Published online Nov 7, 2014. doi: 10.3748/wjg.v20.i41.15423
Revised: March 27, 2014
Accepted: July 22, 2014
Published online: November 7, 2014
Processing time: 332 Days and 19.4 Hours
AIM: To compare the safety of fast-track rehabilitation protocols (FT) and conventional care strategies (CC), or FT and laparoscopic surgery (LFT) and FT and open surgery (OFT) after gastrointestinal surgery.
METHODS: We searched MEDLINE, WHO International Trial Register, Embase and The Cochrane Central Register of Controlled Trials up to 2014 for randomized controlled trials (RCTs) comparing FT and CC or comparing LFT and OFT, with 10 or more randomized participants and about 30 d follow-up. Two reviewers independently extracted data on complications, anastomotic leak, obstruction, wound infection, re-admission between FT and CC or LFT and OFT after gastrointestinal surgery.
RESULTS: Twenty-four RCTs of FT vs CC or LFT vs OFT were included. Compared with CC, FT reduced overall complications and wound infection. However, anastomotic leak, obstruction and re-admission were not significantly reduced. The pooled risk ratio (RR) of 0.69 (95%CI: 0.60-0.78; P < 0.001), pooled RR of 0.71 (95%CI: 0.57-0.88; P < 0.001), pooled RR of 0.93 (95%CI: 0.68-1.25; P > 0.05), a pooled RR of 0.87 (95%CI: 0.67-1.15; P > 0.05) and pooled RR of 0.94 (95%CI: 0.73-1.22; P > 0.05) respectively. Compared with OFT, LFT reduced complications, with a pooled RR of 0.66 (95%CI: 0.54-0.81; P < 0.001).
CONCLUSION: FTs are safe after gastrointestinal surgery. Additional large, prospective RCTs should be conducted to establish further the safety of this approach.
Core tip: Fast-track rehabilitation protocols (FT) after gastrointestinal surgery have become the most fashionable method of treatment for gastrointestinal malignancy. Complications after FT for gastrointestinal resection have been discussed in China as well as other countries. This study clarified that compared with conventional care strategies, FT has a low level of complications and similar incidence of re-admission of about 1 mo.
- Citation: Wang LH, Fang F, Lu CM, Wang DR, Li P, Fu P. Safety of fast-track rehabilitation after gastrointestinal surgery: Systematic review and meta-analysis. World J Gastroenterol 2014; 20(41): 15423-15439
- URL: https://www.wjgnet.com/1007-9327/full/v20/i41/15423.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i41.15423
In recent years, fast-track rehabilitation protocols (FT) have frequently appeared in the literature. The concept of FT was first proposed by a Danish surgeon, Kehlet, with the intent to reduce stress, complications, and hospital stay after gastrointestinal surgery[1,2].
FT was investigated initially in the setting of elective gastrointestinal surgery, where it was shown that, by optimizing and standardizing perioperative care, median length of hospital stay could be reduced from 8-12 d to 2-4 d[3-5]. For the surgical treatment of gastrointestinal malignant disease, conventional elective gastrointestinal resection is associated with a complication rate of 10%-45% and a postoperative hospital stay of 8-13 d[7-10]. It has been established that a higher rate of serious postoperative complications is associated with an excessive response to surgical tress[11-13] and that C-reactive protein (CRP), interleukin (IL)-6, tumor necrosis factor (TNF)-α and resting energy expenditure (REE) may act as markers for the severity of the surgical stress response[14-19]. To improve this problem, FT has been developed[20-22]. FT is based on the reduction of surgical stress by various surgical and anesthetic approaches to enhance recovery.
In a prospective study investigating the value of an accelerated recovery program in elective gastrointestinal resection, Grantcharov and Kehlet[23] demonstrated that a number of the principles of FT, such as avoidance of prophylactic nasogastric tubes and abdominal drains, early postoperative feeding, and use of multimodal analgesia, could be applied successfully in this clinical setting without increasing postoperative morbidity[23]. The safety of FT has been fiercely disputed. Therefore, the primary aim of this meta-analysis was to evaluate the safety of FT vs conventional care strategies (CC) or FT and laparoscopic surgery (LFT) vs FT and open surgery (OFT), as measured by the rate of complications, specifically anastomotic leaks, wound infection, obstruction and re-admission. The secondary aim was to understand the difficulties, limitations, or advantages of FT for gastrointestinal surgery.
Relevant studies were identified by searching the following data: MEDLINE (1985 until present), WHO International Trial Register (1985 until present), Embase (1985 until present) and The Cochrane Central Register of Controlled Trials (1985 until present). The medical subject headings (MeSH) and keywords searched for individually and in combination were as follows: “fast track” or “enhanced recovery” and “colorectal and surgery” or ‘‘gastric and surgery”. The last search was done on March 1, 2014. The search was limited to randomized controlled trials (RCTs) with about 30-d follow-up, but without age, sex and weight. Reference lists from identified trials and review articles were manually scanned to identify any other relevant studies. Internet search engines were also used to perform a manual search for abstracts from international meetings, which were then downloaded and studied.
The inclusion criteria for the study were: (1) RCTs; (2) detailed patient information provided; and (3) to be considered FT, a rehabilitation protocol had to include at least seven of the 20 FT items in the FT group (preoperative counseling, preoperative feeding, no premedication, no bowel preparation, fluid restriction, symbiotics administered before surgery, no preoperative fasting but provision of clear carbohydrate-enriched liquids until 2 h before surgery, multi-way anesthetic techniques, high inspired oxygen concentrations, avoidance of perioperative fluid overload, short/transverse incisions, maintenance of body temperature, no routine use of drains, non-opioid analgesia and nasogastric decompression tubes, standard laxatives, early removal of bladder catheters and prokinetics, and early postoperative mobilization and feeding[6]) to achieve early recovery after gastrointestinal surgery, with no more than two CCs. When a study reporting the same patient cohort was included in several publications, only the most recent or complete study was selected.
The exclusion criteria were: (1) case reports; (2) articles that were not full text or were non-comparative studies; (3) more than two CCs were included; and (4) the FT included no more than seven of the 20 FT items.
RCTs met the inclusion criteria if they involved the FT for gastrointestinal malignant disease in adult patients and used CCs for control. Both full-length publications and abstracts were selected. Letters, reviews without original data and animal studies were excluded. If any doubt about suitability remained after the abstract was examined, the full manuscript was obtained.
All included studies were assessed for the quality of their methodology and relevance to the objective of our meta-analysis. Conduct and reporting were in accordance with the QUOROM statement. Data on complications (anastomotic leak, wound infection, obstruction) and readmission from each trial were extracted and compared independently by the two investigators.
To identify potential sources of bias in the reported events, we followed the Cochrane Collaboration’s risk of bias framework[24] and considered for each trial the following risk domains: (1) selection bias (random sequence generation and allocation concealment); (2) performance bias (blinding of participants and study investigators for the outcomes of interest); (3) detection bias (blinding of outcome assessors); (4) attrition bias (incomplete outcome data); and (5) reporting bias (selective outcome reporting). Risk of bias for each domain was categorized as low, unclear, or high. This information was used to make judgments about the overall risk of bias for each trial. We followed the Cochrane Collaboration’s recommendation to make judgments on the basis of whether the ranking of the level of bias across domains could have led to any material bias on the outcomes of interests and, where applicable, what the direction of the bias would likely be[24].
Statistical analysis was conducted using Review Manager version 5.0.0 (Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). Pooled risk ratios (RRs) with 95% confidence intervals (CIs) were used to assess complications (anastomotic leaks, wound infection, and obstruction) and re-admission. Statistical heterogeneity was assessed with a χ2 test, for which P < 0.1 was considered statistically significant. The I2 statistic was used to assess the impact of heterogeneity on the meta-analysis. If the test of heterogeneity was statistically significant, then the random-effects model was used; otherwise, a fixed-effects model was used. Two-sided P values < 0.05 were considered statistically significant. Funnel plots and Egger’s test were used to evaluate publication bias.
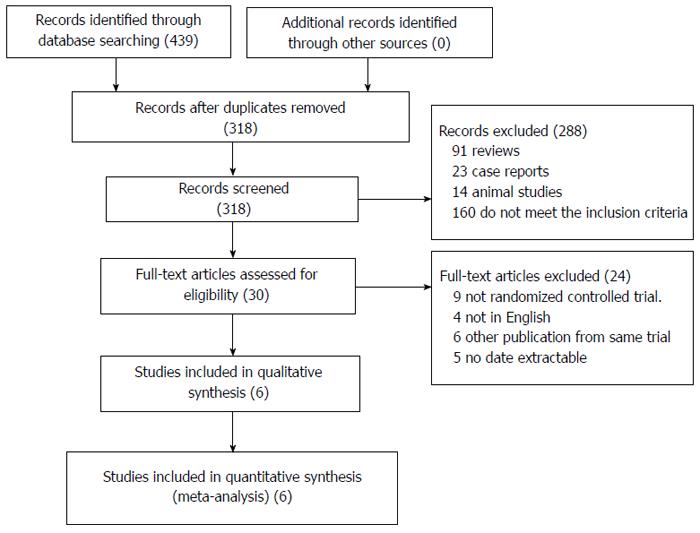
Five hundred and ninety-six references were identified from the medical journal databases. On examination of the abstracts, 485 articles were rejected based on the criteria outlined in Figure 1. Assessment of the complete text of the 87 remaining articles led to the elimination of 25 papers that contained no data pertaining to the outcome of LFT for colorectal resection, and 24 that were not RCTs. Nine had fewer than seven items of FT, 12 papers were of animal studies, and 17 papers explained the effect of analgesia. The remaining 24 nonduplicated RCTs that compared FT with CC were included in the meta-analysis.
Characteristics of the 24 RCTs[2,25-47] included in the meta-analysis are summarized in Table 1. The bias of included studies was low (Table 2). These studies were published between 1985 and 2013 and investigated a total of 3365 patients, 2093 of whom received an FT and 1272 of whom received a CC. Nineteen of the studies compared FT and CC for colorectal resection (2538 patients), 15 of the studies compared OFT and OCC for colorectal resection (1690 patients), eight of the studies compared LFT and LCC for colorectal resection (774 patients), and nine of the studies compared LFT and OFT for colorectal resection (827 patients). Two studies[38,48] published by the same team from the same institute within the same study interval were regarded as one trial, but both studies were included and shared the same study number because some separately published data were complementary.
| Ref. | Way | Year | No. | Age (yr) | Sex (M:F) | |||
| FT | CC | FT | CC | FT | CC | |||
| Ionescu et al[25] | Open | 2009 | 48 | 48 | 60.94 ± 9.9 | 63.1 ± 12.19 | 30:18 | 31:17 |
| Ren et al[26] | Open | 2012 | 299 | 298 | 59 | 61 | 178:121 | 190:108 |
| Yang et al[27] | Open | 2012 | 32 | 30 | 57.2 ± 11.70 | 59.5 ± 12.10 | 20:12 | 22:8 |
| Hübner et al[28] | Open | 2010 | 36 | 31 | 60 | 61 | 18:18 | 17:14 |
| Wang et al[29] | Open | 2012 | 41 | 42 | 57.2 ± 18.1 | 55.4 ± 16.8 | 24:17 | 25:17 |
| Vlug et al[30] | Open | 2011 | 93 | 98 | 66 ± 10.3 | 66 ± 7.1 | 54:39 | 59:39 |
| van Bree et al[31] | Lap | 2011 | 18 | 18 | 64 ± 10.1 | 66 ± 6.9 | 11:7 | 11:7 |
| Veenhof et al[32] | Lap | 2012 | 17 | 20 | 65 | 68 | 9:8 | 14:6 |
| Serclová et al[33] | Lap/open | 2009 | 51 | 52 | 35.1 ± 11.0 | 37.6 ± 12.5 | 20:31 | 32:20 |
| Muller et al[34] | Lap/open | 2009 | 76 | 75 | 62 | 59 | 37:39 | 40:35 |
| Wang et al[35] | Lap/open | 2011 | 40 | 38 | 71 | 72 | 22:18 | 20:18 |
| Wang et al[36] | Lap/open | 2011 | 106 | 104 | 57 | 55 | 65:41 | 60:44 |
| King et al[38] | Lap/open | 2008 | 41 | 19 | 72.3 | 70.4 | 23:18 | 8:11 |
| Faiz et al[37] | Lap/open | 2008 | 191 | 50 | 67.9 ± 14.1 | 66.3 ± 13.7 | 20:30 | 98:93 |
| Srinivasa et al[39] | Lap/open | 2013 | 37 | 37 | 69 ± 16 | 72 ± 12 | 19:18 | 22:15 |
| Basse et al[40] | Lap/open | 2005 | 30 | 30 | 75.5 | 75 | 14:16 | 14:16 |
| MacKay et al[41] | Lap/open | 2006 | 22 | 58 | 72 | 73.2 | 12:10 | 25:33 |
| García-Botello et al[42] | Lap/open | 2011 | 61 | 58 | 62 | 60 | 40:21 | 32:26 |
| Anderson et al[43] | Open | 2003 | 14 | 11 | 64 | 68 | 6:86 | 5:6 |
| Gatt et al[44] | Open | 2005 | 19 | 20 | 67 | 67 | 14:6 | 9:109 |
| Khooet al[2] | Open | 2007 | 35 | 35 | 69.3 | 73 | 12:23 | 15:20 |
| Wang et al[45] | Open | 2010 | 47 | 45 | 58.76 ± 9.66 | 56.87 ± 9.16 | 32:133 | 29:18 |
| Chen Hu et al[46] | Lap/open | 2012 | 40 | 42 | 59/64 | 62.5/64.5 | 19:21 | 22:20 |
| Lemanu et al[47] | Lap | 2012 | 40 | 38 | 43.5 | 43.9 | 13:27 | 10:28 |
| Ref. | Selection bias | Performance bias | Detection bias | Attrition bias | Reporting | Overall risk of bias |
| Ionescu et al[25] | Low | Low | Low | Low | Low | Low |
| Ren et et al[26] | Low | Unclear | Low | Low | Low | Low |
| Yang et al[27] | Low | Low | Low | Unclear | Low | Low |
| Hübner et al[28] | Low | Low | Low | Low | Low | Low |
| Wang et al[29] | Low | Low | Low | Low | Unclear | Low |
| Vlug et al[30] | Unclear | Low | Low | Low | Low | Low |
| van et al[31] | Low | Low | Low | Low | Unclear | Low |
| Veenhof et al[32] | Low | Low | Unclear | Low | Low | Low |
| Serclová et al[33] | Low | Low | Low | Low | Unclear | Low |
| Muller et al[34] | Low | Low | Low | Low | Unclear | Low |
| Wang et al[35] | Unclear | Low | Low | Low | Low | Low |
| Wang et al[36] | Unclear | Low | Low | Low | Low | Low |
| King et al[38] | Low | Low | Unclear | Low | Low | Low |
| Faiz et al[37] | Low | Low | Low | Low | Unclear | Low |
| Srinivasa et al[39] | Low | Unclear | Low | Low | Low | Low |
| Basse et al[40] | Unclear | Low | Low | Low | Low | Low |
| MacKay et al[41] | Low | Low | Low | Low | Low | Low |
| García-Botello et al[42] | Low | Low | Low | Low | Unclear | Low |
| Anderson et al[43] | Low | Low | Low | Low | Low | Low |
| Gatt et al[44] | Low | Unclear | Low | Low | Low | Low |
| Khoo et al[2] | Low | Low | Low | Low | Low | Low |
| Wang et al[45] | Low | Low | Low | Low | Low | Low |
| Chen Hu et al[46] | Low | Low | Low | Low | Low | Low |
| Lemanu et al[47] | Low | Low | Low | Low | Unclear | Low |
Complications: Compared with CC, FT reduced complications. The pooled RR was 0.69 (95%CI: 0.60-0.78; P < 0.001), χ2 = 95.37 (P < 0.001) and I2 = 57% (Figure 2A, Table 3).
| Outcome or subgroup | Studies(n) | Participants(n) | Effect estimateRR (95%CI) | heterogeneity | |
| I2 | P value | ||||
| 1.1 Complication | |||||
| 1.1.1 FT vs CC | 19 | 2538 | 0.67 (0.56, 0.82) | 58% | 0.0009 |
| 1.1.2 OFT vs OCC | 15 | 1690 | 0.73 (0.58, 0.93) | 57% | 0.003 |
| 1.1.3 LFT vs LCC | 8 | 774 | 0.58 (0.38, 0.88) | 62% | 0.01 |
| 1.1.4 LFT vs OFT | 8 | 586 | 0.72 (0.56, 0.92) | 24% | 0.24 |
| 1.2 Anastomotic leak | |||||
| 1.2.1 FT vs CC | 11 | 1939 | 0.92 (0.60, 1.43) | 0% | 0.96 |
| 1.2.2 OFT vs OCC | 9 | 1364 | 0.90 (0.53, 1.53) | 0% | 0.90 |
| 1.2.3 LFT vs LCC | 5 | 575 | 0.98 (0.48, 2.01) | 0% | 0.60 |
| 1.2.4 LFT vs OFT | 6 | 626 | 0.83 (0.40, 1.73) | 0% | 0.78 |
| 1.3 obstruction | |||||
| 1.3.1 FT vs CC | 9 | 1698 | 0.87 (0.59, 1.29) | 0% | 0.96 |
| 1.3.2 OFT vs OCC | 7 | 1160 | 0.97 (0.62, 1.52) | 0% | 1.00 |
| 1.3.3 LFT vs LCC | 4 | 539 | 0.67 (0.32, 1.42) | 0% | 0.62 |
| 1.3.4 LFT vs OFT | 3 | 295 | 1.23 (0.51, 3.00) | 0% | 0.40 |
| 1.4 Wound infection | |||||
| 1.4.1 FT vs CC | 14 | 2133 | 0.72 (0.52, 0.97) | 10% | 0.34 |
| 1.4.2 OFT vs OCC | 12 | 1461 | 0.72 (0.51, 1.02) | 28% | 0.18 |
| 1.4.3 LFT vs LCC | 5 | 539 | 0.64 (0.32, 1.26) | 0% | 0.91 |
| 1.4.4 LFT vs OFT | 4 | 329 | 0.51 (0.26, 1.01) | 35% | 0.20 |
| 1.5 re-admission | |||||
| 1.5.1 FT vs CC | 11 | 1468 | 0.99 (0.71, 1.39) | 0% | 0.80 |
| 1.5.2 OFT vs OCC | 8 | 781 | 1.07 (0.60, 1.91) | 0% | 0.85 |
| 1.5.3 LFT vs LCC | 5 | 613 | 0.74 (0.43, 1.28) | 0% | 0.82 |
| 1.5.4 LFT vs OFT | 6 | 671 | 0.45 (0.29, 0.71) | 14% | 0.32 |
Nineteen studies (2538 patients) provided data on complications for FT vs CC; 26.7% (338/1266 patients) had complications in the FT group and 36.4% (463/1272) in the CC group. Pooling the results indicated that FT significantly reduced complications compared with CC. The weighted mean difference (WMD) was 0.67 (95%CI: 0.56-0.82, P < 0.001), χ2 = 42.76 (P = 0.0009) and I2 = 58%, indicating heterogeneity among the studies.
Fifteen studies (1690 patients) provided data on complications for OFT vs OCC; 25% (211/847 patients) had complications in the OFT group and 32.6% (275/843) in the OCC group. Pooling the results indicated that OFT significantly reduce complications compared with OCC. The WMD was 0.73 (95%CI: 0.58-0.93; P < 0.05), χ2 = 32.76 (P = 0.003) and I2 = 57%, indicating heterogeneity among the studies.
Eight studies (774 patients) provided data on complications for LFT vs LCC; 26.5% (101/382 patients) had complications in the LFT group and 34% (133/392) in the LCC group. Pooling the results indicated that LFT significantly reduce complications compared with LCC. The WMD was 0.58 (95%CI: 0.38-0.88; P < 0.05), χ2 = 18.33 (P = 0.01) and I2 = 62%, indicating heterogeneity among the studies.
Eight studies (586 patients) provided data on complications for LFT vs OFT; 23.9% (69/289 patients) had complications in the LFT group and 33% (98/297) in the OFT group. Pooling the results indicated that LFT significantly reduce complications compared with OFT. The WMD was 0.72 (95%CI: 0.56-0.92; P < 0.05), χ2 = 9.16 (P = 0.01) and I2 =24%, which excludes heterogeneity among the studies.
Anastomotic leak: Compared with CC, FT reduced anastomotic leaks. The pooled RR was 0.93 (95%CI: 0.68-1.25; P > 0.05), χ2 = 9.95 (P = 0.99) and I2 = 0% (Figure 2B, Table 3).
Eleven studies (1939 patients) provided data on anastomotic leaks for FT vs CC; 3.7% (36/966 patients) had anastomotic leaks in the FT group and 4.1% (40/973) in the CC group. Pooling the results indicated that FT did not significantly reduce anastomotic leaks compared with CC. The WMD was 0.92 (95%CI: 0.60-1.43, P > 0.05), χ2 = 3.68 (P = 0.96) and I2 = 0%, which excludes heterogeneity among the studies.
Nine studies (1364 patients) provided data on anastomotic leaks for OFT vs OCC; 3.4% (23/683 patients) had complications in the OFT group and 3.8% (26/681) in the OCC group. Pooling the results indicated that OFT did not significantly reduce anastomotic leaks compared with OCC. The WMD was 0.90 (95%CI: 0.53-1.53, P > 0.05), χ2 = 3.44 (P = 0.90) and I2 = 0%, which excludes heterogeneity among the studies.
Five studies (575 patients) provided data on anastomotic leaks for LFT vs LCC; 4.6% (13/283 patients) had anastomotic leaks in the LFT group and 4.8% (14/292) in the LCC group. Pooling the results indicated that LFT did not significantly reduce anastomotic leaks compared with LCC. The WMD was 0.98 (95%CI: 0.48-2.01, P > 0.05), χ2 = 2.73 (P = 0.60) and I2 = 0%, which excludes heterogeneity among the studies.
Six studies (626 patients) provided data on anastomotic leaks for LFT vs OFT; 3.8% (15/399 patients) had anastomotic leaks in the LFT group and 5.3% (12/227) in the OFT group. Pooling the results indicated that LFT significantly reduce anastomotic leaks compared with OFT. The WMD was 0.83 (95%CI: 0.40-1.73, P > 0.05), χ2 = 2.45 (P = 0.78) and I2 = 0%, which excludes heterogeneity among the studies.
Wound infection: Compared with CC, FT reduced wound infection. The pooled RR was 0.71 (95%CI: 0.57-0.88, P < 0.001), χ2 = 29.43 (P = 0.44) and I2 = 1% (Figure 2C, Table 3).
Fourteen studies (2133 patients) provided data on wound infection for FT vs CC; 5.7% (61/1062 patients) had wound infection in the FT group and 8.1% (87/1071) in the CC group. Pooling the results indicated that FT did not significantly reduce wound infection compared with CC. The WMD was 0.72 (95%CI: 0.52-0.97, P < 0.05), χ2 = 14.43 (P = 0.34) and I2 =10%, which excludes heterogeneity among the studies.
Twelve studies (1461 patients) provided data on wound infection for OFT vs OCC; 6.5% (47/731 patients) had wound infection in the OFT group and 9.0% (66/730) in the OCC group. Pooling the results indicated that OFT did not significantly reduce wound infection compared with OCC. The WMD was 0.72 (95%CI: 0.51-1.02, P > 0.05), χ2 = 13.87 (P = 0.18) and I2 = 28%, which excludes heterogeneity among the studies.
Five studies (580 patients) provided data on wound infection for LFT vs LCC; 4.2% (12/284 patients) had wound infection in the LFT group and 6.8% (20/296) in the LCC group. Pooling the results indicated that LFT did not significantly reduce wound infection compared with LCC. The WMD was 0.64 (95%CI: 0.32-1.26, P > 0.05), χ2 = 0.97 (P = 0.91) and I2 = 0%, which excludes heterogeneity among the studies.
Four studies (329 patients) provided data on wound infection for LFT vs OFT; 6.5% (11/168 patients) had wound infection in the LFT group and 13% (21/161) in the OFT group. Pooling the results indicated that LFT significantly reduce wound infection compared with OFT. The WMD was 0.51 (95%CI: 0.26-1.01, P = 0.05), χ2 = 4.6 (P = 0.20) and I2 =35%, which excludes heterogeneity among the studies.
Obstruction: Compared with CC, FT reduced obstruction. The pooled RR was 0.87 (95%CI: 0.67-1.15, P > 0.05), χ2 = 5.17 (P = 1.00) and I2 = 0% (Figure 2D, Table 3).
Nine studies (1698 patients) provided data on obstruction for FT vs CC; 5.1% (43/844 patients) had obstruction in the FT group and 5.9% (50/854) in the CC group. Pooling the results indicated that FT did not significantly reduce obstruction compared with CC. The WMD was 0.87 (95%CI: 0.59-1.29, P = 0.05), χ2 = 2.58 (P = 0.96) and I2 = 0%, which excludes heterogeneity among the studies.
Seven studies (1160 patients) provided data on obstruction for OFT vs OCC; 5.7% (33/579 patients) had obstruction in the OFT group and 5.9% (34/581) in the OCC group. Pooling the results indicated that OFT did not significantly reduce obstruction compared with OCC. The WMD was 0.97 (95%CI: 0.62-1.52; P > 0.05), χ2 = 0.33 (P = 1.00) and I2 = 0%, which excludes heterogeneity among the studies.
Four studies (539 patients) provided data on obstruction for LFT vs LCC; 3.8% (10/265 patients) had obstruction in the LFT group and 5.8% (16/274) in the LCC group. Pooling the results indicated that LFT did not significantly reduce obstruction compared with LCC. The WMD was 0.67 (95%CI: 0.32-1.42; P > 0.05), χ2 = 1.78 (P = 0.62) and I2 = 0%, which excludes heterogeneity among the studies.
Three studies (295 patients) provided data on obstruction for LFT vs OFT; 6.7% (10/149 patients) had obstruction in the LFT group and 5.5% (8/146) in the OFT group. Pooling the results indicated that LFT significantly reduce obstruction compared with OFT. The WMD was 1.23 (95%CI: 0.51-3.00; P > 0.05), χ2 = 1.86(P = 0.40) and I2 = 0%, which excludes heterogeneity among the studies.
Re-admission: Compared with CC, FT reduced re-admission The pooled RR was 0.94 (95%CI: 0.73-1.22, P > 0.05), χ2 = 11.83 (P = 0.97) and I2 = 0% (Figure 2E, Table 3).
Eleven studies (1468 patients) provided data on readmission for FT vs CC; 7.9% (58/731 patients) had readmission in the FT group and 8% (59/737) in the CC group. Pooling the results indicated that FT did not significantly reduce re-admission compared with CC. The WMD was 0.99 (95%CI: 0.71-1.39, P = 0.05), χ2 = 6.15 (P = 0.80) and I2 = 0%, which excludes heterogeneity among the studies.
Eight studies (781 patients) provided data on readmission for OFT vs OCC; 5.6% (22/390 patients) had obstruction in the OFT group and 5.4% (21/391) in the OCC group. Pooling the results indicated that OFT did not significantly reduce re-admission compared with OCC. The WMD was 1.07 (95%CI: 0.60-1.91, P > 0.05), χ2 = 3.37 (P = 0.85) and I2 = 0%, which excludes heterogeneity among the studies.
Five studies (613 patients) provided data on re-admission for LFT vs LCC; 6.6% (20/304 patients) had re-admission in the LFT group and 8.7% (27/309) in the LCC group. Pooling the results indicated that LFT did not significantly reduce re-admission compared with LCC. The WMD was 0.74 (95%CI: 0.43-1.28, P > 0.05), χ2 = 1.5 5 (P = 0.82) and I2 = 0%, which excludes heterogeneity among the studies.
Six studies (671 patients) provided data on re-admission for LFT vs OFT; 6.7% (27/420 patients) had re-admission in the LFT group and 5.5% (35/251) in the OFT group. Pooling the results indicated that LFT significantly reduced re-admission compared with OFT. The WMD was 0.45 (95%CI: 0.29-0.71; P < 0.001), χ2 = 5.83 (P = 0.32) and I2 =14%, which excludes heterogeneity among the studies.
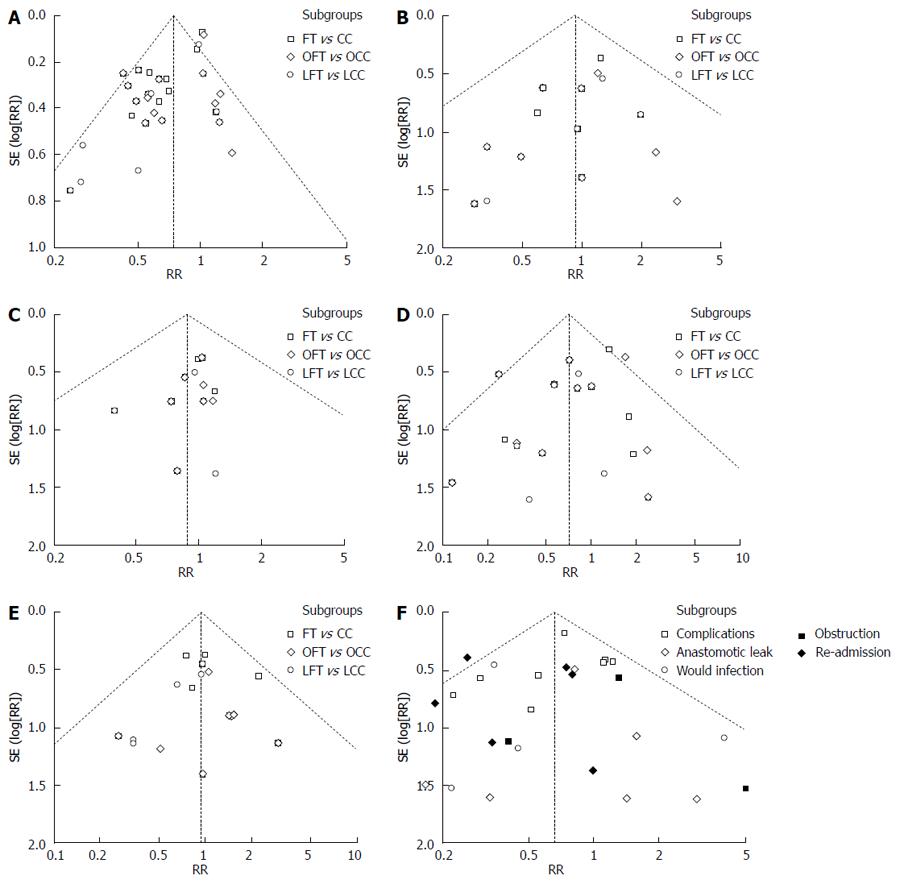
A funnel plot was created to access the publication bias of the literature. The shapes of the funnel plots did not reveal any evidence of obvious asymmetry (Figures 2F and 3).
The present meta-analysis showed that compared with CC, FT reduced complications and re-admission, and had a similar probability of anastomotic leaks, wound infection and obstruction. Compared with OFT, LFT reduced complications and re-admission, the probability of anastomotic leaks, wound infection and obstruction are similar.
The safety of FT after gastrointestinal surgery has been discussed in worldwide. A recent multivariate analysis demonstrated that male sex[49], preoperative education, psychological counseling, anesthesia[50], early postoperative oral nutrition[51] and quality of care were potential risk factors for complications after gastrointestinal surgery. In addition, some studies have found an increased risk of anastomotic leaks in men and 10.1% of the men required reoperation for anastomotic leak vs 3.3% of women[52-55].
Preoperative education and psychological counseling are crucial factors for FT. It is necessary to introduce the detailed treatment program, different steps of FT, and relevant measures, and ease the psychological pressure in order to help patients better understand and coordinate the FT.
Better cooperation of patients can bring better outcomes of FT. Generally, the gastric emptying time of solid meal and fluid is 6 h and 2 h, respectively[56]. Moderate activity after surgery may enhance recover and reduce complications. The patients should be encouraged to have a liquid meal 2 h before the operation instead of fasting. It has been shown that preoperative oral carbohydrate is safe and can efficiently reduce complications[57-59].
The role of epidural or regional anesthesia in FT should be stressed. Postoperative epidural analgesia can avoid stress-induced neurological, endocrinological and homeostatic changes or the blocking of sympathetic nerve-related surgical stress response, reduce postoperative complications such as nausea, vomiting and enteroparalysis, increase early ambulation, improve intestinal function, and shorten hospital stay after resection of gastrointestinal cancer[49,60-65].
Early postoperative oral nutrition is regarded as an essential part of FT. Food intake can stimulate gastrointestinal peristalsis, and early feeding during the first 24 h after surgery promotes recovery of obstruction. It has been illustrated that early postoperative oral nutrition attenuates catabolism and potentially decreases infectious complications[50,66].
FT can improve the rehabilitation of patients after resection of gastrointestinal cancer better than CC can, thus benefiting surgery, anesthesia, pain management, physical therapy, and social work. The primary work of FT is the preoperative education of patients to make them understand the whole plan and the aim of each stage. Therefore, it is necessary to obtain cooperation from nurses.
The pathophysiological mechanisms involved in postoperative obstruction are still not completely understood, but recent studies have stressed the importance of inflammation of the intestinal muscularis resulting from handling during surgery[67-70]. The faster clinical recovery observed after laparoscopic surgery compared with open surgery could be explained by decreased tissue trauma with concomitantly decreased mast cell activation, leading to attenuated intestinal inflammation and thus quicker gastrointestinal recovery[67,68,70]. The mechanisms behind the beneficial effect of FT remain unclear.
Several cytokines, such as IL-6, TNF-α and CRP, are involved in the response to surgical stress and are therefore useful serum markers for evaluating the severity of surgery-induced stress[14-17].
CRP is a nonspecific acute phase protein produced by the liver following trauma or inflammation. Serum CRP level is closely associated with trauma and stress[71]; therefore, measurement of postoperative CRP may reflect the degree of trauma caused by a surgical procedure. The CRP level after colon surgery with LFT was significantly lower than that after other strategies[29], demonstrating that LFT is less traumatic for patients and reduces stress in the perioperative period, protecting the postoperative immune system. IL-6 is produced and activated by monocytes, macrophages and endothelial cells under conditions of surgical trauma and stress. IL-6 levels are positively correlated with the severity of surgical trauma[15]. The increase in serum IL-6 was less after colon surgery with LFT29, suggesting that a suitable surgical mode combined with better perioperative care can lead to less surgical trauma and better prognosis. Surgical trauma causes marked metabolic changes, and REE also acts as the marker for surgical stress[18,19]. The REE rate of patients from the FT group was lower than that in the conventional surgery group, particularly on postoperative days 1 and 3.
Postsurgical complications in patients who underwent FT for colorectal diseases were treated without specific side effects or complications. Compared with CC, FT greatly reduced complications, and no other side effects were found. FT is safe and feasible. Compared with OFT, LTF significantly reduced complications, in addition to reducing hospitalization time and improving quality of life. LFT is not yet practiced widely, but we believe that it will become increasingly popular. Further large studies with more stringent quality criteria may improve the statistical power and confirm that LFT programs reduce morbidity and promote recovery. We believe that FT is significantly advantageous over other procedures for patients after resection of gastrointestinal malignant disease.
There have been eight previous systematic reviews, including meta-analyses on this topic[72-79]. These included three reviews of controlled clinical trials and RCTs[72,74,75], and five reviews of RCTs only[73,76-79]. The present study is the first meta-analysis to have compared FT vs CC or OFT vs OCC or LFT vs LCC or LFT vs OFT in resection for gastrointestinal malignancy, and the first meta-analysis of patients undergoing elective gastrointestinal surgery to demonstrate that FT is associated with a significant reduction in postoperative complications but not re-admission rates. The quality of evidence from the present study was supported by the increased number of included studies. In our study, reports from all trials had previously been subject to external peer review, and the risk of bias in these trials for the outcomes of interest was judged to be low in our assessments (Table 2).
There were several limitations to the present meta-analysis. First, the sample size of some of the studies was low, as was the number of studies included in our meta-analysis. Data extraction and analyses were not blinded to the authors, journals or institutions of the publications; however, the literature screening and data extraction were conducted independently by two investigators, and thus, the selection bias was unlikely. Second, the funnel plots were asymmetrical for some outcomes, which indicated the existence of publication bias. However, other factors such as study size and clinical and statistical heterogeneity may also cause an asymmetric funnel in the present meta-analysis. Finally, the included studies did not adequately evaluate hospital costs and quality of life after surgery, which are important outcomes for patients undergoing elective colorectal surgery.
In conclusion, this study provides a more detailed assessment of the potential effects of FT than has previously been possible. We were unable to confirm the large proportional reduction in risk suggested by some previous studies. However, more modest but perhaps clinically worthwhile reduction of complications in some or all types of patient cannot be ruled out. Implementation of FT and the maintenance of compliance may require collaboration and communication between dietitians, nurses, surgeons, anesthesiologists, and patients. Additional RCTs of FT with long-term follow-up are necessary to assess hospital costs and quality of life after surgery. Future studies may assess the benefits of FT in elderly patients and patients having other gastrointestinal surgery.
Fast-track rehabilitation protocols (FP) have become the most fashionable method of treatment for gastrointestinal malignancy. Complications of FP after gastrointestinal resection have been discussed in China as well as other countries.
Over the past three decades, many studies have assessed the performance of FT. However, comparisons of FT and conventional care strategies (CC) or FT and laparoscopic surgery and FT and open surgery after gastrointestinal surgery have not been published.
Based on this meta-analysis, FT for gastrointestinal malignancy is safe and efficacious. Similar associations were indicated in subgroup analyses of East Asian, Western, cohort, and high-quality studies. These findings were not presented clearly in previous systematic reviews.
FT appears to be neither directly nor indirectly associated with the risk. Further studies should seek to clarify this conclusion.
FT is rapidly becoming the focal point of attraction for specialists worldwide. This article shows the advantages of the procedure. This analysis has great practical value for clinicians
P- Reviewer: Awad RA, Lee HW, Mello ELR, Ozkan OV, Perse M S- Editor: Ma YJ L- Editor: Kerr C E- Editor: Wang CH
| 1. | Wilmore DW, Kehlet H. Management of patients in fast track surgery. BMJ. 2001;322:473-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 539] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 2. | Khoo CK, Vickery CJ, Forsyth N, Vinall NS, Eyre-Brook IA. A prospective randomized controlled trial of multimodal perioperative management protocol in patients undergoing elective colorectal resection for cancer. Ann Surg. 2007;245:867-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 306] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 3. | Kehlet H, Dahl JB. Anaesthesia, surgery, and challenges in postoperative recovery. Lancet. 2003;362:1921-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 947] [Cited by in RCA: 919] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 4. | Kehlet H. Fast-track colonic surgery: status and perspectives. Recent Results Cancer Res. 2005;165:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Kehlet H. Fast-track colorectal surgery. Lancet. 2008;371:791-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 427] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 6. | Lassen K, Soop M, Nygren J, Cox PB, Hendry PO, Spies C, von Meyenfeldt MF, Fearon KC, Revhaug A, Norderval S, Ljungqvist O, Lobo DN, Dejong CH; enhanced Recovery after surgery (ERAS) Group. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg. 2009;144:961-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 752] [Cited by in RCA: 799] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 7. | Lee SI, Choi YS, Park DJ, Kim HH, Yang HK, Kim MC. Comparative study of laparoscopy-assisted distal gastrectomy and open distal gastrectomy. J Am Coll Surg. 2006;202:874-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 132] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 8. | Rohde H, Bauer P, Stützer H, Heitmann K, Gebbensleben B. Proximal compared with distal adenocarcinoma of the stomach: differences and consequences. German Gastric Cancer TNM Study Group. Br J Surg. 1991;78:1242-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Carrère N, Seulin P, Julio CH, Bloom E, Gouzi JL, Pradère B. Is nasogastric or nasojejunal decompression necessary after gastrectomy? A prospective randomized trial. World J Surg. 2007;31:122-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Yoo CH, Son BH, Han WK, Pae WK. Nasogastric decompression is not necessary in operations for gastric cancer: prospective randomised trial. Eur J Surg. 2002;168:379-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg. 2002;183:630-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1195] [Cited by in RCA: 1157] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 12. | Goris RJ. MODS/SIRS: result of an overwhelming inflammatory response? World J Surg. 1996;20:418-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Sato N, Koeda K, Ikeda K, Kimura Y, Aoki K, Iwaya T, Akiyama Y, Ishida K, Saito K, Endo S. Randomized study of the benefits of preoperative corticosteroid administration on the postoperative morbidity and cytokine response in patients undergoing surgery for esophageal cancer. Ann Surg. 2002;236:184-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 141] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 14. | Raeburn CD, Sheppard F, Barsness KA, Arya J, Harken AH. Cytokines for surgeons. Am J Surg. 2002;183:268-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Cruickshank AM, Fraser WD, Burns HJ, Van Damme J, Shenkin A. Response of serum interleukin-6 in patients undergoing elective surgery of varying severity. Clin Sci (Lond). 1990;79:161-165. [PubMed] |
| 16. | Bianchi RA, Silva NA, Natal ML, Romero MC. Utility of base deficit, lactic acid, microalbuminuria, and C-reactive protein in the early detection of complications in the immediate postoperative evolution. Clin Biochem. 2004;37:404-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Molter GP, Soltész S, Kottke R, Wilhelm W, Biedler A, Silomon M. [Procalcitonin plasma concentrations and systemic inflammatory response following different types of surgery]. Anaesthesist. 2003;52:210-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Long CL, Schaffel N, Geiger JW, Schiller WR, Blakemore WS. Metabolic response to injury and illness: estimation of energy and protein needs from indirect calorimetry and nitrogen balance. JPEN J Parenter Enteral Nutr. 1979;3:452-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 261] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Luo K, Li JS, Li LT, Wang KH, Shun JM. Operative stress response and energy metabolism after laparoscopic cholecystectomy compared to open surgery. World J Gastroenterol. 2003;9:847-850. [PubMed] |
| 20. | Bardram L, Funch-Jensen P, Jensen P, Crawford ME, Kehlet H. Recovery after laparoscopic colonic surgery with epidural analgesia, and early oral nutrition and mobilisation. Lancet. 1995;345:763-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 399] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 21. | Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997;78:606-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1691] [Cited by in RCA: 1742] [Article Influence: 62.2] [Reference Citation Analysis (2)] |
| 22. | Kehlet H, Wilmore DW. Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg. 2008;248:189-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1147] [Cited by in RCA: 1195] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 23. | Grantcharov TP, Kehlet H. Laparoscopic gastric surgery in an enhanced recovery programme. Br J Surg. 2010;97:1547-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Cochrane handbook for systematic reviews of interventions. Higgins JPT, Green S, editors. Version 5.1.0 (updated March 2011). London: The Cochrane Collaboration 2011; . |
| 25. | Ionescu D, Iancu C, Ion D, Al-Hajjar N, Margarit S, Mocan L, Mocan T, Deac D, Bodea R, Vasian H. Implementing fast-track protocol for colorectal surgery: a prospective randomized clinical trial. World J Surg. 2009;33:2433-2438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Ren L, Zhu D, Wei Y, Pan X, Liang L, Xu J, Zhong Y, Xue Z, Jin L, Zhan S. Enhanced Recovery After Surgery (ERAS) program attenuates stress and accelerates recovery in patients after radical resection for colorectal cancer: a prospective randomized controlled trial. World J Surg. 2012;36:407-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 163] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 27. | Yang D, He W, Zhang S, Chen H, Zhang C, He Y. Fast-track surgery improves postoperative clinical recovery and immunity after elective surgery for colorectal carcinoma: randomized controlled clinical trial. World J Surg. 2012;36:1874-1880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | Hübner M, Müller S, Schäfer M, Clavien PA, Demartines N. Impact of the nutritional risk score in fast-track colon surgery. Dig Surg. 2010;27:436-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Wang G, Jiang Z, Zhao K, Li G, Liu F, Pan H, Li J. Immunologic response after laparoscopic colon cancer operation within an enhanced recovery program. J Gastrointest Surg. 2012;16:1379-1388. [PubMed] |
| 30. | Vlug MS, Wind J, Hollmann MW, Ubbink DT, Cense HA, Engel AF, Gerhards MF, van Wagensveld BA, van der Zaag ES, van Geloven AA. Laparoscopy in combination with fast track multimodal management is the best perioperative strategy in patients undergoing colonic surgery: a randomized clinical trial (LAFA-study). Ann Surg. 2011;254:868-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 583] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 31. | van Bree SH, Vlug MS, Bemelman WA, Hollmann MW, Ubbink DT, Zwinderman AH, de Jonge WJ, Snoek SA, Bolhuis K, van der Zanden E. Faster recovery of gastrointestinal transit after laparoscopy and fast-track care in patients undergoing colonic surgery. Gastroenterology. 2011;141:872-880.e1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 32. | Veenhof AA, Vlug MS, van der Pas MH, Sietses C, van der Peet DL, de Lange-de Klerk ES, Bonjer HJ, Bemelman WA, Cuesta MA. Surgical stress response and postoperative immune function after laparoscopy or open surgery with fast track or standard perioperative care: a randomized trial. Ann Surg. 2012;255:216-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 247] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 33. | Serclová Z, Dytrych P, Marvan J, Nová K, Hankeová Z, Ryska O, Slégrová Z, Buresová L, Trávníková L, Antos F. Fast-track in open intestinal surgery: prospective randomized study (Clinical Trials Gov Identifier no. NCT00123456). Clin Nutr. 2009;28:618-624. [PubMed] |
| 34. | Muller S, Zalunardo MP, Hubner M, Clavien PA, Demartines N. A fast-track program reduces complications and length of hospital stay after open colonic surgery. Gastroenterology. 2009;136:842-847. [PubMed] |
| 35. | Wang Q, Suo J, Jiang J, Wang C, Zhao YQ, Cao X. Effectiveness of fast-track rehabilitation vs conventional care in laparoscopic colorectal resection for elderly patients: a randomized trial. Colorectal Dis. 2012;14:1009-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 36. | Wang G, Jiang ZW, Xu J, Gong JF, Bao Y, Xie LF, Li JS. Fast-track rehabilitation program vs conventional care after colorectal resection: a randomized clinical trial. World J Gastroenterol. 2011;17:671-676. [PubMed] |
| 37. | Faiz O, Brown T, Colucci G, Kennedy RH. A cohort study of results following elective colonic and rectal resection within an enhanced recovery programme. Colorectal Dis. 2009;11:366-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 38. | King PM, Blazeby JM, Ewings P, Kennedy RH. Detailed evaluation of functional recovery following laparoscopic or open surgery for colorectal cancer within an enhanced recovery programme. Int J Colorectal Dis. 2008;23:795-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Srinivasa S, Taylor MH, Singh PP, Yu TC, Soop M, Hill AG. Randomized clinical trial of goal-directed fluid therapy within an enhanced recovery protocol for elective colectomy. Br J Surg. 2013;100:66-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 40. | Basse L, Jakobsen DH, Bardram L, Billesbølle P, Lund C, Mogensen T, Rosenberg J, Kehlet H. Functional recovery after open versus laparoscopic colonic resection: a randomized, blinded study. Ann Surg. 2005;241:416-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 322] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 41. | MacKay G, Ihedioha U, McConnachie A, Serpell M, Molloy RG, O’Dwyer PJ. Laparoscopic colonic resection in fast-track patients does not enhance short-term recovery after elective surgery. Colorectal Dis. 2007;9:368-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 42. | García-Botello S, Cánovas de Lucas R, Tornero C, Escamilla B, Espí-Macías A, Esclapez-Valero P, Flor-Lorente B, García-Granero E. [Implementation of a perioperative multimodal rehabilitation protocol in elective colorectal surgery. A prospective randomised controlled study]. Cir Esp. 2011;89:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 43. | Anderson AD, McNaught CE, MacFie J, Tring I, Barker P, Mitchell CJ. Randomized clinical trial of multimodal optimization and standard perioperative surgical care. Br J Surg. 2003;90:1497-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 261] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 44. | Gatt M, Anderson AD, Reddy BS, Hayward-Sampson P, Tring IC, MacFie J. Randomized clinical trial of multimodal optimization of surgical care in patients undergoing major colonic resection. Br J Surg. 2005;92:1354-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 226] [Article Influence: 11.3] [Reference Citation Analysis (1)] |
| 45. | Wang D, Kong Y, Zhong B, Zhou X, Zhou Y. Fast-track surgery improves postoperative recovery in patients with gastric cancer: a randomized comparison with conventional postoperative care. J Gastrointest Surg. 2010;14:620-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 46. | Chen Hu J, Xin Jiang L, Cai L, Tao Zheng H, Yuan Hu S, Bing Chen H, Chang Wu G, Fei Zhang Y, Chuan Lv Z. Preliminary experience of fast-track surgery combined with laparoscopy-assisted radical distal gastrectomy for gastric cancer. J Gastrointest Surg. 2012;16:1830-1839. [PubMed] |
| 47. | Lemanu DP, Singh PP, Berridge K, Burr M, Birch C, Babor R, MacCormick AD, Arroll B, Hill AG. Randomized clinical trial of enhanced recovery versus standard care after laparoscopic sleeve gastrectomy. Br J Surg. 2013;100:482-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 168] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 48. | King PM, Blazeby JM, Ewings P, Franks PJ, Longman RJ, Kendrick AH, Kipling RM, Kennedy RH. Randomized clinical trial comparing laparoscopic and open surgery for colorectal cancer within an enhanced recovery programme. Br J Surg. 2006;93:300-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 279] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 49. | Kirchhoff P, Dincler S, Buchmann P. A multivariate analysis of potential risk factors for intra- and postoperative complications in 1316 elective laparoscopic colorectal procedures. Ann Surg. 2008;248:259-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 50. | Wu CL, Cohen SR, Richman JM, Rowlingson AJ, Courpas GE, Cheung K, Lin EE, Liu SS. Efficacy of postoperative patient-controlled and continuous infusion epidural analgesia versus intravenous patient-controlled analgesia with opioids: a meta-analysis. Anesthesiology. 2005;103:1079-1088; quiz 1109-1110. [PubMed] |
| 51. | Gatt M, MacFie J. Randomized clinical trial of the impact of early enteral feeding on postoperative ileus and recovery (Br J Surg 2007; 94: 555-561). Br J Surg. 2007;94:1044-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 52. | Kingham TP, Pachter HL. Colonic anastomotic leak: risk factors, diagnosis, and treatment. J Am Coll Surg. 2009;208:269-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 365] [Article Influence: 21.5] [Reference Citation Analysis (1)] |
| 53. | Lipska MA, Bissett IP, Parry BR, Merrie AE. Anastomotic leakage after lower gastrointestinal anastomosis: men are at a higher risk. ANZ J Surg. 2006;76:579-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 199] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 54. | Rullier E, Laurent C, Garrelon JL, Michel P, Saric J, Parneix M. Risk factors for anastomotic leakage after resection of rectal cancer. Br J Surg. 1998;85:355-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 643] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 55. | Taflampas P, Christodoulakis M, Tsiftsis DD. Anastomotic leakage after low anterior resection for rectal cancer: facts, obscurity, and fiction. Surg Today. 2009;39:183-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 56. | Ljungqvist O, Søreide E. Preoperative fasting. Br J Surg. 2003;90:400-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 200] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 57. | Reissman P, Teoh TA, Cohen SM, Weiss EG, Nogueras JJ, Wexner SD. Is early oral feeding safe after elective colorectal surgery? A prospective randomized trial. Ann Surg. 1995;222:73-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 257] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 58. | Lewis SJ, Egger M, Sylvester PA, Thomas S. Early enteral feeding versus “nil by mouth” after gastrointestinal surgery: systematic review and meta-analysis of controlled trials. BMJ. 2001;323:773-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 502] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 59. | Feo CV, Romanini B, Sortini D, Ragazzi R, Zamboni P, Pansini GC, Liboni A. Early oral feeding after colorectal resection: a randomized controlled study. ANZ J Surg. 2004;74:298-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 60. | Carli F, Mayo N, Klubien K, Schricker T, Trudel J, Belliveau P. Epidural analgesia enhances functional exercise capacity and health-related quality of life after colonic surgery: results of a randomized trial. Anesthesiology. 2002;97:540-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 211] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 61. | Clemente A, Carli F. The physiological effects of thoracic epidural anesthesia and analgesia on the cardiovascular, respiratory and gastrointestinal systems. Minerva Anestesiol. 2008;74:549-563. [PubMed] |
| 62. | Steinberg RB, Liu SS, Wu CL, Mackey DC, Grass JA, Ahlén K, Jeppsson L. Comparison of ropivacaine-fentanyl patient-controlled epidural analgesia with morphine intravenous patient-controlled analgesia for perioperative analgesia and recovery after open colon surgery. J Clin Anesth. 2002;14:571-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 63. | Taqi A, Hong X, Mistraletti G, Stein B, Charlebois P, Carli F. Thoracic epidural analgesia facilitates the restoration of bowel function and dietary intake in patients undergoing laparoscopic colon resection using a traditional, nonaccelerated, perioperative care program. Surg Endosc. 2007;21:247-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 64. | Marret E, Remy C, Bonnet F. Meta-analysis of epidural analgesia versus parenteral opioid analgesia after colorectal surgery. Br J Surg. 2007;94:665-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 212] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 65. | Augestad KM, Delaney CP. Postoperative ileus: impact of pharmacological treatment, laparoscopic surgery and enhanced recovery pathways. World J Gastroenterol. 2010;16:2067-2074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 87] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (2)] |
| 66. | Andersen HK, Lewis SJ, Thomas S. Early enteral nutrition within 24h of colorectal surgery versus later commencement of feeding for postoperative complications. Cochrane Database Syst Rev. 2006;CD004080. [PubMed] |
| 67. | Bauer AJ, Boeckxstaens GE. Mechanisms of postoperative ileus. Neurogastroenterol Motil. 2004;16 Suppl 2:54-60. [PubMed] |
| 68. | Boeckxstaens GE, de Jonge WJ. Neuroimmune mechanisms in postoperative ileus. Gut. 2009;58:1300-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 262] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 69. | Kalff JC, Türler A, Schwarz NT, Schraut WH, Lee KK, Tweardy DJ, Billiar TR, Simmons RL, Bauer AJ. Intra-abdominal activation of a local inflammatory response within the human muscularis externa during laparotomy. Ann Surg. 2003;237:301-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 163] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 70. | The FO, Bennink RJ, Ankum WM, Buist MR, Busch OR, Gouma DJ, van der Heide S, van den Wijngaard RM, de Jonge WJ, Boeckxstaens GE. Intestinal handling-induced mast cell activation and inflammation in human postoperative ileus. Gut. 2008;57:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 71. | Baigrie RJ, Lamont PM, Kwiatkowski D, Dallman MJ, Morris PJ. Systemic cytokine response after major surgery. Br J Surg. 1992;79:757-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 402] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 72. | Wind J, Polle SW, Fung Kon Jin PH, Dejong CH, von Meyenfeldt MF, Ubbink DT, Gouma DJ, Bemelman WA. Systematic review of enhanced recovery programmes in colonic surgery. Br J Surg. 2006;93:800-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 472] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 73. | Eskicioglu C, Forbes SS, Aarts MA, Okrainec A, McLeod RS. Enhanced recovery after surgery (ERAS) programs for patients having colorectal surgery: a meta-analysis of randomized trials. J Gastrointest Surg. 2009;13:2321-2329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 201] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 74. | Gouvas N, Tan E, Windsor A, Xynos E, Tekkis PP. Fast-track vs standard care in colorectal surgery: a meta-analysis update. Int J Colorectal Dis. 2009;24:1119-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 196] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 75. | Walter CJ, Collin J, Dumville JC, Drew PJ, Monson JR. Enhanced recovery in colorectal resections: a systematic review and meta-analysis. Colorectal Dis. 2009;11:344-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 76. | Varadhan KK, Neal KR, Dejong CH, Fearon KC, Ljungqvist O, Lobo DN. The enhanced recovery after surgery (ERAS) pathway for patients undergoing major elective open colorectal surgery: a meta-analysis of randomized controlled trials. Clin Nutr. 2010;29:434-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 782] [Cited by in RCA: 825] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 77. | Adamina M, Kehlet H, Tomlinson GA, Senagore AJ, Delaney CP. Enhanced recovery pathways optimize health outcomes and resource utilization: a meta-analysis of randomized controlled trials in colorectal surgery. Surgery. 2011;149:830-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 420] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 78. | Spanjersberg WR, Reurings J, Keus F, van Laarhoven CJ. Fast track surgery versus conventional recovery strategies for colorectal surgery. Cochrane Database Syst Rev. 2011;CD007635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3573] [Cited by in RCA: 2751] [Article Influence: 1375.5] [Reference Citation Analysis (0)] |
| 79. | Zhuang CL, Ye XZ, Zhang XD, Chen BC, Yu Z. Enhanced recovery after surgery programs versus traditional care for colorectal surgery: a meta-analysis of randomized controlled trials. Dis Colon Rectum. 2013;56:667-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 341] [Article Influence: 28.4] [Reference Citation Analysis (0)] |