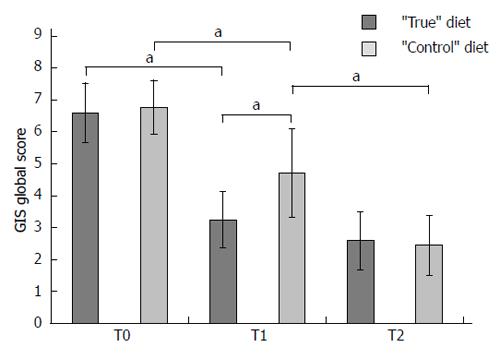
From March 2011 to December 2012 a total of 38 patients were enrolled into the study (mean age: 47.5 years; 18.4% males). At baseline (T0), the mean global GIS score was 6.68 points (range: 5-12). All patients were found to react moderately/severely to at least 1 food item (range: 5-19). Table 1 summarizes the principal characteristics of the patients at baseline. No significant difference emerged between the two groups as regards to age, gender, and severity of symptoms. The number of food items inducing moderate-severe reaction at leukocyotoxic test was higher in the “control” compared with the “true” diet group (11.6 vs 7.0, P < 0.001). The food substances more frequently involved in leukocytotoxic test positivity are reported in Table 2. At T0, the mean GIS global score, as well the mean sub-scores, were similar in the two groups of patients (6.6 ± 1.9 vs 6.7 ± 1.7) (Figure 1). No specific adverse events, nor motivated drop out from the study were recorded in either group.
| True diet group | Control group | Total | |
| Patients (n) | 20 | 18 | 38 |
| Age | 45 ± 9.6 | 50.4 ± 7.7 | 47.5 ± 9.1 |
| Males, n (%) | 5 (25) | 2 (11.1) | 7 (18.4) |
| Time 0: GIS | 6.6 ± 1.9 | 6.7 ± 1.7 | 6.7 ± 1.8 |
| P | 1.3 ± 0.9 | 1.4 ± 0.9 | 1.3 ± 0.9 |
| R | 1.7 ± 0.6 | 2.0 ± 0.9 | 1.8 ± 0.8 |
| B | 1.9 ± 0.8 | 2.0 ± 0.7 | 2.0 ± 0.8 |
| S | 1.8 ± 1.0 | 1.4 ± 1.0 | 1.6 ± 0.9 |
| Foods inducing reaction1 | 7 (6-12) | 11.7 (7-19) | 9.2 (6-19) |
| Level 2 reaction | 5.6 | 9.2 | 7.2 |
| Level 3 reaction | 1.4 | 2.5 | 1.9 |
| Food | Total reactions | Level 2 | Level 3 |
| Milk | 32 | 23 | 9 |
| Lettuce | 20 | 14 | 6 |
| Brewer’s yeast | 15 | 7 | 8 |
| Pork | 12 | 8 | 4 |
| Coffee | 12 | 8 | 4 |
| Rice | 11 | 2 | 9 |
| Sole | 11 | 11 | 0 |
| Asparagus | 10 | 9 | 1 |
| Tuna | 10 | 10 | 0 |
| Egg | 9 | 6 | 3 |
| Tomato | 9 | 8 | 1 |
| Grain | 8 | 5 | 3 |
| Shrimps | 8 | 8 | 0 |
| Chemical yeast | 8 | 6 | 2 |
One month after the beginning of the elimination diet (T1), the “true” test-based diet group experienced an important reduction (-50%) in GIS global score (from 6.6 ± 1.9 to 3.3 ± 1.7, P = 0.001). Patients in the “control” diet group also experienced a significant reduction in GIS global score (-26.9%, from 6.7 ± 1.7 to 4.9 ± 2.8, P = 0.02), although it was much smaller compared to “true” diet group. As a matter of fact, at T1 the difference between the mean GIS scores in the two groups was significant, as regards both the GIS global score (3.3 ± 1.7 vs 4.9 ± 2.8, P = 0.04) and the B sub-score (1.0 ± 0.7 vs 1.5 ± 0.8, P = 0.05) (Figure 1).
Despite a further reduction was observed at T2 (-21.3%) in the “true” diet group the T2 didn’t differ from the T1 GIS score (3.3 ± 1.7 vs 2.6 ± 1.8, P = 0.19) (Figure 1). On the contrary, a further significant reduction in GIS score was observed among patients in the “control” group (-44.9%, 4.9 ± 2.8 vs 2.7 ± 1.9, P = 0.01). At T2, the mean GIS global scores as well as the sub-scores were not statistically different between the two groups (data not shown).
The etiopathogenesis of intestinal functional diseases such as irritable bowel disease (IBS), functional dyspepsia, and GERD are far from being well elucidated. In the last years, however, increasing data has been published consistent with a possible role of food intolerance in these medical conditions. The attention has been particularly focused on IBS, and several studies have confirmed the possible effectiveness of test-based exclusion diets on IBS symptoms[12-14]. To our knowledge, this is the first controlled, double blind, study in which not only a leukocytotoxic test has been used to prepare test-based exclusion diet, but also an attempt to validate the test by a “control” test-based diet has been made. Our preliminary data seem to suggest that pathogenetic mechanisms related to food intolerance may play a role in the etiopathogenesis of GERD. The modifications of the GIS score from T0 to T2 in the two groups of patients clearly shows that the entity of reduction in the “true” diet group at T1 is equal to the reduction observed for the “control” group at T2. This observation seems to confirm the effectiveness of “true” test-based diet in reducing the impact of GERD symptoms of about 4%-50%. Notably, the significant but smaller reduction in GIS score from T0 to T1 observed in the “control” group (-26.9%) is probably due to a placebo effect, in the same way as the slight (not significant) reduction of GIS scores in the “true” diet group from T1 to T2. Placebo effect has been reported to be particularly strong when the treatment consists in a diet, in particular when the condition to cure is a functional disease[15,16]. However, albeit a placebo effect might be present and might justify the reduction of symptoms in patients following the control diet, patients following the “true” test-based diet experienced a significantly greater reduction in symptoms compared to control group, as did people in the control group after the “diet switch”, indicating that the effect of “true” diets cannot be just attributable to the placebo effect.
Our results suggest that, although not all GERD symptoms might be attributed to food intolerance, this pathogenetic condition may play a role in GERD symptoms development. Thus, test-based exclusion diets may be considered an effective and cost-effective therapeutic tool, poor of adverse events, and particularly indicated when PPI treatment is not effective or not indicated. When considering only the moderate-severe reactions, the leukocytotoxic test appears to be effective in identifying the food substances whose exclusion is associated with clinical improvement of GERD symptoms. As a corollary finding, the panel of food items most frequently involved in leukocytotoxic reaction was similar to the one observed in our previous retrospective report[9], and notably different from the panel of food previously related to GERD symptoms (i.e., chocolate, mint, onions and citrus fruit) confirming only the role of mint, dietary fats, coffee, and tomato.
We previously suggested that a rationale for the use of a leukocytotoxic test might be related to the role of T regulatory cells. In recent years, the role of specific receptors and specialized cells of innate immunity in the recognition and identification of antigens has become evident. The complete recognition of an antigen by this receptor system is at the basis of tolerance to a food substance or a microorganism. When an antigen is not suitably recognized, a kind of adaptive immune response may be activated (allergy or intolerance) closely depending on the efficiency of T regulator cell system. Consistent with this, intriguing data[17] indicate that T-regulatory cells induce suppression of allergic disease by suppressing IgE and inducing IgG4. We have previously suggested[9] that an up-regulation of T-regulatory cells may induce an increase in both IgG4 and toxic reactions in blood leukocytes, as a result of T-regulatory cell activity, and these phenomena might represent the pathogenetic bases of intolerance. The regulation state of T-regulatory cells may be the key point: whereas an allergic reaction may depend on a down-regulation of T-regulatory cells, an intolerance reaction may result from an up- regulation of T-regulatory cells.
The outcome of this preliminary trial seem to suggest that elimination diet, based on moderate-severe reactions of the used leukocytotoxic test, can be effective in reducing the severity of GERD symptoms in patients not responders or partially responders to PPI therapy. Since test-based exclusion diets are very cost-effective and lack of serious adverse events, they could be considered as a possible therapeutic approach in GERD when PPI are not effective or not indicated.
Gastro-esophageal reflux disease (GERD) is a very common condition characterized by heartburn and acid regurgitation, with a chronic relapsing disease course. Although it is known to be caused by the abnormal contact between esophageal mucosa and gastric acid secretions, the ethiopathogenesis of this illness remain unknown. We had previously hypothesized a possible role of food intolerance, based on the results of leukocytotoxic tests. Patients younger than 50 years, and free from conditions configuring high risk, can be simply treated on the basis of their symptoms with proton pump inhibitors (PPI); however, there is a proportion of patients considered non-responders or partially responders who could benefit from new therapeutic approaches.
The research hotspot is finding a therapeutic option for GERD patients who do not respond to PPI.
Leukocytotoxic test for food intolerance is one of the available methods to test the presence of intolerance towards a panel of food substances. It is considered to have poor specificity and sensitivity, but this problem might be overcome by considering only the moderate-strong positive reactions. In this way authors produced “true” elimination diets, free from the foods giving moderate-severe positive reactions, and “control” diets, free from foods giving no reactions. They found that GERD patients following the “true” diet had a significant improvement in symptoms compared with “control” group.
Elimination diet, based on moderate-severe reactions of the leukocytotoxic test might be effective in reducing the severity of GERD symptoms in patients not responders/partially responders to PPI treatment. Being very cost-effective, and free from serious adverse event, elimination diet might be considered as a possible therapeutic approach in GERD when PPI are not effective or not indicated.
Leukocytotoxic test is based on the optical evaluation of leukocytes in a blood sample that has come into direct contact with the food substances. On the basis of leukocytes observation, degrees of reaction to the food extracts are rated according to the state of the leukocytes, which react by swelling, then developing vacuoles, and finally deteriorating, and to the total number of leukocytes that react. Elimination diets are diets produced by excluding from the alimentary habits of the patient specific food items. In the study “true” diets excluded food substances giving moderate-severe positive reaction, while “control” diets excluded foods giving no reactions at the leukocytotoxic test.
The authors investigated the effect of the clinical response of GERD symptoms to exclusion diets based on food intolerance tests in a double blind, randomized, controlled pilot trial in patients who were partially or completely non-responders to PPI treatment. They suggest that food intolerance may play a part in GERD symptoms development and leucocytotoxic test-based exclusion diets may be a potential therapeutic approach when PPI therapy is less effective. With the limitation that the test used for food sensitivity is of questionable utility, this is certainly an interesting study. It is also fascinating that both groups, those that eliminated reactive foods and those that eliminated un-reactive foods, showed improvement in their GERD symptoms, indicating a role of placebo effect.
P- Reviewer: Herszenyi L, Parker W S- Editor: Ma N L- Editor: A E- Editor: Ma S
| 1. | Dent J, El-Serag HB, Wallander MA, Johansson S. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2005;54:710-717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1256] [Cited by in F6Publishing: 1200] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 2. | Wiklund I. Review of the quality of life and burden of illness in gastroesophageal reflux disease. Dig Dis. 2004;22:108-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 139] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Ofman JJ, Shaw M, Sadik K, Grogg A, Emery K, Lee J, Reyes E, Fullerton S. Identifying patients with gastroesophageal reflux disease: validation of a practical screening tool. Dig Dis Sci. 2002;47:1863-1869. [PubMed] [Cited in This Article: ] |
| 4. | Spechler SJ. Barrett esophagus and risk of esophageal cancer: a clinical review. JAMA. 2013;310:627-636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 170] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 5. | Lada MJ, Nieman DR, Han M, Timratana P, Alsalahi O, Peyre CG, Jones CE, Watson TJ, Peters JH. Gastroesophageal reflux disease, proton-pump inhibitor use and Barrett’s esophagus in esophageal adenocarcinoma: Trends revisited. Surgery. 2013;154:856-864; discussion 864-866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108:308-328; quiz 329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1136] [Cited by in F6Publishing: 1015] [Article Influence: 92.3] [Reference Citation Analysis (0)] |
| 7. | Kahrilas PJ, Shaheen NJ, Vaezi MF, Hiltz SW, Black E, Modlin IM, Johnson SP, Allen J, Brill JV. American Gastroenterological Association Medical Position Statement on the management of gastroesophageal reflux disease. Gastroenterology. 2008;135:1383-1391, 1391.e1-5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 438] [Cited by in F6Publishing: 371] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 8. | Chassany O, Shaheen NJ, Karlsson M, Hughes N, Rydén A. Systematic review: symptom assessment using patient-reported outcomes in gastroesophageal reflux disease and dyspepsia. Scand J Gastroenterol. 2012;47:1412-1421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Caselli M, Zeni E, Lo Cascio N, Alvisi V, Stanghellini V. A possible role of food intolerance in the pathogenesis of gastroesophageal reflux disease. Am J Gastroenterol. 2009;104:2115-2117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Stapel SO, Asero R, Ballmer-Weber BK, Knol EF, Strobel S, Vieths S, Kleine-Tebbe J. Testing for IgG4 against foods is not recommended as a diagnostic tool: EAACI Task Force Report. Allergy. 2008;63:793-796. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 11. | Jones R, Coyne K, Wiklund I. The gastro-oesophageal reflux disease impact scale: a patient management tool for primary care. Aliment Pharmacol Ther. 2007;25:1451-1459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Atkinson W, Sheldon TA, Shaath N, Whorwell PJ. Food elimination based on IgG antibodies in irritable bowel syndrome: a randomised controlled trial. Gut. 2004;53:1459-1464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 347] [Cited by in F6Publishing: 329] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 13. | Zar S, Mincher L, Benson MJ, Kumar D. Food-specific IgG4 antibody-guided exclusion diet improves symptoms and rectal compliance in irritable bowel syndrome. Scand J Gastroenterol. 2005;40:800-807. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Aydinlar EI, Dikmen PY, Tiftikci A, Saruc M, Aksu M, Gunsoy HG, Tozun N. IgG-based elimination diet in migraine plus irritable bowel syndrome. Headache. 2013;53:514-525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Dobrilla G, Zancanella I, Benvenuti S, Comberlato M, Amplatz S, Di Fede F, De Guelmi A. [Diet and drugs in the therapy of nonorganic dyspepsia: the hypothesis and factual data]. Minerva Gastroenterol Dietol. 1996;42:71-82. [PubMed] [Cited in This Article: ] |
| 16. | Haller D, Antoine JM, Bengmark S, Enck P, Rijkers GT, Lenoir-Wijnkoop I. Guidance for substantiating the evidence for beneficial effects of probiotics: probiotics in chronic inflammatory bowel disease and the functional disorder irritable bowel syndrome. J Nutr. 2010;140:690S-697S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Meiler F, Klunker S, Zimmermann M, Akdis CA, Akdis M. Distinct regulation of IgE, IgG4 and IgA by T regulatory cells and toll-like receptors. Allergy. 2008;63:1455-1463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 217] [Article Influence: 13.6] [Reference Citation Analysis (0)] |