Published online Oct 7, 2014. doi: 10.3748/wjg.v20.i37.13343
Revised: December 29, 2013
Accepted: June 12, 2014
Published online: October 7, 2014
Processing time: 344 Days and 16 Hours
Pancreatic cancer (PC) is a highly lethal disease and notoriously difficult to treat. Only a small proportion of PC patients are eligible for surgical resection, whilst conventional chemoradiotherapy only has a modest effect with substantial toxicity. Gene therapy has become a new widely investigated therapeutic approach for PC. This article reviews the basic rationale, gene delivery methods, therapeutic targets and developments of laboratory research and clinical trials in gene therapy of PC by searching the literature published in English using the PubMed database and analyzing clinical trials registered on the Gene Therapy Clinical Trials Worldwide website (http://www. wiley.co.uk/genmed/ clinical). Viral vectors are main gene delivery tools in gene therapy of cancer, and especially, oncolytic virus shows brighter prospect due to its tumor-targeting property. Efficient therapeutic targets for gene therapy include tumor suppressor gene p53, mutant oncogene K-ras, anti-angiogenesis gene VEGFR, suicide gene HSK-TK, cytosine deaminase and cytochrome p450, multiple cytokine genes and so on. Combining different targets or combination strategies with traditional chemoradiotherapy may be a more effective approach to improve the efficacy of cancer gene therapy. Cancer gene therapy is not yet applied in clinical practice, but basic and clinical studies have demonstrated its safety and clinical benefits. Gene therapy will be a new and promising field for the treatment of PC.
Core tip: This paper tries to present a full picture of gene therapy in pancreatic cancer, providing an unambiguous classification and comprehensive analysis, especially in therapeutic targets and clinical trials worldwide. From our work, you may find the hotspots in related research and the reason why they get there.
- Citation: Liu SX, Xia ZS, Zhong YQ. Gene therapy in pancreatic cancer. World J Gastroenterol 2014; 20(37): 13343-13368
- URL: https://www.wjgnet.com/1007-9327/full/v20/i37/13343.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i37.13343
Pancreatic cancer (PC) is an aggressive and highly lethal malignant disease. The incidence of PC is lower than that of many other types of cancer, but it is the fourth most common cause of death from cancer[1]. PC is highly malignant and invasive, owing to nonspecific incipient symptoms and early metastasis. Most patients have local or metastatic spread at the time of presentation, and less than 15% of patients are candidates for surgery. Therefore, the prognosis of the disease remains poor. Recent statistics from the US National Cancer Institute showed that the overall 5-year relative survival rate for 2002-2008 was 5.8%, and nearly 90% of all patients were dead in 1 year from diagnosis, with a median survival less than 6 mo[2,3].
Surgery is still the first line treatment for PC, because it provides the only curable option. Other adjuvant treatments are chemotherapy, radiotherapy, physiotherapy and biotherapy. However, PC is highly resistant to the currently available chemotherapy and radiotherapy, and it is one of the cancers for which survival rate has not been substantially improved during the past 30 years. Therefore, new effective modalities for the treatment of this disease are urgently required. In recent years, with the outstanding progress of modern molecular biology, tumor immunology and gene engineering technology, tumor biotherapy is becoming a perspective and rapidly developing field of modern medicine, which is expected to improve state of or even cure patients who are not curable by classical methods of therapy.
Generally, tumor biotherapy includes immunotherapy and gene therapy, but there is no explicit boundary between them. Gene therapy can be used to transfer genes into tumor cells to render them more highly immunogenic, while cancer immunotherapy utilizes gene engineering technology to produce immunomodulating agents, such as tumor vaccines.
Since the first gene therapy clinical trial was approved by the National Institutes of Health in May 1990, significant progress in gene therapy technology has been achieved. In September 2006, a successful immunogene therapy of two patients with metastatic melanoma was reported. Up to July 2013, we have entries for 1970 trials undertaken in 31 countries, and most of them had been aimed at the treatment of cancer (64.2% of all gene therapy trials)[4]. In 2008, detailed, global, genomic analyses found that PC contained an average of 63 genetic alterations, the majority of which were point mutations. These alterations defined a core set of 12 cellular signaling pathways and processes that were each genetically altered in 67%-100% of the tumors[5]. PC gene therapy is, therefore, targeting genes involved in these cellular pathways, inducing direct cell apoptosis and/or stimulating host immune defense system against tumor growth and expansion. Highly efficient gene therapy regimen is based on the following key points: efficiency of gene delivery, tumor targeted therapy and selection of efficient targets.
This strategy seeks chances of replacing a mutated gene with a normal gene via in situ homologous recombination. It is the best way to treat or even cure monogenic diseases, but seldom used for cancer gene therapy because of technical limitations and complex genetic alterations in cancer.
This strategy tries to directly modify the mutated gene and rehabilitate functions of target cells. It is an ideal manner of gene therapy but with great difficulties. Rare research related to this strategy has been reported.
Gene augmentation intends to transfer exogenous therapeutic genes into deficient cells and let their expression products make up for the deficiency. This is the most commonly used strategy in gene therapy. Key point of this technology is the selection of therapeutic genes and gene delivery systems. Plenty of efficient delivery systems have been developed to introduce genetic material into eukaryotic cells and get them expressed. The details will be discussed below.
This strategy seeks to prevent the transcription and translation of certain cancer-associated genes by using short nucleotide sequences that bind in a complementary fashion to specific DNA or RNA, which can block aberrant signal transduction pathway and induce tumor differentiation and apoptosis eventually. It is also known as antisense gene therapy. Common materials used in this strategy include antisense oligonucleotides, ribozymes and small interfering RNAs (siRNAs).
Antisense oligonucleotides: Antisense oligonucleotides are short single-stranded segments of DNA or RNA artificially synthesized in vitro, which can selectively inhibit the transcription and translation of the target gene through the Watson-Crick base pairing between the antisense nucleotide and the target RNA or DNA. Since DNA is more easily synthesized and stable in body fluid, antisense oligodeoxyribonucleotides have become the most common material used in practice. Various chemical modifications to its backbone have been used to improve oligonucleotide stability, targeting and transduction efficiency[6].
Ribozymes: Ribozymes are RNA molecules with catalytic activity that are capable of sequence specific cleaving of mRNA molecules. They can selectively bind to target mRNAs through the Watson-Crick base pairing and form a duplex, which includes a highly distorted conformation that is easily hydrolyzed. The hydrolysis of the mRNA can be used for targeted suppression of specific genes[6].
However, a shortcoming of ribozymes is that their RNA backbone makes them easy targets for degradation by the ubiquitous RNAases, so these molecules are biologically unstable in vivo. Then researchers found another category of ribozymes, which are called DNAzymes or deoxyribozymes. They are analogs of ribozymes with greater biological stability, employing DNA motifs to replace the RNA backbone. Moreover, these DNAzymes are also easy to modify synthetically, thereby generating even stronger, resilient second-generation analogs, which makes them powerful tools for gene suppression applications[6].
siRNAs: siRNAs are short double-stranded RNA segments with typically 21- to 23-nucleotide bases that are complementary to the target mRNA sequence. siRNAs can be artificially synthesized in vitro and directly transferred into target cells, or be produced in the genetically modified target cells, in which a gene encoding siRNA is introduced via appropriate vectors, with the help of endogenous RNAase. When entering into the target cell, siRNAs bind to ribozyme compounds and form RNA-induced silencing complexes (RISCs), which bind to the target mRNA and stimulate mRNA degradation mechanisms, such as nuclease activity, that lead to silencing of the particular gene. Compared with other gene blockade technologies, siRNAs are remarkably superior because of their high degree of specificity to mRNAs, nonimmunogenic nature and high resistance to ribonucleases. Since siRNAs do not integrate into the genome, they offer greater safety than plasmid molecules. Furthermore, siRNAs do not have to transfer through the nuclear membrane and therefore require less sophisticated delivery systems, promising faster development and higher efficiencies[6]. Thanks to these advantages, RNA interfering technique has become one of the hotspots in research of gene therapy.
In this system, the recipient cells which are previously explanted from the target tissue or bone marrow are cultured or proliferated in vitro and subsequently reinfused into the patient after therapeutic gene transfer. Obviously, only transplantable cells, such as lymphocytes and medullary cells, are acceptable in this method. In cancer therapy, tumor cells can also be cultured and engineered in vitro, but usually they are used to secrete cytokines or act as a vaccine. To improve the therapeutic efficacy, positively transfected cells are screened from the total cells for implantation, which gives ex vivo delivery higher transduction efficiency than in vivo delivery. However, the shortcomings of ex vivo delivery are complex operational process and a low survival rate of reimplanted cells[7,8].
In this system, gene vectors carrying therapeutic genes are directly delivered into the target tissues or organs, via systemic injection, in situ injection, oral agents or spray, of which in situ injection into local tumor tissue mediated by imaging methods is the most commonly used and ripest technology. Almost all the clinical trials on in vivo cancer gene therapy are based on this method, which includes intratumoral injection mediated by CT or ultrasound, tumor main vascular perfusion and gene-eluting stent implantation.
In vivo delivery is superior for its simple operation, easy preparation, independence on cell culture systems and wide range of application, whereas low efficiency of transduction, short curative effect, poor target cell specificity and immunologic problems are the main problems of this system. In vivo delivery might be the most useful strategy in clinical application. If only we overcome the shortcomings of this technique, gene therapy can truly be widespread applied in clinical treatment[9].
The core problem on whether we choose in vivo delivery or ex vivo delivery is how to achieve specific gene transfection and highly efficient gene expression in recipient cells. As a consequence, establishing an efficient, safe and specialized delivery system has become the foundation of gene therapy. An ideal gene delivery system should have these characters: (1) non-invasive mode of administration; (2) tumor-specific targeting, including primary lesion and distant metastatic lesion, especially site specific lesion, such as the central nervous system and testis; (3) sustained gene expression; and (4) high insertion capacity, bio-safety, stability and easy preparation.
These vector systems can be divided into two categories: non-viral and viral vector systems. Both of them have been investigated and each of them presents distinct advantages and weaknesses. Viral methods normally offer higher transduction efficiency and long-term gene expression, but it may be associated with toxicity, immunogenicity, mutagenicity, inability to transfer large size genes and high costs. Non-viral methods provide advantages including relative safety, ability to transfer large size genes, less toxicity and easy preparation; they can also be modified with ligands for tissue or cell specific targeting. However, non-viral methods show limitations of low transfection efficiency and poor transgene expression[10,11].
Non-viral vectors consist of chemical vectors, biological vectors and physical methods of gene transfer to introduce naked DNA (in the form of plasmid DNA), RNA molecules, or oligonucleotides into recipient cells.
Physical delivery mainly includes microinjection, microparticle bombardment and electroporation.
Microinjection involves the utilization of a micropipette to inject nucleic acid directly into a single living cell at a microscopic level. It is highly efficient since one cell at a time is targeted for DNA transfer. However, this precision is achieved at the expense of time. Microparticle bombardment, also known as ballistic DNA injection, gene gun technology or DNA-coated particle bombardment, is used to transfer plasmid DNA coated with heavy metals, usually gold, tungsten or silver, which are used as payload. These particles can be accelerated by pressurized gas and fire at the target cells or tissues without injuring them. Nevertheless, since direct exposure of target tissues is required, its application is restricted in internal organs. In addition, low efficiency of transfection into the nucleus and plasmid DNA integration into host genome are also problems to be solved. Electroporation uses high-voltage electrical current to generate transient disruption on the membrane of target cells, which allows the entry of plasmid DNA by diffusion. This technique results in high cell mortality and therefore is not suitable for clinical use.
In conclusion, though significant transfection efficiencies have been achieved using physical techniques, they are extremely difficult to standardize in a clinical setting and are considered laborious, impractical, and invasive[11].
Commonly used chemical vectors can be classified into two major types based on the nature of the synthetic material, including cationic lipids and cationic polymers. In recent years, chemical vectors have been widely studied due to their advantages, including safety, large size gene transfer ability, less toxicity, low cost and easiness in preparation.
Cationic lipids (liposomes) are vesicles that consist of an aqueous compartment enclosed in a phospholipid bilayer[6]. DNA is bound by cationic lipids as a result of electrostatic interaction, which allows for fusion of the liposome with the target cell membrane, endocytosis, and delivery of the DNA into the cytoplasm. Cationic lipids mediated gene transfer is the most promising method in non-viral delivery systems. Although this approach has already been applied in clinical trials, some problems still need to be solved for its better application, including the toxicity and lower transfection efficiency in vivo[11].
Cationic polymer is an umbrella term of a wide range of chemical compounds, including: (1) natural polymers such as chitosan; (2) dendrimers such as polyamidoamine (PAMAM); (3) polypeptides such as poly-L-lysine (PLL), polyarginine, polyornithine, histones and protamines; and (4) other polymers such as polyethylenimine (PEI) and polyphosphoester[11]. Their transfection activity and toxicity vary dramatically. When mixed with negatively-charged DNA, positive charges of the polymers allow the formation of polymer/DNA complexes (polyplexes) through electrostatic interaction. Polyplexes are nanosized transfection units that normally have higher stability than lipoplexes. The contributions of cationic polymers are enhancing the DNA uptake via endocytosis, protecting DNA from nuclease degradation and facilitating DNA escape from endosomes. Finally, DNA is released into the cytoplasm and migrates into the nucleus in which transgene expression takes place.
Recently, a combination of cationic polymers with liposomes, called polymer/lipid hybrid system, has also been developed and showed some superiority. To prepare this 3-part (lipid/polymer/DNA) system, DNA is precondensed by cationic polymers, followed by the subsequent complexation with liposomes. Using cationic lipids, the polymer/DNA complexes can be further condensed and protected, which can facilitate endocytosis and increase circulating half-life in vivo[11].
Furthermore, to improve the tissue or cell specificity of chemical vectors, they are also manipulated with the supplement of ligands or fusogenic peptides, such as transferrin, lectin and epidermal growth factor, which can bind to the receptors on the surface of target cells specifically. This approach is also known as receptor-directed gene transfer.
Bacteria can be used as gene therapy vectors. When engineered to express the therapeutic transgene, bacteria can introduce both the therapeutic gene and protein product to recipient cells. The types of bacteria used include attenuated strains of Salmonella, Shigella, Listeria, and Yersinia, as well as non-pathogenic Escherichia coli. For some of these vectors, the mechanism of DNA transfer from the bacteria to the mammalian cell is not yet fully understood, but their potential to deliver therapeutic molecules has been demonstrated in vitro and in vivo in experimental models[12]. A bacterial cancer vaccine for pancreatic cancer - a live attenuated Listeria strain expressing mesothelin - has entered early-phase clinical trial and demonstrated antitumor effects[13].
In addition, many mammalian cell types can be used as carriers of gene therapy vectors, such as hematological cells and mesenchymal stem cells (MSCs)[14]. MSCs possess natural tropism towards tumors, making them a vehicle for targeted delivery of therapeutic genes into tumors. Many experiments have identified their significant antitumor effects in vitro and in vivo. However, to effectively use this therapeutic strategy in clinic, we still have to solve a number of technical problems[15].
Viral vectors: Viral vectors are the most commonly studied and applied gene delivery systems. More than two-thirds of clinical trials of gene therapy reported are viral therapies. These viruses can use their innate mechanism of infection to enter the cell and transfer DNA molecules into cells without any physical or chemical processing. The therapeutic gene then enters the nucleus, integrates into the host gene pool, and is eventually expressed.
The most common viral vectors in cancer gene therapy are adenovirus (AdV), retrovirus (RV), adeno-associated virus (AAV), lentivirus, herpes simplex virus (HSV), influenza virus, Newcastle disease virus, pox virus, and Epstein-Barr virus (EBV). Both advantages and disadvantages should be considered when selecting a viral vector, including insertion capacity, host range of infection, state of integration into host genome, efficiency of transfection and expression, immunogenicity, bio-safety and difficulty of preparation. The comparison of common viral vectors is shown in Table 1[6,9,16-18].
| Virus | Viral genome | Insertion capacity | Host range of infection | State of integration into host genome | Efficiency of gene transfection and expression | Immunogenicity | Titers of preparation in vitro(PFU/mL) | Bio-safety |
| AdV | Double-stranded DNA | 38 kb | Broad spectrum (both dividing and non-dividing cells) | No integration | High | High | 1011-1012 | Safe |
| AAV | Single-stranded DNA | 4.9 kb | Broad spectrum | Site-specific integration on chromosome 19q13.3 | High | Low | 1012, dependent on helper virus | Safe |
| RV | Single-stranded RNA | 8 kb | Dividing cells only | Integrate randomly | Low | Low | 106-107 | Risk of insertional mutagenesis |
| Lentivirus | Single-stranded RNA | 8 kb | Broad spectrum | Integrate randomly | High | Low | 109-1010 | Risk of viral infection and insertional mutagenesis |
| Pox virus | Souble-stranded DNA | 25 kb | Broad spectrum | No integration | High | High, function as immunologic adjuvant | 106-107 | Safe |
| HSV | Double-stranded DNA | 15-30 kb | Nerve cells and epithelial cells, especially neuro- tropic speciality | No integration | High | Moderate | 1011-1012 | Risk of viral infection |
Oncolytic virus: Referring to viral treatment, there is another related field called oncolytic virotherapy in tumor-targeted gene therapy, which is an emerging treatment modality that uses replication-selective virus (or conditionally replicating virus) to destroy cancers. These natural viruses are genetically modified to be non-pathogenic but selectively infectious and cytotoxic to cancer cells. Viruses hijack the host cell’s protein factory, disabling its production in favor of viral products, which are intrinsically cytotoxic. The infected host cell eventually lyses, releasing new virions capable of infecting other cells in a “bystander” effect, amplifying and propagating the initial effect of infection. These viruses can also evoke an immune response in the host, but in a tolerable morbidity. In conclusion, two main characteristics of oncolytic viruses are: (1) they replicate selectively in cancer cells and have self-amplification properties; and (2) they have cancer-cell-specific toxicity.
Meanwhile, these oncolytic viruses can also be engineered to carry exogenous genetic materials to produce therapeutic effects such as secreting cytokines and enhancing antitumor immune responses prior to eventual cytolysis[19,20].
Nowadays, oncolytic virotherapy has shown its great potential in cancer therapy, and dozens of clinical trials are under way, some of which have been in phase III. The rate of publication of manuscripts on oncolytic virotherapy has now surpassed that on viral cancer gene therapy[20]. In 2005, the Chinese State Food and Drug Administration approved the world’s first oncolytic virus for treatment of cancer, an engineered human adenovirus (Oncorine; Shanghai Sunway Biotech, Shanghai, China) for treatment of head and neck carcinoma[21].
At present, cancer gene therapies are mainly based on two principles: gene augumentation and gene blockade. The former is introducing exogenous genetic materials (therapeutic genes) into cancer cells and let their expression products play a therapeutic role to prevent or reverse the growth of cancer cells, while the latter is inhibiting the excessive expression of intrinsic genes (target genes) in cancer cells. Therefore, the investigated genes of interest can be divided into same categories: therapeutic genes utilized for gene augumentation and target genes for gene blockade (Table 2). Since PC is one of the malignant diseases that have the most complicated genetics and pathogenesis, gene therapy of PC has encompassed almost all these categories mentioned above.
| Strategy | Categories | Examples |
| Gene augumentation | Tumor suppressor genes | p16INK4a, p21CIP1/WAF1, p14ARF, Retinoblastoma Protein (pRb), p53, p84 /Thoc1, p73, Smad4/DPC4 |
| Drug sensitivity genes/ suicide genes | Herpes Simplex Virus Thymidine Kinase (HSV-TK), Cytosine Deaminase (CD), Nitroreductase (NTR), Cytochrome P450 (CYP) | |
| Anti-angiogenesis genes | Soluble VEGFR, Soluble FGFR, Endostatin, Thrombospondin-1, Angiostatin, Vasostatin, NK4, Matrix metalloproteinases inhibitors (MMIPs/TIMPs), Somatostatin receptors (SSTR) | |
| Immune related genes | MHC molecules, Co-stimulatory molecules (B7 family, ICAM-1, LFA-3), Inflammatory cytokines (IL-2, IL-12, GM-CSF, IFN-α, IFN-β, IFN-γ, TNF-α), Tumor antigen (CEA, MUC-1, etc.) | |
| Apoptosis related genes | TRAIL | |
| Gene blockade/antisense therapy | Oncogenes | K-ras, LSM1/CaSm, HER-2/EerB-2 |
| MDR | MDR1, MRP family, BCRP | |
| Proliferation related genes | VEGF, hTERT, COX-2 |
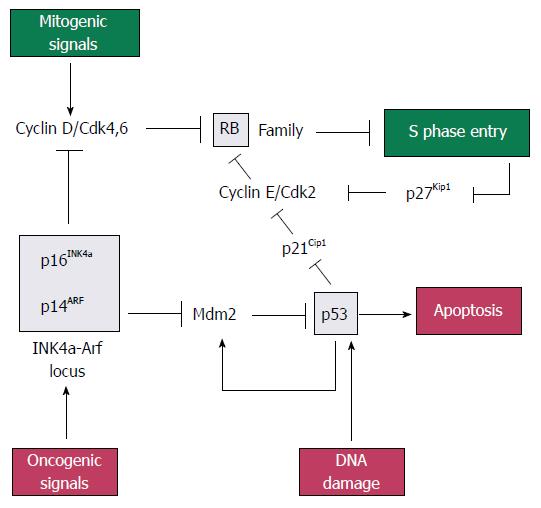
p16INK4a, p21CIP1/WAF1, p14ARF, retinoblastoma protein (pRb) and p53: The pRb gene is a part of gene family that includes two other members, p107 and p130, which collectively repress genes that regulates the G1 to S checkpoint of the cell cycle. Rb family proteins interact with transcription factor E2Fs, which induce the expression of genes needed for DNA synthesis. When bound at E2F-responsive promoters, Rb family proteins help to repress gene expression[22]. Rb proteins can be phosphorylated by cyclin dependent kinases 2, 4 and 6 (CDK2, 4 and 6), resulting in the release of E2F and gene expression[23].
The p16INK4a gene (p16), located on chromosome 9p21, is deleted in 85% of pancreatic adenocarcinomas[24]. It is the first member to be identified in the INK4 family of CDK inhibitors. The p16 gene product is tight-binding and inhibitory protein for CDK4 to induce G1 arrest of the cell cycle[25], while p21CIP1/WAF1 (p21) gene product acts as a downstream effector of p53 and mediates G1 cell cycle arrest by inhibiting CDK2. Loss of p21 activity has been observed in approximately 30%-60% of PC specimens[26].
The p14ARF (p14) gene, also located on chromosome 9p21, shares an exon with p16INK4a in different reading frames[27]. Mdm2 (murine double minute) is a p53-inducible gene that normally acts to terminate the p53 response. The p14ARF protein inhibits Mdm2 to induce p53, leading to p53-dependent apoptosis[22].
The p53 gene, located on chromosome 17p, is inactivated by mutation in 70% of pancreatic adenocarcinomas. It can induce apoptosis or G1 cell cycle arrest via p21CIP1/WAF1. It is normally maintained at a very low level by Mdm2, which targets p53 for ubiquitin-mediated degradation. Stress or mitogenic signals increase the level of p14ARF, which in turn inhibits Mdm2 and lead to the stabilisation and activation of p53.
In short, pRb, p53, p16INK4a, p21CIP1/WAF1 and p14ARF form part of a signaling network that monitors mitogenic signaling and restrains aberrant growth-promoting signals from driving cell cycle progression inappropriately (Figure 1)[22].
In research of PC therapy, both p16 and p21 have successfully been transduced into pancreatic cancer cell lines by means of adenoviral vectors, which results in growth inhibition and induction of apoptosis in vitro[25,28]. One study proved that p16-mediated cytotoxicity is tightly associated with the presence of functional pRb[29]. Liposome-mediated delivery of the p14ARF gene to pancreatic cancer cell lines was capable of resulting in the enhancement of their sensitivity to 5-Fu contrasting with cells devoid of p14ARF expression, which successfully inhibits PC cell proliferation[30].
Of all these tumor suppressor genes, p53 is the most important and extensively studied one. The first gene therapy for the treatment of cancer in China in 2004 is a replication-defective adenovirus 5 expressing p53 used for squamous cell carcinoma of the head and neck[21]. Transfer of the p53 gene using an adenoviral vector also suppressed the growth of human pancreatic cancer cell lines in vitro[31]. Reintroduction of p53 also increased cytotoxicity of gemcitabine in vitro and in vivo[32], and that of temozolomide in vitro[33]. In nude mouse model, intraperitoneal administration of the retroviral p53 vector resulted in significant inhibition of the growth of primary pancreatic tumor and peritoneal deposits compared to controls[34]. What is more, the inhibitors of Mdm2 are also investigated as new agents for PC treatment, since they induced the growth inhibition through reactivation of the p53 pathway[35].
p84 /Thoc1: The p84/Thoc1 gene encodes proteins that have similar death domains with other proteins involved in the regulation of apoptosis, which can bind to an amino terminal domain of the Rb1 protein, regulating transcriptional elongation and RNA processing. Its overexpression induces apoptosis of cancer cells. A recent study found that infection of pancreatic adenocarcinoma with adenovirus encoding p53 and p84/Thoc1 inhibited growth of cancer cells both in vitro and in vivo to a greater extent than treatment with either one alone[36].
p73: The p73 gene, located on chromosome 1p36, is identified as a p53 family member observed in 45.6% of pancreatic adenocarcinomas. It can induce cell cycle arrest and apoptosis in a p53 manner by binding to p53 DNA target sites, and transactivates p53-responsive genes[37]. One research found that an adenoviral vector encoding p73 was capable of effectively killing several pancreatic cancer cell lines, including those that were completely resistant to p53-mediated apoptosis[38].
Smad4/DPC4: The Smad4 gene, designated as tumor suppressor gene DPC4, is located on chromosome 18q21.1 and deleted in about 50% of pancreatic adenocarcinomas but only in about 10% or less of other cancers, which suggests that Smad4/ DPC4 may have a specific role in pancreatic tumorigenesis[39]. Smad4 is a member of the Smad family of transcription factors, which potentiates tumor growth, angiogenesis and invasion and is associated with poor prognosis[23]. Restoration of the Smad4 gene using an adenoviral vector showed inhibition of pancreatic tumor growth in mice[40], while the same effect was seen in vitro through a retroviral vector pLXSN containing DPC4[41] .
Drug sensitivity gene therapy, also known as gene-directed enzyme prodrug therapy (GDEPT) or suicide gene therapy, attempts to selectively transduce tumor cells with a gene which, when express an enzyme, will convert a systemically administered nontoxic prodrug into a toxic metabolite.
A large number of enzyme-prodrug systems have been developed for suicide gene therapy in recent years. Examples of enzymes include viral thymidine kinase (TK), bacterial cytosine deaminase (CD), bacterial carboxypeptidase G2 (CPG2), purine nucleotide phosphorylase (PNP), thymidine phosphorylase (TP), nitroreductase (NR), D-amino-acid oxidase (DAAO), xanthine-guanine phosphoribosyl transferase (XGPRT), penicillin-G amidase (PGA), β-lactamase (β-L), multiple-drug activation enzyme (MDAE), β-galactosidase (β-Gal), horseradish peroxidase (HRP), deoxyribonucleotide kinase (DRNK), deoxycytidine kinase (dCK), carboxypeptidase A (CPA), β-glucuronidase (β-Glu), and cytochrome P450 (CYP)[42]. However, among these dazzling choices, the most classic paradigm in PC therapy is herpes simplex virus thymidine kinase (HSV-TK).
The HSV-TK gene codes for an enzyme that converts the nontoxic prodrug ganciclovir into monophosphorylated ganciclovir, which is subsequently further converted by cellular guanylate kinases to the triphosphorylated forms, blocking DNA synthesis and inducing cell death[43]. The therapeutic effect can also be amplified by a “bystander effect”, which means HSV-TK transduced tumor cells are toxic to neighbouring unmodified tumor cells. The reason may be related to the uptake of toxic metabolites via intercellular communication paths such as gap junctions[44]. It is, in some extent, compensable for the low efficacy of gene transfer.
The HSK-TK delivered by retrovirus and adenovirus has been proved efficient in killing PC cells in vitro and in vivo[45,46]. The combination of adenovirus- and retrovirus-mediated delivery of HSV-TK appeared to be more effective in tumor reduction compared to either one alone in vivo[47]. Liposome mediated transfer of HSV-TK was able to cause regression of tumors in nude mice with peritoneal dissemination of PC[48]. However, there were some studies showing that retrovirally transduced HSV-TK had limited efficacy in PC cell lines both in vitro and in vivo[49,50]. The main reason of this controversial fact may be related to a poor efficiency of gene transfection in vivo and a limited bystander cell killing effect, so further study is required for its clinical application.
CD is a bacterial enzyme that converts the prodrug 5-FC into the cytotoxic and radiosensitising agent 5-FU, which inhibits DNA replication and protein synthesis. Several studies have proved that the adenovirus carrying CD gene is efficient in inhibiting the growth of murine PC cell lines in vitro and in vivo when associated with 5-FC[51-53]. Furthermore, when combined with radiation, the adenoviral vector carrying a mutant bacterial CD gene (AdbCD-D314A) plus 5-FC significantly increased frequency of tumor regression and the persistence of tumor growth inhibition compared with either radiation or AdbCD-D314A/5-FC therapy alone[54].
There is another gene called FUR1, which encodes uracil phosphoribosyltransferase (UPRT), playing a role in CD/5-FC suicide therapy. Since the CD gene, also called FCY1 gene, often demonstrates resistant to 5-FU, UPRT has an additional advantage as it catalyses the conversion of 5-FU into the toxic metabolite 5-fluorouridine-5’-monophosphate[55]. Combined treatment with 5-FU and E1B-55kDa-deleted adenovirus carrying the UPRT gene (AxE1AdB-UPRT) dramatically reduced the disseminated tumor burden in mice with peritoneal dissemination of AsPC-1 without causing toxicity in normal tissues[56]. One study showed that the FCY1 gene alone was ineffective in the treatment of PC in vitro and plasmid vectors expressing chimera CD-UPRT (pRSV-CD-UPRT) only increased 5-FC sensitivity to some PC cell lines[57]. Another study demonstrated that adenoviral vectors carrying the CD: uracil phosphoribosyltransferase fusion gene (Ad-CD: UPRT) resulted in increased 5-FC-mediated cell killing, compared with Ad-CD[58]. Moreover, Ad-CD: UPRT/5-FC combined with monoclonal antibody TRA-8 produces an additive cytotoxic effect in cancer cells both in vitro and in vivo[58].
The Escherichia coli enzyme nitroreductase (NTR) is able to convert the prodrug CB1954 (5-[aziridin-1-yl]- 2,4-dinitrobenzamide) to 2- and 4-hydroxylamino derivatives, which react with cellular thioesters to generate a potent alkylating agent capable of cross-linking DNA, inducing cell apoptosis eventually[59]. Retrovirus-mediated NTR gene delivery showed increased sensitivity up to 500-fold to CB1954 in PC cell lines in vitro, through associated bystander effect[60]. In a nude mouse model with subcutaneous PC xenografts, retrovirus vectors expressing the NTR gene with administration of CB1954 resulted in tumor regression, growth delay and significantly increased median survival[61]. Another research of nude mouse xenograft model for disseminated peritoneal carcinomatosis with ascites (PC cell line SUIT2) showed that combination of replication-defective adenovirus vectors carrying the NTR gene (Ad-CMV-NTR) and CB1954 almost doubled the median survival from 14 to 26 d[62]. The NTR/CB1954 treatment has been tested in clinical trials of gastrointestinal and liver malignancies, but none for PC.
Cytochrome P450 enzyme converts the chemotherapeutic prodrugs cyclophosphamide (CPA) or iphosphamide (IPA) to toxic metabolites phosphoramide mustard, which is an alkylating agent able to form DNA cross-links in a cell cycle-independent manner. Since cytochrome P450 is predominantly produced in the liver and toxicity of the metabolite is rather systemic and not tumor-specific in human body, the transduction of the CYP gene into the tumor tissue shows its advantages to enhance tumor-specific toxicity.
In vitro study demonstrated that expression of CYP 2B1 enzymes (retrovirus-mediated transduction) led to an up to 13-fold increase in susceptibility to IPA in a range of PC cell lines (BxPC-3, MIA PaCa-2, Hs-766T, PaCa-44 and PANC-1)[63]. In vivo, retroviral CYP 2B1 transfer with CPA treatment highly sensitized PC cells NP-9, NP-18, and NP-31, and led to significant differences in tumor volume at the end of the treatment when compared with CPA alone[64]. Furthermore, in tumor-bearing mice model, intratumoral injection of encapsulated cells, which were genetically modified to express the CYP, was able to cause significant tumor reduction[65,66]. In addition, these encapsulated cells have also been used in phase I/II trials in patients with PC and have shown remarkable early success, with median survival doubled and 1-year survival improved by 3-fold[67].
Tumor growth is dependent on angiogenesis, in which vascular endothelia growth factor (VEGF) plays a leading role. VEGF is a glycoprotein that has a huge impact on endothelial cell survival, mitogenesis, migration, differentiation, and vascular permeability. It is overexpressed in over 90% of PC and is associated with increased microvessel density, tumor progression and poor prognosis[68]. The VEGF receptor (VEGFR), which is a transmembrane receptor tyrosine kinase of the ErbB family, including VEGFR-1 (FMS-like tyrosine kinase-1, flt-1) and VEGFR-2 [fetal liver kinase-1 (flk-1) or kinase insert domain receptor (KDR)], is also overexpressed in the vasculature of tumors that express VEGF[69,70].
Mechanisms that lead to inappropriate activation of the VEGF pathway include receptor overexpression, activating mutations, overexpression of receptor ligands, and loss of their negative regulatory pathways. Both VEGF and VEGFR are, therefore, appealing targets for anti-angiogenesis therapy. Many molecular targeted agents and monoclonal antibody interfering with VEGF signal system have been developed for cancer therapy, such as Bevacizumab (a humanized antibody against VEGF), Sorafenib (a multi-targeted kinase inhibitor), Erlotinib (an inhibitor of EGFR, the only molecular targeted drug approved by the FDA in 2005 for PC), Axitinib (an inhibitor of both VEGFR and related tyrosine kinase receptors), and Aflibercept (a recombinant fusion protein that functions as a soluble decoy receptor and inhibits VEGF). However, these agents seem unlikely to confer sufficient benefit in the PC clinical trials and their cost-effectiveness has been questioned[71].
Compared to classic non-gene therapy, gene therapy represents a powerful tool for therapeutic intervention to angiogenesis in terms of specific targeting, cost-effectiveness and safety. In this new approach, the VEGF/ VEGFR pathway is still the main hotspot. Stimulators and inhibitors that up-regulate and down-regulate VEGF signal pathways are all possible therapeutic targets in cancer treatment (Table 3)[72,73]. Therefore, strategies for anti-angiogenesis gene therapy can be divided into two categories: (1) delivery of genes encoding endogenous angiogenesis inhibitors or their receptors; and (2) blockage of the excessive angiogenesis genes encoding growth factors or growth factor receptors. The former is based on transfer of exogenous genes whereas the latter seeks to block excessive genes in tumor. Materials involved in these two are totally different.
| Stimulators | Inhibitors |
| Hypoxia | Angiostatin |
| Oncogenic proteins such as Ras | TSP-1 |
| Inflammatory cytokines such as IL-8 and IL-6 | Endostatin |
| FGF | Arrestin |
| TGF-β | Canstatin |
| HGF | MMPIs |
| PDGF | Somatostatin |
| G-CSF | Tumstatin |
| Angiogenin | VEGI |
| Leptin | Decoy receptors such as soluble VEGFR |
| Proliferin | Inflammatory cytokines such as IL-12 |
Soluble forms of VEGFR-1 and VEGFR-2 are a kind of decoy receptor, which can inhibit VEGF dependent tumor angiogenesis, by binding to VEGF and acting as a dominant negative receptor[74]. Recombinant adenoviruses encoding soluble VEGFR-2 (Ad Flk1-Fc) and soluble VEGFR-1 (Ad sflt1) showed significant tumor inhibition when injected intravenously and directly into the tumors, respectively[75,76]. Crosslinked polyplex micelles modified by RGD (Arg-Gly-Asp) peptide ligands, a non-viral vector, carrying plasmid DNA expressing a soluble form of VEGFR-1 (sFlt-1), demonstrated significant inhibition of tumor growth via anti-angiogenic effect when systemically injected into pancreatic adenocarcinoma bearing mice[77]. A truncated dominant negative mutant of VEGFR-2, which binds to VEGF and decreases the angiogenic stimulus of VEGF, when delivered by replication-defective retroviruses, could also lead to inhibition of tumor growth in each of three human PC cell lines in vivo[78]. Similar results were also found in an in vitro study with herpes simplex virus (HSV) amplicon mediated delivery of a hypoxia-inducible soluble VEGFR-2 (sFlk-1)[79]. In an comparative research of the antitumor activity of anti-angiogenic proteins, recombinant adenoviruses encoding angiostatin, endostatin, neuropilin, and soluble forms of VEGFR (Flk1, Flt1) all resulted in inhibition of tumor growth through intravenous injection in murine models involving lung cancer, fibrosarcoma and PC, but soluble forms of VEGFR were significantly more effective (approximately 80% inhibition of preexisting tumor growth) than the others[80].
FGFRs, encoded by four genes (FGFR1, FGFR2, FGFR3, and FGFR4), are involved in the regulation of organ development, cell proliferation and migration, angiogenesis and other processes. Recent studies have shown that FGFR-activating mutations and overexpression are closely associated with the development and progression of tumors in human[81]. Several monoclonal antibodies and small-molecule FGFR inhibitors have been developed, some of which have already entered early clinical development, such as AZD4547 (AstraZeneca), BGJ398 (Novartis), LY2874455 (Eli Lilly), GP369 (Aveo) and HuGAL-FR21 (Galaxy). Soluble forms of FGFR (sFGFR) had similar mechanism of action with sVEGFR. One study found that replication-defective adenoviral vectors carrying sFGFR1 gene could effectively suppress tumor angiogenesis and enhance apoptosis among lung cancer cells and pancreatic cancer cells both in vitro and in vivo, especially in sVEGFR-resistant cancers. Furthermore, the combined usage of sVEGFR plus sFGFR1 produced an enhanced inhibitory effect compared to their individual effects[82].
ES, AS, vasostatin and TSP-1 are the most important endogenous angiogenesis inhibitors that have been studied extensively. ES is a cleavage product from the C-terminal portion of collagen XVIII, which has been shown to inhibit endothelial cell migration and proliferation and induce apoptosis. The apoptotic effect of ES is associated with down-regulation of anti-apoptotic proteins, such as Bcl-2 and Bcl-xl, while inhibition of endothelial cell proliferation and migration is via interacting with several pathways, such as binding to integrin α5, associating with heparan sulfate proteoglycans on cell surface, and inhibiting VEGFR-2, PDGF, cyclin-D1 and metalloproteinases[83,84]. Recombinant human ES has been developed for cancer treatment and entered in clinical trials[85,86]. TSP-1 is a multifunctional extracellular matrix protein with pivotal roles in the regulation of vascular development and angiogenesis[87], while AS is a potent inhibitor of angiogenesis, selectively inhibiting endothelial cell proliferation and migration through binding a cell surface ATP synthase or inhibiting extracellular matrix (ECM)-stimulated plasminogen[72]. Vasostatin, the N-terminal domain of calreticulin, was also reported to have tumor suppressor and anti-angiogenic function in vitro and in vivo.
A double-regulated duplicative adenovirus expressing human ES (AdTPHre-hEndo) limited PC growth both in vitro and in vivo, and the inhibition was significantly higher than non-duplicative adenovirus vectors carrying the ES gene[88]. A eukaryotic expression vector pRC/CMV carrying the AS gene, when delivered into human PC cell line mediated by liposome in vitro, also notedly reduced the volume of tumors[89]. A replication deficient recombinant adenovirus encoding vasostatin (Ad-vasostatin) showed less neovascularisation and inhibited tumor growth in vivo and in vitro[90]. Same results were also discovered in a study with intratumoral delivery of recombinant AAV expressing vasostatin (rAAV-VAS)[91]. The Lister vaccine strain of vaccinia virus armed with the ES-AS fusion gene (VVhEA), which displayed high selectivity for cancer cells with effective infection of tumors after both intravenous and intratumoral administrations, showed a significant antitumor effect with evidence of inhibition of angiogenesis both in vitro and in vivo[92]. A recombinant AAV-mediated gene delivery of 3TSR (the antiangiogenic domain of TSP-1) or ES (rAAV-ES), via intratumoral injection, intramuscular injection or intrasplenic injection, also inhibited tumor growth by anti-angiogenic effect[87].
NK4, composed of the N-terminal hairpin and subsequent 4-kringle fragment of hepatocyte growth factor (HGF), is an HGF antagonist that acts as an angiogenesis inhibitor. NK4 strongly inhibits the infiltration, metastasis, and tumor growth of PC[93]. HGF is overexpressed in 61%-87% of PC cases[94] but frequently expressed in tumor-associated fibroblasts rather than PC cells. HGF promotes growth and enhances cell motility and extracellular matrix breakdown, leading to invasion and metastasis of cancer cells[95].
Strong antiangiogenic activity of an NK4-expressing adenvirus vector (Ad-NK) has been tested on PC cells in both in vitro and in vivo studies of subcutaneous and orthotopic transplantation, peritoneal dissemination, and liver metastasis[96]. Ad-NK potently inhibited the growth and invasion of cancer cells in response to HGF[97]. Intrasplenic injection of Ad-NK4 suppressed the number and growth of hepatic metastases[98]. When injected into the peritumoral region combined with gemcitabine, Ad-NK4 completely suppressed peritoneal dissemination and liver metastases, leading to significantly increased survival, compared to Ad-NK or gemcitabine alone[96]. Intraperitoneal injection of Ad-NK4 could also suppress the development of peritoneal dissemination of PC in nude mice[99].
MMPs are a family of zinc-dependent proteolytic enzymes that degrade the extracellular matrix and play an important role in tumor progression, angiogenesis, immune evasion, invasion and metastasis[100]. Natural tissue inhibitors of metalloproteinases (TIMPs) regulate physiologic MMP activity and are implicated in malignancy. The imbalance between MMPs and TIMPs, which denotes overexpression of MMPs and reduced expression of TIMPs, is frequently found in PC[101]. Some synthetic MMPIs have been exploited for antitumor therapy, such as BAY 12-9566 and Marimastat, but showed limited survival benefit in clinical trials in spite of their effectiveness in animal models[102-104]. When undergoing gene transfection to overexpress TIMP-1 in vitro, human PC cells were less likely to implant, grow and migrate in nude mice and showed increased apoptosis, and decreased angiogenesis[105]. Adenoviral vectors encoding human TIMP1 or TIMP2 limited development of PC and led to prolonged survival in vivo[106].
SST is a natural peptide hormone secreted in various parts of the human body, and participates in a wide variety of biological processes including neurotransmission and negative control of exocrine and endocrine secretions. Meanwhile, SST exerts a strong antiproliferative effect in normal as well as tumor cells by interacting with SSTRs, including apoptotic effects, growth factor inhibition, antiangiogenic and immuno-modulating activities[107,108]. SSTRs consist of five different G-protein coupled receptor subtypes (SSTR1-5), which are differently expressed in the various types of tumor. The antineoplastic activity of SST and its analogues depends on the receptor subtypes they are bound to. Among these receptor subtypes, SSTR-1 and SSTR-2 play a predominant role in mediating the anti-proliferative effect[109]. However, only SSTR2 expression is significantly inactivated in 90% of PC cases and SSTR-2, therefore, occupies the majority of gene therapy studies of SSTRs[110,111].
When introduced into PC cell line PC-3 with lipofectamine, SSTR2 showed inhibition of VEGF and MMP-2 expression in vitro[112]. A targeting adenoviral vector driven by MUC1-promoter expressing SSTR2 gene showed significant cell proliferation inhibition in vitro, though there was no AdMUC1-SSTR2-induced apoptosis[113]. Intratumoral transfer of SSTR2 using the synthetic vector linear polymers of ethylenimine (PEI), strongly inhibited tumor progression of pancreatic adenocarcinoma in vivo, while depleting SST by RNA interference completely reversed SSTR2’s antitumoral effect on VEGF expression and tumor angiogenesis[107]. Another study demonstrated that SSTR2 restoration mediated by oncolytic adenovirus (ZD55-hSSTR2) alone had a minor antitumor effect, but antitumor efficacy can be enhanced with the combination of ZD55-TRAIL (TNF-related apoptosis-inducing ligand) in vitro and in vivo[114]. Gene transfer using SSTR1 also displayed growth inhibition of PC by inducing cell cycle arrest in vitro and in vivo[115]. What is more, cotransfection of SSTR1 and SSTR2 showed a synergistic inhibitory effect on tumor proliferation and rendered Panc-1 cells more responsive to an SST analogue[116].
Tumor cells generally have a low immunogenicity and are able to escape surveillance by the host. Cancer immunotherapy has been developed to overcome this immune tolerance, including active and passive immunity. Passive approach encompasses administration of cytokines, activated effector cells or specific monoclonal antibodies targeting tumor cells. Active immunotherapy involves stimulation of immune response to tumor-associated antigens (TAAs), via employing cancer vaccines. Meanwhile, gene therapy is helpful by transferring genes into tumor cells or immune cells to render them more immunogenic and more effective, respectively. This combination is also known as immunogene therapy (Figure 2)[117].
In pathway A, allogeneic or autologous tumor cell vaccines that are genetically modified to secrete cytokines or co-stimulatory molecules are designed to elicit systemic immune responses to attack tumor tissue. Though autologous tumor cell vaccines showed promising results in clinical trials, a number of technical problems were uncovered, including the requirement of labor-intensive procedures for production of an individualized vaccine and the difficulty of expanding primary human tumor cells to the high numbers required for vaccination[118]. Fortunately, allogeneic whole tumor cell vaccines had been also proved to successfully induce systemic tumor-specific immune responses without the need to be HLA compatible with the host, and became the major way in study of tumor vaccines[119]. In pathway C, immune cells, especially dendritic cells (DCs), are ex vitro cultured autologous cells that are genetically modified, stimulated by specific antigens or activated by multiple cytokines to bypass the dysfunction of endogenous immune cells, restore immune surveillance, induce cancer regression or stabilization or delay and prevent its recurrence.
Host immune responses against a tumor antigen include cellular and/or humoral immune responses, starting with the processing of tumor antigens by antigen presenting cells (APCs) and recognition by T lymphocytes. T-cell activation requires not only the interaction between major histocompatibility complex (MHC) antigens bearing a specific peptide and the T-cell receptors, but also non-antigen-specific co-stimulatory activation by interaction of molecules expressed on the T-cells and APCs. Mechanisms of tumor immune tolerance include: under-expression of MHC antigens, loss of co-stimulatory molecule expression, alteration of tumor antigens, and secretion of immunosuppressive factors. Aiming at these mechanisms, at least four kinds of genes can be utilized in immunogene therapy: (1) major histocompatibility complex (MHC) antigens/human leukocyte antigens (HLAs), (2) co-stimulatory molecule genes, (3) tumor antigen genes; and (4) inflammatory cytokine genes.
Tumors may be capable of delivering antigen-specific signals to T cells, but may not deliver the co-stimulatory signals necessary for full activation of T cells. Therefore, one approach to induce tumor-specific immune responses is to genetically modify tumor cells to express MHC molecules and co-stimulatory molecules on their cell surface. This kind of modification makes tumor cells to function as professional APCs and enhance their ability to directly stimulate T cells. In addition, this way is also appropriate for manipulation of DC cancer vaccines[120].
Loss or reduced expression of MHC-class-I molecules in many cancer cells have been found in mice and humans and have been identified as an important form of immune evasion. Despite that a large number of potential cancer antigens were discovered, clinical trials of immunization were disappointing due to the loss of MHC-I expression in tumors[121]. In animal models, in vivo gene transfer of foreign MHC-class-I H-2K gene into tumors successfully induced a cytotoxic T-cell response and attenuated tumor growth and caused complete tumor regression in murine models[122,123]. Transfection of tumor cells with syngeneic MHC-class-II or allogeneic MHC-class-I genes improved tumor-specific immunity in the autologous host[124]. Therefore, combining MHC molecules with other tumor antigens could emerge as an attractive approach in cancer immunotherapy. No correlative research in PC has been reported yet.
In studies of costimulatory molecules, B7 family is one of the most important members and is constitutively expressed by most APCs, as the ligand for two receptors expressed on T cells, CD28 and CTLA-4. In animal studies, transfection of B7.1 into some tumors resulted in tumor rejection and generated systemic immunity against wild-type tumor challenges via stimulating CD8+ T cells[125]. Cotransfection of B7 with MHC-II molecules was capable of inducing potent systemic immunity via both CD4+ and CD8+ T cells pathways[126]. A combinatory vaccine regimen (PANVAC-VF) composed of vaccinia virus and fowlpox virus expressing tumor-associated antigens (CEA and MUC1) and costimulatory molecules [B7.1, intercellular adhesion molecule-1 (ICAM-1) and leukocyte function-associated antigen-3 (LFA-3)], when administered by subcutaneous injection with adjuvant GM-CSF, showed a significantly great therapeutic effect both in animal models and in clinical trials with advanced PC patients[127,128].
Numerous cytokines have been studied in in vivo gene transfer. Human PC cells that underwent retrovirus-mediated gene transfer of IL-2, IL-4, IL-6, IL-27 and granulocyte macrophage-colony stimulating factor (GM-CSF) showed significant retardation and even regression when inoculated into BALB/c nude mice[129,130]. In vivo gene delivery of IL-1β, IL-24, IFN-α, IFN-β and IFN-γ by different viral vectors have been proved to lead to significant tumor growth inhibition in many PC models[131-135]. In addition, combined application of cytokine transfection and traditional chemotherapy, and combining immune genes with either tumor suppressor genes or suicide genes, are also common strategies in cancer immunogen therapy. For instance, combination of intratumoral human TNF-α gene delivery with gemcitabine produced marked delays in the growth of human pancreatic xenograft tumors relative to either agent alone in vivo[136,137]. Combination of IFN-α gene delivery with 5-FU had similar results as well[137]. An in vitro study of combining INF-β gene tranfection with gemcitabine also showed tumor growth inhibition[138]. In a phase I/II trial, intratumoral gene delivery of replication-deficient adenovirus encoding TNF-α, combined with standard chemoradiation, showed promising clinical outcome with dose-limiting effect and toxicity[139].
In studies of tumor cell based vaccines, cytokine genes have been also introduced into tumor cells to render them tumorigenicity and immunogenicity. One study that directly compared and contrasted effects of different cytokines in murine tumor models demonstrated that the tumors transduced with GM-CSF produced the greatest degree of systemic immunity relative to irradiated non-transduced tumor cells[140]. In a phase I trial of allogeneic GM-CSF-secreting cancer vaccines in 14 patients with pancreatic adenocarcinoma, no local or systemic dose-limiting toxicities were observed and three subjects who received the highest two dose levels showed increased disease-free survival time (more than 2 years)[141]. In a comparative clinical trial of GM-CSF-secreting cancer vaccines alone and combined with cyclophosphamide in patients with metastatic PC, minimal treatment-related toxicity was found and cyclophosphamide cohort exhibited longer progression-free survival and overall survival[142]. Another clinical trial of ipilimumab (anti-CTLA-4) in combination with allogeneic PC cells transfected with a GM-CSF gene also showed prolonged disease stabilization in previously treated advanced PC[143].
Clinical studies of immunotherapy in cancer have focused on five classes of tumor antigens: (1) tumor-specific antigens (MAGE-1, NY-ESO-1, TRAG-3, PSA); (2) mutated oncogene products (p53, K-ras, HER2, BCR/abl, WT-1); (3) reactivated embryonic gene products (CEA, AFP); (4) self-antigens overexpressed in tumors (MUC1, survivin); and (5) oncogenic virus antigens (EBV, HPV, HBV)[120,144]. Many antigen-specific vaccines that urge the host immune system to recognize the primary tumor have been developed, including recombinant viral and bacterial vaccines that encode tumor antigens, peptide-or protein-based vaccines that mixed with adjuvants, DNA-based vaccines expressing tumor antigens, and antigen-pulsed DC vaccines[120]. Up to now, at least 75 antigens were identified that had many of the study-defined characteristics of an ideal candidate antigen for cancer therapy. Many of these antigens are the focus of targeted therapy in clinical trials in cancer (Table 4)[144-149].
| Peptide or protein vaccines | Mutant K-ras peptide |
| WT1 peptide | |
| CAP1-6D | |
| G17DT | |
| HLA-A*0201 restricted VEGF receptor-1 and -2 peptides | |
| HLA-A24 restricted survivin-2B80-88 peptide (AYACNTSTL) | |
| Autologous heat shock protein HSPPC-96 | |
| TELOVAC | |
| Whole cell vaccines or dendritic cell vaccines | α-Gal transferase transfected allogeneic tumor cells (including Algenpantucel-L) |
| GVAX | |
| MUC-1 pulsed autologous dendritic cells | |
| Viral or bacterial vaccines | PANVAC-VF-MUC-1, CEA, and TRICOM transfected virus |
| Mesothelin (CRS-207) transfected live-attenuated Listeria | |
| DNA vaccines | VXMO1 |
It is encouraging that many of the results of PC vaccine trials verified the safety and immunogenicity of these vaccines. In a mutated K-ras peptide vaccine clinical trial with 48 patients in PC, when combined with adjuvant GM-CSF, more than 50% of patients demonstrated a tumor-specific immune response and significantly improved median overall survival vs their non-responding counterparts[150]. A multi-institution double-blinded placebo-controlled trial of gastrin peptide vaccine in 154 patients with advanced-stage PC demonstrated a nearly 2-fold increase in the median overall survival in the treatment compared to the placebo group[151]. A phase I/II clinical trial of telomerase peptide vaccines also demonstrated prolonged survival in immune responders vs non-responders[152]. In a multi-institutional, open-label, dose-finding, phase II trial of Algenpantucel-L, 70 patients with resected PC were administered by two different doses of the vaccine in combination with gemcitabine and 5-fluorouracil. Of the PC patients who received a higher dose of vaccine, 96% survived for at least one year in comparison to the historical control of 69%, showing a statistically significant difference[153].
Although encouraging, results from single-agent immunotherapy clinical trials have been underwhelming. As more and more tumor antigens are identified, more specific and potent vaccines will be developed. The ideal vaccine will target multiple antigens that are crucial to the growth and progression of tumors. Perhaps combinatorial therapeutic approach, which includes chemotherapy, radiation, surgery, and immunotherapy, will result in even greater survival benefits for patients with PC[13].
TRAIL or Apo-2 ligand (Apo-2L) is a type II transmembrane protein belonging to the TNF superfamily and serves as an effective anticancer agent due to its cancer cell specificity and potent antitumor activity. TRAIL interacts with death receptor 4 (DR4) and DR5, which form the death-inducing signal complex (DISC) by binding to Fas associated death domain (FADD), thereby activates caspase-8 and results in activation of downstream caspases-3, -6, and -7, and apoptosis induction[154]. Recombinant human TRAIL has been developed as a novel anticancer agent and is being clinically evaluated for the treatment of both solid tumors and hematological malignancies, such as colorectal, melanoma, lung, ovarian cancers and non-Hodgkins lymphoma[155]. However, its use in vivo is limited by a short half-life in plasma due to a rapid clearance by the kidney.
Gene delivery of TRAIL into tumors may overcome this limitation. An adenoviral vector expressing TRAIL driven by a human telomerase reverse transcriptase (hTERT) promoter showed specific antitumor efficacy in PC cell lines in vitro and significantly suppressed tumor growth in vivo[156]. Systemic administration of this vector in combination with chemotherapy (gemcitabine) exhibited a synergistic effect in the induction of apoptosis[157]. TRAIL-engineered pancreas-derived mesenchymal stem cells (MSCs) were able to induce PC cell apoptosis in vitro[158]. Another study of adipose-derived MSCs that were transduced with the TRAIL gene showed that, when injected intravenously or subcutaneously into mice, these MSCs localized into tumors and mediated apoptosis without significant apparent toxicities to normal tissues[159]. These studies implied that MSCs may serve as a stable source of TRAIL delivery in PC therapy.
Ras is the most common oncogene detected in human cancers. It comprises 3 families, H-ras, K-ras, and N-ras. Of these, the K-ras family is responsible for almost all of the PC mutations, with mutations in the other families occurring rarely. Studies suggested that K-ras, which is located on chromosome 12p13, is mutated in up to 95% of PC cases[16,160]. The K-ras encodes membrane-bound GTP-binding proteins, which can be activated by signaling partners to regulate many cellular functions, including cell growth, proliferation, and differentiation. Mutations of K-ras result in malfunction of GTPase and participate in the initiation or early phase of pancreatic tumorigenesis[71].
To blockade the Ras signaling pathway, cancer vaccines that stimulate immunity against mutant Ras proteins and antisense therapy that blocks the translation of mutant Ras gene are two common strategies. Antisense therapy involves the use of oligonucleotides, ribozymes and siRNAs. Retroviral delivery of K-ras siRNA to human tumor cells induced loss of expression of the K-ras gene, leading to loss of anchorage-independent growth and tumorigenicity in vitro[161]. Another study of K-ras siRNA delivered by electroporation demonstrated significant tumor growth inhibition both in vitro and in vivo. When K-ras siRNA is combined with gemcitabine, survival rate was significantly prolonged and the mean tumor volume was dramatically reduced when compared with single agents[162].
K-ras antisense oligodeoxynucleotide (K-ras-ASODN) was identified to successfully inhibit K-ras expression[163] and suppress the growth and invasiveness of PC cell lines in vitro[164]. In peritoneal dissemination models of PC, intraperitoneal injection of adenovirus expressing antisense K-ras RNA significantly suppressed the peritoneal growth with no significant systemic toxicity[165]. K-ras ASODN combined with type I insulin-like growth factor receptor (IGF-IR) antisense oligodeoxynucleotide (IGF-IR-ASODN) showed a significant inhibitory effect on tumor growth and induced apoptosis in vitro and in vivo, compared with each agent alone[166]. In a phase II trial of patients with locally advanced and metastatic PC, ISIS 2503, a phosphorothioate oligonucleotide antisense inhibitor of human H-ras mRNA, showed a response rate of 10.4% and a median survival of 6.6 mo in combination with gemcitabine, which is promising but of unclear benefit[167]. Initial enthusiasm for this approach is currently diminishing following the failures of antisense inhibitors such as ISIS 3521 (a protein kinase C-alpha antisense oligonucleotide) and oblimersen (a bcl-2 antisense oligonucleotide) in lung cancer and melanoma, respectively.
The cancer-associated Sm-like (CaSm) oncogene LSM1 has been reported to be overexpressed in 87% of PC cases[168]. An adenovirus expressing CaSm antisense RNA (Ad-alpha CaSm) reduced endogenous CaSm mRNA expression in vitro, and a single intratumoural dose of Ad-alpha CaSm inhibited tumor growth and extended survival time in an in vivo SCID mouse model of human PC. The antitumor effect was further enhanced by gemcitabine[168]. In a metastatic tumor model, systemic administration of Ad-alpha CaSm resulted in a significant decrease in the number of hepatic metastases and increased survival time through both direct and bystander effects[169].
Human epidermal growth factor receptor 2 (HER-2) is a transmembrane receptor tyrosine kinase of the ErbB family. It participates in the EGFR signaling pathway and has roles in cell proliferation, survival, motility, invasion and adhesion. Blocking of overexpressed HER-2 oncogene was able to improve survival in breast and gastroesophageal cancers. Some studies found that HER-2 amplification occurs in 2% of pancreatic ductal adenocarcinomas (PDACs) but has distinct features with implications for clinical practice, which represents an attractive target for anti-HER2 therapies[170]. However, clinical trials of anti-HER2 antibodies such as trastuzumab showed no benefits compared with standard chemotherapy and did not recommend further evaluation of anti-HER2 treatment in patients with metastatic PC[171].
Drug resistance is a major cause of treatment failure in cancer chemotherapy. One of the important mechanisms of tumor multidrug resistance is increased drug efflux and decreased accumulation of drugs in the cell. Efflux transporters of the ATP-binding cassette (ABC) family such as ABCB1 (multidrug resistance 1, MDR1), the ABCC (multidrug resistance-associated protein, MRP) family, and ABCG2 (breast cancer resistance protein, BCRP) have been identified as major determinants of chemoresistance in tumor cells[172].
MDR1 is a classic paradigm in the ABC family and has been analyzed in detail in PC. Its product, P-glycoprotein, is a membrane protein that functions as an ATP-dependent exporter of drugs from cells. Some studies demonstrated a high rate (73.2%) of MDR1 expression in PC[173,174], and other studies reported that MDR1 is associated with sensitivity to gemcitabine[175]. The MRP family consists of 9 members (MRP1-9) and is involved in exporting a variety of endogenous substrates as well as organic anions of xenobiotics, conferring cells resistance to cytotoxic and antiviral drugs[176]. It has been shown that the expression of MRP3 and MRP5 mRNAs was upregulated in PC and MRP3 was even correlated with tumor grade[176]. BCRP was first derived from a resistant breast cancer cell line, but is present in a wide range of human solid tumors, including PC[177]. One study proved that BCRP expression was frequent (73.1%) in PC, and high BCRP expression was a significant prognostic factor for early tumor recurrence and poor survival[178]. Conversely, a study demonstrated low BCRP mRNA levels in both normal pancreatic tissues and PC[176].
In fields of anti-MDR gene therapy for PC, some studies have showed that siRNAs against MDR1 could specifically inhibit MDR1 expression at the mRNA and protein levels and decreased resistance against daunorubicin in PC cell lines in vitro[179]. Similar results were found in another study of a hammerhead ribozyme against MDR1 mRNA[180]. However, in vivo studies and clinical trials are still scarce in this field and there is much room for advancement to validate their clinical applicability.
Another important approach of making use of the MDR gene is transducing the MDR gene into hematopoietic stem cells to strengthen the host’s resistance to myelosuppression caused by chemotherapeutic drugs. An advantage of this approach is that it permits higher doses of chemotherapy without severe adverse effects, thus providing a better chance to achieve remission. In vitro, lentiviral or retroviral vectors encoding MGMT (P140K) and MDR1 or MRP1 resulted in significant survival advantage of human hematopoietic stem cells when undergoing intensification chemotherapy, compared with untransduced cells or either single vector alone[181-183]. In mouse models, MDR1 gene modified hematopoietic cells also showed chemoprotective effect from various anticancer drugs[184,185]. So far, several clinical trials of MDR1 transfected autologous hematopoietic stem cell transplantation have been approved for the treatment of patients with breast cancer, ovarian cancer or leukemia. However, since the expression rate of the MDR1 gene in PC is very high, this system may not be useful for treating this cancer.
As mentioned above, the VEGF signal pathway plays a leading role in tumor angiogenesis. Therefore, knockdown of VEGF gene expression is a promising way in anti-angiogenesis therapy in PC. Materials in this approach include antisense oligonucleotides, ribozymes and siRNAs.
One study of antisense oligodeoxynucleotide of VEGF-C showed that it decreased the expression levels of VEGF-C and inhibited lymphangiogenesis in nude mice with orthotopically xenografted human PC, but had no significant effect on angiogenesis[186]. This result was verified in another study with short hairpin RNA (shRNA) targeting VEGF-C, and the VEGF-C shRNA significantly inhibited cell proliferation and tumor growth in vivo[187]. On the other hand, antisense oligonucleotide of VEGF was proved to significantly decrease neoangiogenesis and vascular permeability in orthotopic xenograft models; furthermore, it reduced tumor growth and metastasis and improved survival[188]. Systemic or intratumoral injection of VEGF specific siRNAs also led to the significant reduction in the subcutaneous tumor growth through down-regulating VEGF expression and decreasing microvascular density[189,190]. A study with hammerhead ribozymes against VEGF gene transcripts showed inhibition of tumor growth and liver metastasis of a PC cell line in vivo[191]. However, no clinical trial in this approach has been reported in PC.
Telomere is a region of repetitive nucleotide sequences at each end of a chromatid, which plays a critical role in maintaining chromosome stability. Telomere is shortened progressively during normal cell division. When its length becomes critically short, it triggers replicative senescence or apoptosis. Telomerase is a ribonucleoprotein polymerase that maintains the length of telomere. Human telomerase complex consists of telomerase reverse transcriptase (hTERT), telomerase RNA (hTR or TERC), telomerase associated protein-1 (TEP-1), hsp90 and p23, of which the RNA subunit and hTERT constitute the core of telomerase. It has been tested that hTERT is the limiting component of telomerase and its expression levels parallel to those of telomerase activity[192,193]. Generally, telomerase activity is detectable only in germ line cells and certain stem cells but is repressed in somatic cells. Upregulated telomerase activity is associated with promotion of tumorigenesis, neoplastic growth and metastasis of human cancer[194,195]. In fact, approximately 85% of human cancers exhibit reactivation of telomerase activity, which is even as high as 92%-95% in PC[196,197]. Recently, one new viewpoint declares that hTERT is also implicated in DNA repair and regulation of the expression of genes that control cell proliferation, which promotes tumorigenesis independent on the stability of telomere[198]. In a nutshell, hTERT is an important proliferation-related factor and can serve as a tumor marker and a prognostic indicator.
In vitro, hTERT antisense oligonucleotide (hTERT-ASODN) could down-regulate expression of hTERT mRNA and increase cell apoptosis rate in a concentration- and time-dependent manner in PC cell lines[199,200]. Therefore, consecutive transfections were performed in order to inhibit telomerase activity and result in a continuous reduction in cell viability[201]. Cell cycle analysis indicated that the cells were mainly arrested at the G0/G1 phase with the treatment of hTERT-ASODN. In addition, hTERT ASODN synergized with gemcitabine to exert an anti-proliferation effect[200]. Similar results were demonstrated in another study of hTERT-siRNA transfection in pancreatic cancer cell line Capan-2, and the inhibitory effect was associated with the downregulation of Bcl-2 and cyclooxygenase-2 (COX-2)[202]. Hammerhead ribozyme targeting hTR was also found to depress telomerase activity, and ribozyme targeting hTERT mRNA showed stronger inhibition. Since the level of hTERT mRNA expression is less than that of hTR expression in cancer cells, hTERT might be a more useful therapeutic target[203]. An hTERT peptide vaccine GV1001 was tested in a phase I/II study of 48 patients with unresectable PC. The patients received intradermal injection in combination with GM-CSF. In the end, 24 of 38 evaluable patients demonstrated immune responses with the highest percentage (75%) in the intermediate dose group. Approximately 8.6 mo of mean survival for this group was significantly longer and one-year survival rate was 25%[152]. These promising results have led to commencement of another phase III trial of GV1001.
COX-2 is a key enzyme of the metabolic process of arachidonic acid and an early response protein that is induced rapidly by growth factors, tumor promoters, oncogenes, and carcinogens. It plays an important role in tumorigenesis and angiogenesis. It has been shown that COX-2 mRNA and protein expression was highly up-regulated in up to 90% of PC cases but was undetectable in nontumorous pancreatic tissue[204,205], and this indicated that COX-2 may be a potential target for treatment of PC. One study showed that COX-2 siRNA transfection could inhibit cell proliferation, induce cell apoptosis and regulate cell cycle of a PC cell line in vitro and decrease its tumorigenicity when inoculated subcutaneously into nude mice[206]. Nevertheless, inhibition of COX-2 is more applied in anti-inflammation treatment as nonsteroidal anti-inflammatory drugs (selective inhibitors of COX-2) than gene therapy.
Up to July 2013, 1970 gene therapy clinical trials have been completed, are ongoing or have been approved worldwide. Of these, 1264 (64.2%) have been made for treatment of cancers, including lung, gynecological, skin, urological, neurological and gastrointestinal tumors, as well as hematological malignancies and pediatric tumors. In this part, we summarized the finished and ongoing clinical trials of PC gene therapy worldwide (Tables 5 and 6) and the administration routes they employed (Figure 3). Meanwhile, since a variety of cancers have common characteristics, such as mutation of tumor suppressor genes or oncogenes and overexpression of tumor antigens, one therapeutic transgene can be effective for different tumors. In fact, many clinical trials are indicated for several cancers simultaneously, like all solid tumors or advanced tumors. Therefore, here we also collected such kind of trials and obtained a list of them (Table 7). It is worth noting that our analysis is mainly based on the records in The Journal of Gene Medicine Gene Therapy Clinical Trials Worldwide website (http://www.wiley.co.uk/genmed/clinical), so trials in some countries (e.g., Japan) are not available.
| Trial ID and investigator | Phase | Clinical indication | Patients(n) | Transgenes | Vectors and target cells | Administration route | Combined therapy | Results |
| DE-0009 Löhr et al[67] | I/II | Inoperable PC | 14 | Cytochrome p450 | Naked/plasmid DNA transfected allogeneic human 293 embryonic kidney cells | Inject into the tumor vasculature via supraselective angiography | In combination with low-dose ifosfamide | 4 patients showed tumor regression, the other ten individuals remained stable. Median survival was doubled compared with historic controls. 1-yr survival rate was three times better |
| DE-0024 Pecher et al[207] | I/II | Advanced breast cancer, PC and gallbladder carcinoma | 10 (2 PC) | MUC-1 | Naked/plasmid DNA transfected autologus dendritic cells | Subcutaneous injection | None | 9 patients showed signs of progression. Only one remained stable for 3 mo until she was transferred to another therapy. 3 of 10 patients developed vaccine-specific delayed-type hypersensitivity reaction (DTH). 4 of 10 patients showed increased mucinspecific INF-gamma- secreting CD8+ T cells |
| DE-0063 Kubuschok et al[208] | I | PC | 3 healthy donors and 1 PC patient | Mutated ras oncoprotein | EB virus transformed autologous lymphoblastoid cells | Subcutaneous injection | None | All the subjects showed strong vaccine-induced muRas-specific cytotoxic T lymphocytes |
| DE-0083 Niethammer et al[209] | I | Advanced PC | 45 | VEGFR-2 | Naked/plasmid DNA (oral DNA vaccine) | Oral administration | In combination with gemcitabine | Not reported |
| ES-0004 Mazzolini et al[210] | I | Liver cancer, PC, colorectal cancer | 17 (3 PC) | Interleukin-12 (IL-12) | Adenovirus transfected autologous dendritic cells | Intratumoral injection | None | Treatment was well tolerated. 11 of 17 were assessable for response. A partial response was observed in 1 case with PC. Stable disease was observed in 2 patients and progression in 8 patients |
| FR-0018 Gilly et al[211] | I/II | Unresectable digestive cancer | 6 (3 PC) | Interleukin-2 (IL-2) | Adenovirus | Intratumoral injection | None | Good safety. But final results of tumor responses were not reported |
| US-0853 Le et al[212] | I | Ovarian cancer, PC, lung cancer, mesothelioma | 28 | Mesothelin | Listeria monocytogenes | Intravenous injection | 1: Live attenuated Listeria vaccine (n = 9); 2: live attenuated mesothein expressing Listeria vaccine ( n = 17) | In arm 2, Listeriolysin O and mesothelin-specific T-cell responses were seen and 37% of subjects lived ≥ 15 mo |
| US-0700 Galanis et al[213] | I | Gemcitabine-refractory, metastatic PC | 12 | Cyclin G1 | Retrovirus | Intravenous injection | None | Good safety. But there was no evidence of anti-tumor activity |
| Kaufman et al[214] | I | Advanced PC | 10 | CEA, MUC-1, and TRICOM (including B7.1, ICAM-1, LFA-3) | Poxvirus | Subcutaneous injection | In combination with GM-CSF | Antigen-specific T-cell responses in 5 of 8 evaluated patients. 15.1 mo of median survival in responders vs 3.9 mo in non-responders. Overall median survival is 6.3 mo |
| Jaffee et al[141] | I | Resected PC | 14 | GM-CSF | Naked/plasmid DNA transfected allogeneic tumor cell vaccine | Intradermal injection | In combination with standard chemoradiation | 3 of 14 patients developed DTH, and 3 patients had increased disease-free survival time (at least 25 mo after diagnosis) |
| US-0475 Lutz et al[215] | II | Resected PC | 60 | GM-CSF | Naked/plasmid DNA transfected allogeneic tumor cell vaccine | Intradermal injection | In combination with standard chemoradiation | The median disease-free survival is 17.3 mo with median survival of 24.8 mo. Induction of CD8+ mesothelin-specific T cells correlated with disease-free survival |
| Laheru et al[142] | A pilot study | Advanced PC | 50 | GM-CSF | Naked/plasmid DNA transfected allogeneic tumor cell vaccine | Intradermal injection | A: vaccine alone (n = 20) B: vaccine plus cyclophosphamide (n = 30) | Cohort B showed enhanced mesothelin-specific T-cell responses. Median survival values in cohort A and cohort B were 2.3 and 4.3 mo |
| Le et al[143] | Ib | Advanced PC | 30 | CM-CSF | Naked/plasmid DNA transfected allogeneic tumor cell vaccine | Intradermal injection | 1: Ipilimumab alone (n = 15); 2: Ipilimumab plus vaccine (n = 15) | Median overall survival was 3.6 mo for arm 1 and 5.7 mo for arm 2. Mesothelin-specific T cells responses were associated with increased disease-free survival in arm 2 |
| Phase | Transgenes |
| I | Oncolytic adenovirus and oncolytic herpesvirus1 |
| Somatostatin receptor 2 (sst2), Deoxycitidine kinase :: uridylmonophosphate kinase (dck::umk) | |
| Somatostatin receptor 2 (sst2) | |
| Mesothelin-scFv with signaling domains comprised of TCR , CD28, and 4-1BB (CD137) cDNA | |
| GM-CSF | |
| PANVAC (CEA, MUC-1, and TRICOM) | |
| Cytosine deaminase, Herpes simplex virus thymidine kinase (HSV-TK) | |
| Cyclin G1 | |
| p53 | |
| BikDD (DOTAP-cholesterol mediated gene transfection) | |
| Mutated Ras | |
| I/II | Cytochrome p450 |
| AEG 35156: antisense oligonucleotide to X-linked inhibitor of apoptosis protein (XIAP) | |
| Herpes simplex virus thymidine kinase (HSV-TK) | |
| Diphtheria Toxin A Chain (DTA) gene with H19 promoter | |
| Alpha-(1,3) galactosyltransferase | |
| II | CEA, MUC-1, and PANVAC |
| Mutated Ras | |
| Alpha-(1,3) galactosyltransferase | |
| III | Alpha-(1,3) galactosyltransferase |
| PANVAC (CEA, MUC-1, and TRICOM)2 |
| Phase | Clinical indication | Transgenes |
| II | Adenocarcinoma | CEA, TCRzeta and CD28 |
| I | Cachexia | GHRH |
| I | CEA- or HER-2-expressing malignancies | HER-2, CEA |
| I | CEA-expressing malignancies | CEA |
| I/II | CEA-expressing malignancies | CAP-1 peptide from CEA |
| I/II | CEA-expressing malignancies | T cell receptor alpha and beta chains cDNA |
| I | CEA-expressing malignancies | CEA, B7.1, ICAM-1, LFA-3, GM-CSF |
| I | CEA-expressing malignancies | Anti-CEA-SFv-Zeta T cell receptor |
| I | CEA-expressing malignancies | B7.1 (CD80) |
| II | Advanced cancer with overexpression of p53 | Anti-p53 T cell receptor |
| I | MUC-1- expressing tumors | MUC-1, IL-2 |
| I | Advanced cancer | Cytochrome P450 |
| I | Advanced cancer | Endostatin |
| I | Advanced cancer | Heat shock protein 70 |
| I | Advanced cancer | T cell receptor alpha and beta chain |
| I | Advanced cancer | Bifunctional shRNA specific for stathmin 1 oncoprotein |
| I/II | Advanced cancer | GM-CSF |
| I | Advanced cancer | IL-12 |
| I/II | Advanced cancer | CYP1B1 |
| I | Advanced cancer | GM-CSF, CD154 (CD40-ligand) |
| I/II | Advanced cancer | IL-2 |
| I | Advanced cancer | p53 |
| I | Advanced cancer | AMEP |
| I | Advanced cancer | B7.1 (CD80) ,ICAM-1, LFA-3 |
| I/II | Advanced cancer | Cytosine deaminase |
| I/II | Advanced cancer | CEA |
| I | Solid tumors | Tumor antigen |
| I | Solid tumors | Interferon-gamma |
| I | Solid tumors | TNF |
| I | Solid tumors | Brachyury oncoprotein |
| I | Solid tumors | Human telomerase reverse transcriptase |
| I/II | Solid tumors | Oncolytic virus (no transgene) |
| I | Solid tumors | Retinoblastoma 94 |
| I | Solid tumors | p53 |
| I | Solid tumors | O6-methylguanine DNA methyltransferase (MGMT) |
| I | Solid tumors | GM-CSF |
| I | Solid tumors | GM-CSF, bi-shRNA-furin |
| I | Solid tumors | GM-CSF, TGF-beta 2 antisense |
| I | Solid tumors | GM-CSF, humanized Escherichia coli beta-galactosidase |
Over the past decades, plenty of experimental works and clinical trials have demonstrated the safety and efficacy of cancer gene therapy. Strategies encompass tumor suppressor, suicide, anti-angiogenesis and immune related genes, as well as activated oncogenes and multidrug resistance genes. Proliferation and apoptosis related signal pathways are also possible targets. However, among these therapeutic targets, only a small part were tested in clinical settings and proved to be effective, and even for them, outcomes were far from curative and treatments have to be combined with standard chemo- or radio-therapy for maximum benefits.
In this field, tumor suppressor gene p53 and mutant oncogene K-ras are two members that have been most deeply studied in all kinds of cancers and have yielded encouraging results in vitro and in animal models. Unfortunately, these findings have not been translated to improve outcomes in clinical trials. In suicide strategy, HSV-TK, CD and cytochrome p450 have been proved to be efficacious. A closed clinical trial of cytochrome p450 showed exciting results when combined with ifosfamide, and more trials are ongoing. As to anti-angiogenesis approach, VEGFR is of course the best choice, clinical trials of peptide vaccine derived from VEGFR-2 and DNA vaccine targeting VEGFR-2 did show benefits, but as a monotherapy it is not a “magic bullet” for all tumor patients since anti-angiogenesis is far more effective in preventing tumor growth than causing regression of established tumors, thus this method may be more suitable for patients with minimal residual disease. Nowadays immunotherapy has become the mainstream direction of studies in searching new cancer treatment regimens, and the cooperation of gene therapy and immunotherapy has created even more inspiring achievements, such as adoptive transfer of genetically modified T-cells or DCs, DNA vaccines (CEA, MUC-1, B7.1, ICAM-1, LFA-3), and direct transfer of cytokine genes (IL-2, IL-12, GM-CSF, INF-gamma). Of course, combining different targets or combination of gene therapy and traditional chemoradiotherapy may lead to improved efficacy of gene therapy in PC.
Considering that low efficiency of gene transfer is the main obstacle in application and popularization of gene therapy, the selection of high-efficient and tumor-targeted vectors should be emphasized. In general, viral vectors offer higher transduction efficiency and long-term gene expression and this is why more than two-thirds of clinical trials reported employ viral vectors. Recently, oncolytic viruses that infect and replicate selectively in cancer cells have become the hotspot in this field and show a bright future. Oncolytic viruses produced from initially infected cancer cells can spread to surrounding cancer tissue and distant tissue, thus intratumoural injections could be efficient, even for the treatment of disseminated tumors. Systemic administration of oncolytic viruses has also shown good safety and this is of great interest particularly in PC, since it is difficult to inject viruses into primary lesions directly in PC patients.
Gene therapy of cancer is not yet applied in routine clinical practice, but good safety records and validated clinical benefits make it a good candidate for all clinical settings including neo-adjuvant, adjuvant and palliative treatment. Despite these promising therapeutic strategies, greatest advance in the treatment of PC may still come from improved early detection and diagnosis.
P- Reviewer: Cordelier P, Fillat C, Gu DS S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1151] [Cited by in RCA: 1335] [Article Influence: 111.3] [Reference Citation Analysis (0)] |
| 2. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7953] [Cited by in RCA: 8093] [Article Influence: 505.8] [Reference Citation Analysis (2)] |
| 3. | Laheru D, Biedrzycki B, Jaffee EM. Development of a cytokine-modified allogeneic whole cell pancreatic cancer vaccine. Methods Mol Biol. 2013;980:175-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Gene Therapy Clinical Trials Worldwide. 20 October 2013. Available from: http://www.wiley.co.uk/genmed/clinical/. |
| 5. | Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801-1806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3216] [Cited by in RCA: 3017] [Article Influence: 177.5] [Reference Citation Analysis (0)] |
| 6. | Patil SD, Rhodes DG, Burgess DJ. DNA-based therapeutics and DNA delivery systems: a comprehensive review. AAPS J. 2005;7:E61-E77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 422] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 7. | Mann MJ, Whittemore AD, Donaldson MC, Belkin M, Conte MS, Polak JF, Orav EJ, Ehsan A, Dell’Acqua G, Dzau VJ. Ex-vivo gene therapy of human vascular bypass grafts with E2F decoy: the PREVENT single-centre, randomised, controlled trial. Lancet. 1999;354:1493-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 282] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 8. | Bowie KM, Chang PL. Development of engineered cells for implantation in gene therapy. Adv Drug Deliv Rev. 1998;33:31-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Nayerossadat N, Maedeh T, Ali PA. Viral and nonviral delivery systems for gene delivery. Adv Biomed Res. 2012;1:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 591] [Cited by in RCA: 552] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 10. | Touchefeu Y, Harrington KJ, Galmiche JP, Vassaux G. Review article: gene therapy, recent developments and future prospects in gastrointestinal oncology. Aliment Pharmacol Ther. 2010;32:953-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Wang W, Li W, Ma N, Steinhoff G. Non-viral gene delivery methods. Curr Pharm Biotechnol. 2013;14:46-60. [PubMed] |
| 12. | Vassaux G, Nitcheu J, Jezzard S, Lemoine NR. Bacterial gene therapy strategies. J Pathol. 2006;208:290-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Soares KC, Zheng L, Edil B, Jaffee EM. Vaccines for pancreatic cancer. Cancer J. 2012;18:642-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Power AT, Bell JC. Cell-based delivery of oncolytic viruses: a new strategic alliance for a biological strike against cancer. Mol Ther. 2007;15:660-665. [PubMed] |
| 15. | Karshieva SSh, Krasikov LS, Beliavskiĭ AV. [Mesenchymal stem cells as an antitumor therapy tool]. Mol Biol (Mosk). 2008;47:50-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Ramírez PJ, Vickers SM. Current status of gene therapy for pancreatic cancer. Curr Surg. 2004;61:84-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Kim JW, Gulley JL. Poxviral vectors for cancer immunotherapy. Expert Opin Biol Ther. 2012;12:463-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Saydam O, Glauser DL, Fraefel C. Construction and packaging of herpes simplex virus/adeno-associated virus (HSV/AAV) Hybrid amplicon vectors. Cold Spring Harb Protoc. 2012;2012:352-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Sze DY, Reid TR, Rose SC. Oncolytic virotherapy. J Vasc Interv Radiol. 2013;24:1115-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Bourke MG, Salwa S, Harrington KJ, Kucharczyk MJ, Forde PF, de Kruijf M, Soden D, Tangney M, Collins JK, O’Sullivan GC. The emerging role of viruses in the treatment of solid tumours. Cancer Treat Rev. 2011;37:618-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Garber K. China approves world’s first oncolytic virus therapy for cancer treatment. J Natl Cancer Inst. 2006;98:298-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 339] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 22. | Sherr CJ. Principles of tumor suppression. Cell. 2004;116:235-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 677] [Cited by in RCA: 659] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 23. | Wong HH, Lemoine NR. Biological approaches to therapy of pancreatic cancer. Pancreatology. 2008;8:431-461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Caldas C, Hahn SA, da Costa LT, Redston MS, Schutte M, Seymour AB, Weinstein CL, Hruban RH, Yeo CJ, Kern SE. Frequent somatic mutations and homozygous deletions of the p16 (MTS1) gene in pancreatic adenocarcinoma. Nat Genet. 1994;8:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 785] [Cited by in RCA: 754] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 25. | Kobayashi S, Shirasawa H, Sashiyama H, Kawahira H, Kaneko K, Asano T, Ochiai T. P16INK4a expression adenovirus vector to suppress pancreas cancer cell proliferation. Clin Cancer Res. 1999;5:4182-4185. [PubMed] |
| 26. | Garcea G, Neal CP, Pattenden CJ, Steward WP, Berry DP. Molecular prognostic markers in pancreatic cancer: a systematic review. Eur J Cancer. 2005;41:2213-2236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 186] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 27. | Zhang Y, Xiong Y, Yarbrough WG. ARF promotes MDM2 degradation and stabilizes p53: ARF-INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways. Cell. 1998;92:725-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1167] [Cited by in RCA: 1152] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 28. | Joshi US, Dergham ST, Chen YQ, Dugan MC, Crissman JD, Vaitkevicius VK, Sarkar FH. Inhibition of pancreatic tumor cell growth in culture by p21WAF1 recombinant adenovirus. Pancreas. 1998;16:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Craig C, Kim M, Ohri E, Wersto R, Katayose D, Li Z, Choi YH, Mudahar B, Srivastava S, Seth P. Effects of adenovirus-mediated p16INK4A expression on cell cycle arrest are determined by endogenous p16 and Rb status in human cancer cells. Oncogene. 1998;16:265-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 82] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Zhang Q, Ni Q, Gan J, Shen Z, Luo J, Jin C, Zhang N, Zhang Y. p14ARF upregulation of p53 and enhanced effects of 5-fluorouracil in pancreatic cancer. Chin Med J (Engl). 2003;116:1150-1155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Bouvet M, Bold RJ, Lee J, Evans DB, Abbruzzese JL, Chiao PJ, McConkey DJ, Chandra J, Chada S, Fang B. Adenovirus-mediated wild-type p53 tumor suppressor gene therapy induces apoptosis and suppresses growth of human pancreatic cancer [seecomments]. Ann Surg Oncol. 1998;5:681-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Hill R, Rabb M, Madureira PA, Clements D, Gujar SA, Waisman DM, Giacomantonio CA, Lee PW. Gemcitabine-mediated tumour regression and p53-dependent gene expression: implications for colon and pancreatic cancer therapy. Cell Death Dis. 2013;4:e791. [PubMed] |
| 33. | Gupta S, Sathishkumar S, Ahmed MM. Influence of cell cycle checkpoints and p53 function on the toxicity of temozolomide in human pancreatic cancer cells. Pancreatology. 2010;10:565-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Hwang RF, Gordon EM, Anderson WF, Parekh D. Gene therapy for primary and metastatic pancreatic cancer with intraperitoneal retroviral vector bearing the wild-type p53 gene. Surgery. 1998;124:143-150; discussion 150-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 35. | Azmi AS, Philip PA, Aboukameel A, Wang Z, Banerjee S, Zafar SF, Goustin AS, Almhanna K, Yang D, Sarkar FH. Reactivation of p53 by novel MDM2 inhibitors: implications for pancreatic cancer therapy. Curr Cancer Drug Targets. 2010;10:319-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | Yin S, Goodrich DW. Combination gene therapy with p53 and Thoc1/p84 is more effective than either single agent in an animal model of human pancreatic adenocarcinoma. Int J Oncol. 2006;28:781-785. [PubMed] |
| 37. | Stiewe T, Pützer BM. p73 in apoptosis. Apoptosis. 2001;6:447-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Rödicker F, Pützer BM. p73 is effective in p53-null pancreatic cancer cells resistant to wild-type TP53 gene replacement. Cancer Res. 2003;63:2737-2741. [PubMed] |
| 39. | Schutte M, Hruban RH, Hedrick L, Cho KR, Nadasdy GM, Weinstein CL, Bova GS, Isaacs WB, Cairns P, Nawroz H. DPC4 gene in various tumor types. Cancer Res. 1996;56:2527-2530. [PubMed] |
| 40. | Duda DG, Sunamura M, Lefter LP, Furukawa T, Yokoyama T, Yatsuoka T, Abe T, Inoue H, Motoi F, Egawa S. Restoration of SMAD4 by gene therapy reverses the invasive phenotype in pancreatic adenocarcinoma cells. Oncogene. 2003;22:6857-6864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 41. | Shen W, Tao GQ, Li DC, Zhu XG, Bai X, Cai B. Inhibition of pancreatic carcinoma cell growth in vitro by DPC4 gene transfection. World J Gastroenterol. 2008;14:6254-6260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 42. | Niculescu-Duvaz I, Springer CJ. Introduction to the background, principles, and state of the art in suicide gene therapy. Mol Biotechnol. 2005;30:71-88. [PubMed] |
| 43. | Craperi D, Vicat JM, Nissou MF, Mathieu J, Baudier J, Benabid AL, Verna JM. Increased bax expression is associated with cell death induced by ganciclovir in a herpes thymidine kinase gene-expressing glioma cell line. Hum Gene Ther. 1999;10:679-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | Yang L, Chiang Y, Lenz HJ, Danenberg KD, Spears CP, Gordon EM, Anderson WF, Parekh D. Intercellular communication mediates the bystander effect during herpes simplex thymidine kinase/ganciclovir-based gene therapy of human gastrointestinal tumor cells. Hum Gene Ther. 1998;9:719-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 45. | Mäkinen K, Loimas S, Wahlfors J, Alhava E, Jänne J. Evaluation of herpes simplex thymidine kinase mediated gene therapy in experimental pancreatic cancer. J Gene Med. 2000;2:361-367. [PubMed] |
| 46. | Wang J, Lu XX, Chen DZ, Li SF, Zhang LS. Herpes simplex virus thymidine kinase and ganciclovir suicide gene therapy for human pancreatic cancer. World J Gastroenterol. 2004;10:400-403. [PubMed] |
| 47. | Carrió M, Romagosa A, Mercadé E, Mazo A, Nadal M, Gómez-Foix AM, Fillat C. Enhanced pancreatic tumor regression by a combination of adenovirus and retrovirus-mediated delivery of the herpes simplex virus thymidine kinase gene. Gene Ther. 1999;6:547-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 48. | Aoki K, Yoshida T, Matsumoto N, Ide H, Hosokawa K, Sugimura T, Terada M. Gene therapy for peritoneal dissemination of pancreatic cancer by liposome-mediated transfer of herpes simplex virus thymidine kinase gene. Hum Gene Ther. 1997;8:1105-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 49. | Fogar P, Greco E, Basso D, Habeler W, Navaglia F, Zambon CF, Tormen D, Gallo N, Cecchetto A, Plebani M. Suicide gene therapy with HSV-TK in pancreatic cancer has no effect in vivo in a mouse model. Eur J Surg Oncol. 2003;29:721-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | Greco E, Fogar P, Basso D, Stefani AL, Navaglia F, Zambon CF, Mazza S, Gallo N, Piva MG, Scarpa A. Retrovirus-mediated herpes simplex virus thymidine kinase gene transfer in pancreatic cancer cell lines: an incomplete antitumor effect. Pancreas. 2002;25:e21-e29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 51. | Li ZS, Pan X, Xu GM, Cui L, Dai GR, Gong YF, Tu ZX. Killing effects of cytosine deaminase gene mediated by adenovirus vector on human pancreatic cancer cell lines in vitro. Hepatobiliary Pancreat Dis Int. 2003;2:147-151. [PubMed] |
| 52. | Evoy D, Hirschowitz EA, Naama HA, Li XK, Crystal RG, Daly JM, Lieberman MD. In vivo adenoviral-mediated gene transfer in the treatment of pancreatic cancer. J Surg Res. 1997;69:226-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 53. | Zhang SN, Yuan SZ, Zhu ZH, Wen ZF, Huang ZQ, Zeng ZY. Apoptosis induced by 5-flucytosine in human pancreatic cancer cells genetically modified to express cytosine deaminase. Acta Pharmacol Sin. 2000;21:655-659. [PubMed] |
| 54. | Kaliberova LN, Della Manna DL, Krendelchtchikova V, Black ME, Buchsbaum DJ, Kaliberov SA. Molecular chemotherapy of pancreatic cancer using novel mutant bacterial cytosine deaminase gene. Mol Cancer Ther. 2008;7:2845-2854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 55. | Erbs P, Regulier E, Kintz J, Leroy P, Poitevin Y, Exinger F, Jund R, Mehtali M. In vivo cancer gene therapy by adenovirus-mediated transfer of a bifunctional yeast cytosine deaminase/uracil phosphoribosyltransferase fusion gene. Cancer Res. 2000;60:3813-3822. [PubMed] |
| 56. | Sunamura M, Hamada H, Motoi F, Oonuma M, Abe H, Saitoh Y, Hoshida T, Ottomo S, Omura N, Matsuno S. Oncolytic virotherapy as a novel strategy for pancreatic cancer. Pancreas. 2004;28:326-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 57. | Fogar P, Navaglia F, Basso D, Greco E, Zambon CF, Fadi E, Falda A, Stranges A, Vannozzi F, Danesi R. Suicide gene therapy with the yeast fusion gene cytosine deaminase/uracil phosphoribosyltransferase is not enough for pancreatic cancer. Pancreas. 2007;35:224-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 58. | Kaliberov SA, Chiz S, Kaliberova LN, Krendelchtchikova V, Della Manna D, Zhou T, Buchsbaum DJ. Combination of cytosine deaminase suicide gene expression with DR5 antibody treatment increases cancer cell cytotoxicity. Cancer Gene Ther. 2006;13:203-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 59. | Grove JI, Searle PF, Weedon SJ, Green NK, McNeish IA, Kerr DJ. Virus-directed enzyme prodrug therapy using CB1954. Anticancer Drug Des. 1999;14:461-472. [PubMed] |
| 60. | Green NK, Youngs DJ, Neoptolemos JP, Friedlos F, Knox RJ, Springer CJ, Anlezark GM, Michael NP, Melton RG, Ford MJ. Sensitization of colorectal and pancreatic cancer cell lines to the prodrug 5-(aziridin-1-yl)-2,4-dinitrobenzamide (CB1954) by retroviral transduction and expression of the E. coli nitroreductase gene. Cancer Gene Ther. 1997;4:229-238. [PubMed] |
| 61. | McNeish IA, Green NK, Gilligan MG, Ford MJ, Mautner V, Young LS, Kerr DJ, Searle PF. Virus directed enzyme prodrug therapy for ovarian and pancreatic cancer using retrovirally delivered E. coli nitroreductase and CB1954. Gene Ther. 1998;5:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 62. | Weedon SJ, Green NK, McNeish IA, Gilligan MG, Mautner V, Wrighton CJ, Mountain A, Young LS, Kerr DJ, Searle PF. Sensitisation of human carcinoma cells to the prodrug CB1954 by adenovirus vector-mediated expression of E. coli nitroreductase. Int J Cancer. 2000;86:848-854. [PubMed] |
| 63. | Hlavaty J, Petznek H, Holzmüller H, Url A, Jandl G, Berger A, Salmons B, Günzburg WH, Renner M. Evaluation of a gene-directed enzyme-product therapy (GDEPT) in human pancreatic tumor cells and their use as in vivo models for pancreatic cancer. PLoS One. 2012;7:e40611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 64. | Carrió M, Visa J, Cascante A, Estivill X, Fillat C. Intratumoral activation of cyclophosphamide by retroviral transfer of the cytochrome P450 2B1 in a pancreatic tumor model. Combination with the HSVtk/GCV system. J Gene Med. 2002;4:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 65. | Löhr M, Müller P, Karle P, Stange J, Mitzner S, Jesnowski R, Nizze H, Nebe B, Liebe S, Salmons B. Targeted chemotherapy by intratumour injection of encapsulated cells engineered to produce CYP2B1, an ifosfamide activating cytochrome P450. Gene Ther. 1998;5:1070-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 66. | Karle P, Müller P, Renz R, Jesnowski R, Saller R, von Rombs K, Nizze H, Liebe S, Günzburg WH, Salmons B. Intratumoral injection of encapsulated cells producing an oxazaphosphorine activating cytochrome P450 for targeted chemotherapy. Adv Exp Med Biol. 1998;451:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 67. | Löhr M, Hoffmeyer A, Kröger J, Freund M, Hain J, Holle A, Karle P, Knöfel WT, Liebe S, Müller P. Microencapsulated cell-mediated treatment of inoperable pancreatic carcinoma. Lancet. 2001;357:1591-1592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 138] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 68. | Seo Y, Baba H, Fukuda T, Takashima M, Sugimachi K. High expression of vascular endothelial growth factor is associated with liver metastasis and a poor prognosis for patients with ductal pancreatic adenocarcinoma. Cancer. 2000;88:2239-2245. [PubMed] |
| 69. | Bloomston M, Bhardwaj A, Ellison EC, Frankel WL. Epidermal growth factor receptor expression in pancreatic carcinoma using tissue microarray technique. Dig Surg. 2006;23:74-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 70. | Korc M, Chandrasekar B, Yamanaka Y, Friess H, Buchier M, Beger HG. Overexpression of the epidermal growth factor receptor in human pancreatic cancer is associated with concomitant increases in the levels of epidermal growth factor and transforming growth factor alpha. J Clin Invest. 1992;90:1352-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 352] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 71. | Wong HH, Lemoine NR. Pancreatic cancer: molecular pathogenesis and new therapeutic targets. Nat Rev Gastroenterol Hepatol. 2009;6:412-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 166] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 72. | Persano L, Crescenzi M, Indraccolo S. Anti-angiogenic gene therapy of cancer: current status and future prospects. Mol Aspects Med. 2007;28:87-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 73. | Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6747] [Cited by in RCA: 6918] [Article Influence: 314.5] [Reference Citation Analysis (0)] |
| 74. | Takayama K, Ueno H, Nakanishi Y, Sakamoto T, Inoue K, Shimizu K, Oohashi H, Hara N. Suppression of tumor angiogenesis and growth by gene transfer of a soluble form of vascular endothelial growth factor receptor into a remote organ. Cancer Res. 2000;60:2169-2177. [PubMed] |
| 75. | Tseng JF, Farnebo FA, Kisker O, Becker CM, Kuo CJ, Folkman J, Mulligan RC. Adenovirus-mediated delivery of a soluble form of the VEGF receptor Flk1 delays the growth of murine and human pancreatic adenocarcinoma in mice. Surgery. 2002;132:857-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 76. | Hoshida T, Sunamura M, Duda DG, Egawa S, Miyazaki S, Shineha R, Hamada H, Ohtani H, Satomi S, Matsuno S. Gene therapy for pancreatic cancer using an adenovirus vector encoding soluble flt-1 vascular endothelial growth factor receptor. Pancreas. 2002;25:111-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 77. | Büchler P, Reber HA, Ullrich A, Shiroiki M, Roth M, Büchler MW, Lavey RS, Friess H, Hines OJ. Pancreatic cancer growth is inhibited by blockade of VEGF-RII. Surgery. 2003;134:772-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 78. | Vachutinsky Y, Oba M, Miyata K, Hiki S, Kano MR, Nishiyama N, Koyama H, Miyazono K, Kataoka K. Antiangiogenic gene therapy of experimental pancreatic tumor by sFlt-1 plasmid DNA carried by RGD-modified crosslinked polyplex micelles. J Control Release. 2011;149:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 79. | Reinblatt M, Pin RH, Bowers WJ, Federoff HJ, Fong Y. Herpes simplex virus amplicon delivery of a hypoxia-inducible soluble vascular endothelial growth factor receptor (sFlk-1) inhibits angiogenesis and tumor growth in pancreatic adenocarcinoma. Ann Surg Oncol. 2005;12:1025-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 80. | Kuo CJ, Farnebo F, Yu EY, Christofferson R, Swearingen RA, Carter R, von Recum HA, Yuan J, Kamihara J, Flynn E. Comparative evaluation of the antitumor activity of antiangiogenic proteins delivered by gene transfer. Proc Natl Acad Sci USA. 2001;98:4605-4610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 228] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 81. | Brooks AN, Kilgour E, Smith PD. Molecular pathways: fibroblast growth factor signaling: a new therapeutic opportunity in cancer. Clin Cancer Res. 2012;18:1855-1862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 331] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 82. | Ogawa T, Takayama K, Takakura N, Kitano S, Ueno H. Anti-tumor angiogenesis therapy using soluble receptors: enhanced inhibition of tumor growth when soluble fibroblast growth factor receptor-1 is used with soluble vascular endothelial growth factor receptor. Cancer Gene Ther. 2002;9:633-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 83. | Braga MS, Turaça TL, Foguer K, Chaves KC, Pesquero JB, Chammas R, Schor N, Bellini MH. Vascular endothelial growth factor as a biomarker for endostatin gene therapy. Biomed Pharmacother. 2013;67:511-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 84. | Abdollahi A, Hlatky L, Huber PE. Endostatin: the logic of antiangiogenic therapy. Drug Resist Updat. 2005;8:59-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 85. | Kulke MH, Bergsland EK, Ryan DP, Enzinger PC, Lynch TJ, Zhu AX, Meyerhardt JA, Heymach JV, Fogler WE, Sidor C. Phase II study of recombinant human endostatin in patients with advanced neuroendocrine tumors. J Clin Oncol. 2006;24:3555-3561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 135] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 86. | Eder JP, Supko JG, Clark JW, Puchalski TA, Garcia-Carbonero R, Ryan DP, Shulman LN, Proper J, Kirvan M, Rattner B. Phase I clinical trial of recombinant human endostatin administered as a short intravenous infusion repeated daily. J Clin Oncol. 2002;20:3772-3784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 177] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 87. | Zhang X, Xu J, Lawler J, Terwilliger E, Parangi S. Adeno-associated virus-mediated antiangiogenic gene therapy with thrombospondin-1 type 1 repeats and endostatin. Clin Cancer Res. 2007;13:3968-3976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 88. | Shan YF, Fang YF, Wang XQ, Jin R, Zhang QY, Andersson R. Experimental studies on treatment of pancreatic cancer with double-regulated duplicative adenovirus AdTPHre-hEndo carrying human endostatin gene. Pancreatology. 2013;13:393-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 89. | Nie SL, Yuan SZ. Experimental study of gene therapy with angiostatin gene in pancreatic cancer. Hepatobiliary Pancreat Dis Int. 2002;1:452-457. [PubMed] |
| 90. | Li L, Yuan YZ, Lu J, Xia L, Zhu Y, Zhang YP, Qiao MM. Treatment of pancreatic carcinoma by adenoviral mediated gene transfer of vasostatin in mice. Gut. 2006;55:259-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 91. | Diao Y, Ma J, Li X, Sun X, Xu R. [Construction and activity of recombinant adeno-associated virus expressing vasostatin]. Shengwu Gongcheng Xuebao. 2008;24:1949-1954. [PubMed] |
| 92. | Tysome JR, Briat A, Alusi G, Cao F, Gao D, Yu J, Wang P, Yang S, Dong Z, Wang S. Lister strain of vaccinia virus armed with endostatin-angiostatin fusion gene as a novel therapeutic agent for human pancreatic cancer. Gene Ther. 2009;16:1223-1233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 93. | Kuba K, Matsumoto K, Date K, Shimura H, Tanaka M, Nakamura T. HGF/NK4, a four-kringle antagonist of hepatocyte growth factor, is an angiogenesis inhibitor that suppresses tumor growth and metastasis in mice. Cancer Res. 2000;60:6737-6743. [PubMed] |
| 94. | Furukawa T, Duguid WP, Kobari M, Matsuno S, Tsao MS. Hepatocyte growth factor and Met receptor expression in human pancreatic carcinogenesis. Am J Pathol. 1995;147:889-895. [PubMed] |
| 95. | Tomioka D, Maehara N, Kuba K, Mizumoto K, Tanaka M, Matsumoto K, Nakamura T. Inhibition of growth, invasion, and metastasis of human pancreatic carcinoma cells by NK4 in an orthotopic mouse model. Cancer Res. 2001;61:7518-7524. [PubMed] |
| 96. | Ogura Y, Mizumoto K, Nagai E, Murakami M, Inadome N, Saimura M, Matsumoto K, Nakamura T, Maemondo M, Nukiwa T. Peritumoral injection of adenovirus vector expressing NK4 combined with gemcitabine treatment suppresses growth and metastasis of human pancreatic cancer cells implanted orthotopically in nude mice and prolongs survival. Cancer Gene Ther. 2006;13:520-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 97. | Maehara N, Nagai E, Mizumoto K, Sato N, Matsumoto K, Nakamura T, Narumi K, Nukiwa T, Tanaka M. Gene transduction of NK4, HGF antagonist, inhibits in vitro invasion and in vivo growth of human pancreatic cancer. Clin Exp Metastasis. 2002;19:417-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 98. | Murakami M, Nagai E, Mizumoto K, Saimura M, Ohuchida K, Inadome N, Matsumoto K, Nakamura T, Maemondo M, Nukiwa T. Suppression of metastasis of human pancreatic cancer to the liver by transportal injection of recombinant adenoviral NK4 in nude mice. Int J Cancer. 2005;117:160-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 99. | Saimura M, Nagai E, Mizumoto K, Maehara N, Okino H, Katano M, Matsumoto K, Nakamura T, Narumi K, Nukiwa T. Intraperitoneal injection of adenovirus-mediated NK4 gene suppresses peritoneal dissemination of pancreatic cancer cell line AsPC-1 in nude mice. Cancer Gene Ther. 2002;9:799-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 100. | Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1323] [Cited by in RCA: 1313] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 101. | Bramhall SR, Neoptolemos JP, Stamp GW, Lemoine NR. Imbalance of expression of matrix metalloproteinases (MMPs) and tissue inhibitors of the matrix metalloproteinases (TIMPs) in human pancreatic carcinoma. J Pathol. 1997;182:347-355. [PubMed] |
| 102. | Bramhall SR, Rosemurgy A, Brown PD, Bowry C, Buckels JA. Marimastat as first-line therapy for patients with unresectable pancreatic cancer: a randomized trial. J Clin Oncol. 2001;19:3447-3455. [PubMed] |
| 103. | Bramhall SR, Schulz J, Nemunaitis J, Brown PD, Baillet M, Buckels JA. A double-blind placebo-controlled, randomised study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. Br J Cancer. 2002;87:161-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 402] [Cited by in RCA: 397] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 104. | Moore MJ, Hamm J, Dancey J, Eisenberg PD, Dagenais M, Fields A, Hagan K, Greenberg B, Colwell B, Zee B. Comparison of gemcitabine versus the matrix metalloproteinase inhibitor BAY 12-9566 in patients with advanced or metastatic adenocarcinoma of the pancreas: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2003;21:3296-3302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 276] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 105. | Bloomston M, Shafii A, Zervos EE, Rosemurgy AS. TIMP-1 overexpression in pancreatic cancer attenuates tumor growth, decreases implantation and metastasis, and inhibits angiogenesis. J Surg Res. 2002;102:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 106. | Rigg AS, Lemoine NR. Adenoviral delivery of TIMP1 or TIMP2 can modify the invasive behavior of pancreatic cancer and can have a significant antitumor effect in vivo. Cancer Gene Ther. 2001;8:869-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 107. | Carrere N, Vernejoul F, Souque A, Asnacios A, Vaysse N, Pradayrol L, Susini C, Buscail L, Cordelier P. Characterization of the bystander effect of somatostatin receptor sst2 after in vivo gene transfer into human pancreatic cancer cells. Hum Gene Ther. 2005;16:1175-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 108. | Appetecchia M, Baldelli R. Somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine tumours, current aspects and new perspectives. J Exp Clin Cancer Res. 2010;29:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 109. | Schaer JC, Waser B, Mengod G, Reubi JC. Somatostatin receptor subtypes sst1, sst2, sst3 and sst5 expression in human pituitary, gastroentero-pancreatic and mammary tumors: comparison of mRNA analysis with receptor autoradiography. Int J Cancer. 1997;70:530-537. [PubMed] |
| 110. | Buscail L, Saint-Laurent N, Chastre E, Vaillant JC, Gespach C, Capella G, Kalthoff H, Lluis F, Vaysse N, Susini C. Loss of sst2 somatostatin receptor gene expression in human pancreatic and colorectal cancer. Cancer Res. 1996;56:1823-1827. [PubMed] |
| 111. | Kumar M, Liu ZR, Thapa L, Qin RY. Anti-angiogenic effects of somatostatin receptor subtype 2 on human pancreatic cancer xenografts. Carcinogenesis. 2004;25:2075-2081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 112. | Kumar M, Liu ZR, Thapa L, Chang Q, Wang DY, Qin RY. Antiangiogenic effect of somatostatin receptor subtype 2 on pancreatic cancer cell line: Inhibition of vascular endothelial growth factor and matrix metalloproteinase-2 expression in vitro. World J Gastroenterol. 2004;10:393-399. [PubMed] |
| 113. | Chen L, Liu Q, Qin R, Le H, Xia R, Li W, Kumar M. Amplification and functional characterization of MUC1 promoter and gene-virotherapy via a targeting adenoviral vector expressing hSSTR2 gene in MUC1-positive Panc-1 pancreatic cancer cells in vitro. Int J Mol Med. 2005;15:617-626. [PubMed] |
| 114. | Zhang Z, Huang Y, Newman K, Gu J, Zhang X, Wu H, Zhao M, Xianyu Z, Liu X. Reexpression of human somatostatin receptor gene 2 gene mediated by oncolytic adenovirus increases antitumor activity of tumor necrosis factor-related apoptosis-inducing ligand against pancreatic cancer. Clin Cancer Res. 2009;15:5154-5160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 115. | Li M, Wang X, Li W, Li F, Yang H, Wang H, Brunicardi FC, Chen C, Yao Q, Fisher WE. Somatostatin receptor-1 induces cell cycle arrest and inhibits tumor growth in pancreatic cancer. Cancer Sci. 2008;99:2218-2223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 116. | Li M, Zhang R, Li F, Wang H, Kim HJ, Becnel L, Yao Q, Chen C, Fisher WE. Transfection of SSTR-1 and SSTR-2 inhibits Panc-1 cell proliferation and renders Panc-1 cells responsive to somatostatin analogue. J Am Coll Surg. 2005;201:571-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 117. | Cross D, Burmester JK. Gene therapy for cancer treatment: past, present and future. Clin Med Res. 2006;4:218-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 275] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 118. | Simons JW, Jaffee EM, Weber CE, Levitsky HI, Nelson WG, Carducci MA, Lazenby AJ, Cohen LK, Finn CC, Clift SM. Bioactivity of autologous irradiated renal cell carcinoma vaccines generated by ex vivo granulocyte-macrophage colony-stimulating factor gene transfer. Cancer Res. 1997;57:1537-1546. [PubMed] |
| 119. | Thomas MC, Greten TF, Pardoll DM, Jaffee EM. Enhanced tumor protection by granulocyte-macrophage colony-stimulating factor expression at the site of an allogeneic vaccine. Hum Gene Ther. 1998;9:835-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 120. | Greten TF, Jaffee EM. Cancer vaccines. J Clin Oncol. 1999;17:1047-1060. [PubMed] |
| 121. | Alpizar YA, Chain B, Collins MK, Greenwood J, Katz D, Stauss HJ, Mitchison NA. Ten years of progress in vaccination against cancer: the need to counteract cancer evasion by dual targeting in future therapies. Cancer Immunol Immunother. 2011;60:1127-1135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 122. | Wallich R, Bulbuc N, Hämmerling GJ, Katzav S, Segal S, Feldman M. Abrogation of metastatic properties of tumour cells by de novo expression of H-2K antigens following H-2 gene transfection. Nature. 1985;315:301-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 339] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 123. | Plautz GE, Yang ZY, Wu BY, Gao X, Huang L, Nabel GJ. Immunotherapy of malignancy by in vivo gene transfer into tumors. Proc Natl Acad Sci USA. 1993;90:4645-4649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 217] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 124. | Ostrand-Rosenberg S, Roby C, Clements VK, Cole GA. Tumor-specific immunity can be enhanced by transfection of tumor cells with syngeneic MHC-class-II genes or allogeneic MHC-class-I genes. Int J Cancer Suppl. 1991;6:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 125. | Townsend SE, Allison JP. Tumor rejection after direct costimulation of CD8+ T cells by B7-transfected melanoma cells. Science. 1993;259:368-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 855] [Cited by in RCA: 830] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 126. | Cayeux S, Beck C, Dörken B, Blankenstein T. Coexpression of interleukin-4 and B7.1 in murine tumor cells leads to improved tumor rejection and vaccine effect compared to single gene transfectants and a classical adjuvant. Hum Gene Ther. 1996;7:525-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 127. | Petrulio CA, Kaufman HL. Development of the PANVAC-VF vaccine for pancreatic cancer. Expert Rev Vaccines. 2006;5:9-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 128. | Madan RA, Arlen PM, Gulley JL. PANVAC-VF: poxviral-based vaccine therapy targeting CEA and MUC1 in carcinoma. Expert Opin Biol Ther. 2007;7:543-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 129. | Kimura M, Tagawa M, Takenaga K, Kondo F, Yamaguchi T, Saisho H, Nakagawara A, Sakiyama S. Loss of tumorigenicity of human pancreatic carcinoma cells engineered to produce interleukin-2 or interleukin-4 in nude mice: a potentiality for cancer gene therapy. Cancer Lett. 1998;128:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 130. | Liu L, Meng J, Zhang C, Duan Y, Zhao L, Wang S, Shan B. Effects on apoptosis and cell cycle arrest contribute to the antitumor responses of interleukin-27 mediated by retrovirus in human pancreatic carcinoma cells. Oncol Rep. 2012;27:1497-1503. [PubMed] |
| 131. | Peplinski GR, Tsung K, Meko JB, Norton JA. In vivo gene therapy of a murine pancreas tumor with recombinant vaccinia virus encoding human interleukin-1 beta. Surgery. 1995;118:185-190; discussion 190-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 132. | Xie FJ, Zhao P, Zhang YP, Liu FY, Nie XL, Zhu YH, Yu XM, Zheng QQ, Mao WM, Lu HY. Adenovirus-mediated interferon-γ gene therapy induced human pancreatic carcinoma Capan-2 cell apoptosis in vitro and in vivo. Anat Rec (Hoboken). 2013;296:604-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 133. | Kidd S, Caldwell L, Dietrich M, Samudio I, Spaeth EL, Watson K, Shi Y, Abbruzzese J, Konopleva M, Andreeff M. Mesenchymal stromal cells alone or expressing interferon-beta suppress pancreatic tumors in vivo, an effect countered by anti-inflammatory treatment. Cytotherapy. 2010;12:615-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 145] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 134. | Ravet E, Lulka H, Gross F, Casteilla L, Buscail L, Cordelier P. Using lentiviral vectors for efficient pancreatic cancer gene therapy. Cancer Gene Ther. 2010;17:315-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 135. | Armstrong L, Davydova J, Brown E, Han J, Yamamoto M, Vickers SM. Delivery of interferon alpha using a novel Cox2-controlled adenovirus for pancreatic cancer therapy. Surgery. 2012;152:114-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 136. | Murugesan SR, King CR, Osborn R, Fairweather WR, O’Reilly EM, Thornton MO, Wei LL. Combination of human tumor necrosis factor-alpha (hTNF-alpha) gene delivery with gemcitabine is effective in models of pancreatic cancer. Cancer Gene Ther. 2009;16:841-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 137. | Zhu Y, Tibensky I, Schmidt J, Hackert T, Ryschich E, Jäger D, Büchler MW, Märten A. Interferon-alpha in combination with chemotherapy has potent antiangiogenic properties in an orthotopic mouse model for pancreatic adenocarcinoma. J Immunother. 2008;31:28-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 138. | Endou M, Mizuno M, Nagata T, Tsukada K, Nakahara N, Tsuno T, Osawa H, Kuno T, Fujita M, Hatano M. Growth inhibition of human pancreatic cancer cells by human interferon-beta gene combined with gemcitabine. Int J Mol Med. 2005;15:277-283. [PubMed] |
| 139. | Hecht JR, Farrell JJ, Senzer N, Nemunaitis J, Rosemurgy A, Chung T, Hanna N, Chang KJ, Javle M, Posner M. EUS or percutaneously guided intratumoral TNFerade biologic with 5-fluorouracil and radiotherapy for first-line treatment of locally advanced pancreatic cancer: a phase I/II study. Gastrointest Endosc. 2012;75:332-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 140. | Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan RC. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539-3543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2032] [Cited by in RCA: 2010] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 141. | Jaffee EM, Hruban RH, Biedrzycki B, Laheru D, Schepers K, Sauter PR, Goemann M, Coleman J, Grochow L, Donehower RC. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol. 2001;19:145-156. [PubMed] |
| 142. | Laheru D, Lutz E, Burke J, Biedrzycki B, Solt S, Onners B, Tartakovsky I, Nemunaitis J, Le D, Sugar E. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res. 2008;14:1455-1463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 285] [Cited by in RCA: 268] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 143. | Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, Zheng L, Diaz LA, Donehower RC, Jaffee EM. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother. 2013;36:382-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 415] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 144. | Kudrin A. Overview of cancer vaccines: considerations for development. Hum Vaccin Immunother. 2012;8:1335-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 145. | Plate JM. Advances in therapeutic vaccines for pancreatic cancer. Discov Med. 2012;14:89-94. [PubMed] |
| 146. | Kameshima H, Tsuruma T, Kutomi G, Shima H, Iwayama Y, Kimura Y, Imamura M, Torigoe T, Takahashi A, Hirohashi Y. Immunotherapeutic benefit of α-interferon (IFNα) in survivin2B-derived peptide vaccination for advanced pancreatic cancer patients. Cancer Sci. 2013;104:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 147. | Okusaka T, Ueno M, Sato T, Heike Y. Possibility of immunotherapy for biliary tract cancer: how do we prove efficacy? Introduction to a current ongoing phase I and randomized phase II study to evaluate the efficacy and safety of adding Wilms tumor 1 peptide vaccine to gemcitabine and cisplatin for the treatment of advanced biliary tract cancer (WT-BT trial). J Hepatobiliary Pancreat Sci. 2012;19:314-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 148. | Maki RG, Livingston PO, Lewis JJ, Janetzki S, Klimstra D, Desantis D, Srivastava PK, Brennan MF. A phase I pilot study of autologous heat shock protein vaccine HSPPC-96 in patients with resected pancreatic adenocarcinoma. Dig Dis Sci. 2007;52:1964-1972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 149. | Rong Y, Qin X, Jin D, Lou W, Wu L, Wang D, Wu W, Ni X, Mao Z, Kuang T. A phase I pilot trial of MUC1-peptide-pulsed dendritic cells in the treatment of advanced pancreatic cancer. Clin Exp Med. 2012;12:173-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 150. | Gjertsen MK, Buanes T, Rosseland AR, Bakka A, Gladhaug I, Søreide O, Eriksen JA, Møller M, Baksaas I, Lothe RA. Intradermal ras peptide vaccination with granulocyte-macrophage colony-stimulating factor as adjuvant: Clinical and immunological responses in patients with pancreatic adenocarcinoma. Int J Cancer. 2001;92:441-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 197] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 151. | Gilliam AD, Broome P, Topuzov EG, Garin AM, Pulay I, Humphreys J, Whitehead A, Takhar A, Rowlands BJ, Beckingham IJ. An international multicenter randomized controlled trial of G17DT in patients with pancreatic cancer. Pancreas. 2012;41:374-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 152. | Bernhardt SL, Gjertsen MK, Trachsel S, Møller M, Eriksen JA, Meo M, Buanes T, Gaudernack G. Telomerase peptide vaccination of patients with non-resectable pancreatic cancer: A dose escalating phase I/II study. Br J Cancer. 2006;95:1474-1482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 220] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 153. | Hardacre JM, Mulcahy M, Small W, Talamonti M, Obel J, Krishnamurthi S, Rocha-Lima CS, Safran H, Lenz HJ, Chiorean EG. Addition of algenpantucel-L immunotherapy to standard adjuvant therapy for pancreatic cancer: a phase 2 study. J Gastrointest Surg. 2013;17:94-100; discussion 100-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 154. | Kong R, Jia G, Cheng ZX, Wang YW, Mu M, Wang SJ, Pan SH, Gao Y, Jiang HC, Dong DL. Dihydroartemisinin enhances Apo2L/TRAIL-mediated apoptosis in pancreatic cancer cells via ROS-mediated up-regulation of death receptor 5. PLoS One. 2012;7:e37222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 155. | Wu GS. TRAIL as a target in anti-cancer therapy. Cancer Lett. 2009;285:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 156. | Katz MH, Spivack DE, Takimoto S, Fang B, Burton DW, Moossa AR, Hoffman RM, Bouvet M. Gene therapy of pancreatic cancer with green fluorescent protein and tumor necrosis factor-related apoptosis-inducing ligand fusion gene expression driven by a human telomerase reverse transcriptase promoter. Ann Surg Oncol. 2003;10:762-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 157. | Jacob D, Davis JJ, Zhang L, Zhu H, Teraishi F, Fang B. Suppression of pancreatic tumor growth in the liver by systemic administration of the TRAIL gene driven by the hTERT promoter. Cancer Gene Ther. 2005;12:109-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 158. | Moniri MR, Sun XY, Rayat J, Dai D, Ao Z, He Z, Verchere CB, Dai LJ, Warnock GL. TRAIL-engineered pancreas-derived mesenchymal stem cells: characterization and cytotoxic effects on pancreatic cancer cells. Cancer Gene Ther. 2012;19:652-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 159. | Grisendi G, Bussolari R, Cafarelli L, Petak I, Rasini V, Veronesi E, De Santis G, Spano C, Tagliazzucchi M, Barti-Juhasz H. Adipose-derived mesenchymal stem cells as stable source of tumor necrosis factor-related apoptosis-inducing ligand delivery for cancer therapy. Cancer Res. 2010;70:3718-3729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 187] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 160. | Gnoni A, Licchetta A, Scarpa A, Azzariti A, Brunetti AE, Simone G, Nardulli P, Santini D, Aieta M, Delcuratolo S. Carcinogenesis of pancreatic adenocarcinoma: precursor lesions. Int J Mol Sci. 2013;14:19731-19762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 161. | Brummelkamp TR, Bernards R, Agami R. Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell. 2002;2:243-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 843] [Cited by in RCA: 820] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 162. | Réjiba S, Wack S, Aprahamian M, Hajri A. K-ras oncogene silencing strategy reduces tumor growth and enhances gemcitabine chemotherapy efficacy for pancreatic cancer treatment. Cancer Sci. 2007;98:1128-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 163. | Wang YX, Gao L, Ji ZZ. Inhibitary effects of antisense oligonucleotide specific to K-ras point mutation on the target gene expression in human pancreatic carcinoma cells. Chin Med J (Engl). 2007;120:1448-1450. [PubMed] |
| 164. | Morioka CY, Machado MC, Saito S, Nakada Y, Matheus AS, Jukemura J, Bacchella T, Takahara T, Watanabe A. Suppression of invasion of a hamster pancreatic cancer cell line by antisense oligonucleotides mutation-matched to K-ras gene. In Vivo. 2005;19:535-538. [PubMed] |
| 165. | Miura Y, Ohnami S, Yoshida K, Ohashi M, Nakano M, Ohnami S, Fukuhara M, Yanagi K, Matsushita A, Uchida E. Intraperitoneal injection of adenovirus expressing antisense K-ras RNA suppresses peritoneal dissemination of hamster syngeneic pancreatic cancer without systemic toxicity. Cancer Lett. 2005;218:53-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 166. | Shen YM, Yang XC, Yang C, Shen JK. Enhanced therapeutic effects for human pancreatic cancer by application K-ras and IGF-IR antisense oligodeoxynucleotides. World J Gastroenterol. 2008;14:5176-5185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 167. | Alberts SR, Schroeder M, Erlichman C, Steen PD, Foster NR, Moore DF, Rowland KM, Nair S, Tschetter LK, Fitch TR. Gemcitabine and ISIS-2503 for patients with locally advanced or metastatic pancreatic adenocarcinoma: a North Central Cancer Treatment Group phase II trial. J Clin Oncol. 2004;22:4944-4950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 168. | Kelley JR, Fraser MM, Hubbard JM, Watson DK, Cole DJ. CaSm antisense gene therapy: a novel approach for the treatment of pancreatic cancer. Anticancer Res. 2003;23:2007-2013. [PubMed] |
| 169. | Yan Y, Rubinchik S, Wood AL, Gillanders WE, Dong JY, Watson DK, Cole DJ. Bystander effect contributes to the antitumor efficacy of CaSm antisense gene therapy in a preclinical model of advanced pancreatic cancer. Mol Ther. 2006;13:357-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 170. | Chou A, Waddell N, Cowley MJ, Gill AJ, Chang DK, Patch AM, Nones K, Wu J, Pinese M, Johns AL. Clinical and molecular characterization of HER2 amplified-pancreatic cancer. Genome Med. 2013;5:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 171. | Harder J, Ihorst G, Heinemann V, Hofheinz R, Moehler M, Buechler P, Kloeppel G, Röcken C, Bitzer M, Boeck S. Multicentre phase II trial of trastuzumab and capecitabine in patients with HER2 overexpressing metastatic pancreatic cancer. Br J Cancer. 2012;106:1033-1038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 139] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 172. | Tanaka M, Okazaki T, Suzuki H, Abbruzzese JL, Li D. Association of multi-drug resistance gene polymorphisms with pancreatic cancer outcome. Cancer. 2011;117:744-751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 173. | Lu Z, Kleeff J, Shrikhande S, Zimmermann T, Korc M, Friess H, Büchler MW. Expression of the multidrug-resistance 1 (MDR1) gene and prognosis in human pancreatic cancer. Pancreas. 2000;21:240-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 174. | Suwa H, Ohshio G, Arao S, Imamura T, Yamaki K, Manabe T, Imamura M, Hiai H, Fukumoto M. Immunohistochemical localization of P-glycoprotein and expression of the multidrug resistance-1 gene in human pancreatic cancer: relevance to indicator of better prognosis. Jpn J Cancer Res. 1996;87:641-649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 175. | Bergman AM, Pinedo HM, Talianidis I, Veerman G, Loves WJ, van der Wilt CL, Peters GJ. Increased sensitivity to gemcitabine of P-glycoprotein and multidrug resistance-associated protein-overexpressing human cancer cell lines. Br J Cancer. 2003;88:1963-1970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 176. | König J, Hartel M, Nies AT, Martignoni ME, Guo J, Büchler MW, Friess H, Keppler D. Expression and localization of human multidrug resistance protein (ABCC) family members in pancreatic carcinoma. Int J Cancer. 2005;115:359-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 147] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 177. | Diestra JE, Scheffer GL, Català I, Maliepaard M, Schellens JH, Scheper RJ, Germà-Lluch JR, Izquierdo MA. Frequent expression of the multi-drug resistance-associated protein BCRP/MXR/ABCP/ABCG2 in human tumours detected by the BXP-21 monoclonal antibody in paraffin-embedded material. J Pathol. 2002;198:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 203] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 178. | Lee SH, Kim H, Hwang JH, Lee HS, Cho JY, Yoon YS, Han HS. Breast cancer resistance protein expression is associated with early recurrence and decreased survival in resectable pancreatic cancer patients. Pathol Int. 2012;62:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 179. | Nieth C, Priebsch A, Stege A, Lage H. Modulation of the classical multidrug resistance (MDR) phenotype by RNA interference (RNAi). FEBS Lett. 2003;545:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 143] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 180. | Holm PS, Scanlon KJ, Dietel M. Reversion of multidrug resistance in the P-glycoprotein-positive human pancreatic cell line (EPP85-181RDB) by introduction of a hammerhead ribozyme. Br J Cancer. 1994;70:239-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 181. | Maier P, Heckmann D, Spier I, Laufs S, Zucknick M, Allgayer H, Fruehauf S, Zeller WJ, Wenz F. F2A sequence linking MGMT(P140K) and MDR1 in a bicistronic lentiviral vector enables efficient chemoprotection of haematopoietic stem cells. Cancer Gene Ther. 2012;19:802-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 182. | Maier P, Spier I, Laufs S, Veldwijk MR, Fruehauf S, Wenz F, Zeller WJ. Chemoprotection of human hematopoietic stem cells by simultaneous lentiviral overexpression of multidrug resistance 1 and O(6)-methylguanine-DNA methyltransferase(P140K). Gene Ther. 2010;17:389-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 183. | Southgate TD, Garside E, Margison GP, Fairbairn LJ. Dual agent chemoprotection by retroviral co-expression of either MDR1 or MRP1 with the P140K mutant of O6-methylguanine-DNA-methyl transferase. J Gene Med. 2006;8:972-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 184. | Carpinteiro A, Peinert S, Ostertag W, Zander AR, Hossfeld DK, Kühlcke K, Eckert HG, Baum C, Hegewisch-Becker S. Genetic protection of repopulating hematopoietic cells with an improved MDR1-retrovirus allows administration of intensified chemotherapy following stem cell transplantation in mice. Int J Cancer. 2002;98:785-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 185. | Licht T, Gottesman MM, Pastan I. Transfer of the MDR1 (multidrug resistance) gene: protection of hematopoietic cells from cytotoxic chemotherapy, and selection of transduced cells in vivo. Cytokines Mol Ther. 1995;1:11-20. [PubMed] |
| 186. | Li K, Tao J, Li T, Yu Z, Yang Z, Wu H, Xiong J, Wang C. Effect of antisense oligodeoxynucleotide of vascular endothelial growth factor C on lymphangiogenesis and angiogenesis of pancreatic cancer. J Huazhong Univ Sci Technolog Med Sci. 2007;27:51-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 187. | Shi Y, Tong M, Wu Y, Yang Z, Hoffman RM, Zhang Y, Tian Y, Qi M, Lin Y, Liu Y. VEGF-C ShRNA inhibits pancreatic cancer growth and lymphangiogenesis in an orthotopic fluorescent nude mouse model. Anticancer Res. 2013;33:409-417. [PubMed] |
| 188. | Hotz HG, Hines OJ, Masood R, Hotz B, Foitzik T, Buhr HJ, Gill PS, Reber HA. VEGF antisense therapy inhibits tumor growth and improves survival in experimental pancreatic cancer. Surgery. 2005;137:192-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 189. | Pittella F, Miyata K, Maeda Y, Suma T, Watanabe S, Chen Q, Christie RJ, Osada K, Nishiyama N, Kataoka K. Pancreatic cancer therapy by systemic administration of VEGF siRNA contained in calcium phosphate/charge-conversional polymer hybrid nanoparticles. J Control Release. 2012;161:868-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 190. | Wang J, Shi YQ, Yi J, Ye S, Wang LM, Xu YP, He M, Kong XM. Suppression of growth of pancreatic cancer cell and expression of vascular endothelial growth factor by gene silencing with RNA interference. J Dig Dis. 2008;9:228-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 191. | Tokunaga T, Abe Y, Tsuchida T, Hatanaka H, Oshika Y, Tomisawa M, Yoshimura M, Ohnishi Y, Kijima H, Yamazaki H. Ribozyme mediated cleavage of cell-associated isoform of vascular endothelial growth factor inhibits liver metastasis of a pancreatic cancer cell line. Int J Oncol. 2002;21:1027-1032. [PubMed] |
| 192. | Deeb D, Gao X, Liu Y, Kim SH, Pindolia KR, Arbab AS, Gautam SC. Inhibition of cell proliferation and induction of apoptosis by oleanane triterpenoid (CDDO-Me) in pancreatic cancer cells is associated with the suppression of hTERT gene expression and its telomerase activity. Biochem Biophys Res Commun. 2012;422:561-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 193. | Sato N, Maehara N, Mizumoto K, Nagai E, Yasoshima T, Hirata K, Tanaka M. Telomerase activity of cultured human pancreatic carcinoma cell lines correlates with their potential for migration and invasion. Cancer. 2001;91:496-504. [PubMed] |
| 194. | Hiyama E, Kodama T, Shinbara K, Iwao T, Itoh M, Hiyama K, Shay JW, Matsuura Y, Yokoyama T. Telomerase activity is detected in pancreatic cancer but not in benign tumors. Cancer Res. 1997;57:326-331. [PubMed] |
| 195. | Myung SJ, Kim MH, Kim YS, Kim HJ, Park ET, Yoo KS, Lim BC, Wan Seo D, Lee SK, Min YI. Telomerase activity in pure pancreatic juice for the diagnosis of pancreatic cancer may be complementary to K-ras mutation. Gastrointest Endosc. 2000;51:708-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 196. | Hiyama E, Hiyama K. Telomerase as tumor marker. Cancer Lett. 2003;194:221-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 128] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 197. | Janknecht R. On the road to immortality: hTERT upregulation in cancer cells. FEBS Lett. 2004;564:9-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 198. | Chung HK, Cheong C, Song J, Lee HW. Extratelomeric functions of telomerase. Curr Mol Med. 2005;5:233-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 199. | Wang YF, Guo KJ, Huang BT, Liu Y, Tang XY, Zhang JJ, Xia Q. Inhibitory effects of antisense phosphorothioate oligodeoxynucleotides on pancreatic cancer cell Bxpc-3 telomerase activity and cell growth in vitro. World J Gastroenterol. 2006;12:4004-4008. [PubMed] |
| 200. | Liu YP, Hu YD, Wang F, Ling Y, Kong YZ, Li P. [Effect of human telomerase reverse transcriptase gene antisense oligonucleotide on sensitivity of gemcitabine in pancreatic cancer cell]. Zhonghua Yixue Zazhi. 2009;89:2391-2394. [PubMed] |
| 201. | Liu Y, Ling Y, Hu Y, Kong Y, Li P, Zhou Z, Liu B. Antitumor efficacy of human telomerase reverse transcriptase gene antisense oligonucleotide in pancreatic cancer cells. Mol Med Rep. 2009;2:515-521. [PubMed] |
| 202. | Zhong YQ, Xia ZS, Fu YR, Zhu ZH. Knockdown of hTERT by SiRNA suppresses growth of Capan-2 human pancreatic cancer cell via the inhibition of expressions of Bcl-2 and COX-2. J Dig Dis. 2010;11:176-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 203. | Hayashidani Y, Hiyama E, Murakami Y, Sueda T. Attenuation of telomerase activity by hammerhead ribozymes targeting human telomerase RNA and telomerase reverse transcriptase in pancreatic carcinoma cells. Hiroshima J Med Sci. 2005;54:21-27. [PubMed] |
| 204. | Tucker ON, Dannenberg AJ, Yang EK, Zhang F, Teng L, Daly JM, Soslow RA, Masferrer JL, Woerner BM, Koki AT, Fahey TJ. Cyclooxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Res. 1999;59:987-990. [PubMed] |
| 205. | Yip-Schneider MT, Barnard DS, Billings SD, Cheng L, Heilman DK, Lin A, Marshall SJ, Crowell PL, Marshall MS, Sweeney CJ. Cyclooxygenase-2 expression in human pancreatic adenocarcinomas. Carcinogenesis. 2000;21:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 224] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 206. | Zhong Y, Xia Z, Liu J, Lin Y, Zan H. The effects of cyclooxygenase-2 gene silencing by siRNA on cell proliferation, cell apoptosis, cell cycle and tumorigenicity of Capan-2 human pancreatic cancer cells. Oncol Rep. 2012;27:1003-1010. [PubMed] |
| 207. | Pecher G, Häring A, Kaiser L, Thiel E. Mucin gene (MUC1) transfected dendritic cells as vaccine: results of a phase I/II clinical trial. Cancer Immunol Immunother. 2002;51:669-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 116] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 208. | Kubuschok B, Cochlovius C, Jung W, Schmits R, Trümper L, Hartmann F, Renner C, Pfreundschuh M. Gene-modified spontaneous Epstein-Barr virus-transformed lymphoblastoid cell lines as autologous cancer vaccines: mutated p21 ras oncogene as a model. Cancer Gene Ther. 2000;7:1231-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 209. | Niethammer AG, Lubenau H, Mikus G, Knebel P, Hohmann N, Leowardi C, Beckhove P, Akhisaroglu M, Ge Y, Springer M. Double-blind, placebo-controlled first in human study to investigate an oral vaccine aimed to elicit an immune reaction against the VEGF-Receptor 2 in patients with stage IV and locally advanced pancreatic cancer. BMC Cancer. 2012;12:361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 210. | Mazzolini G, Alfaro C, Sangro B, Feijoó E, Ruiz J, Benito A, Tirapu I, Arina A, Sola J, Herraiz M. Intratumoral injection of dendritic cells engineered to secrete interleukin-12 by recombinant adenovirus in patients with metastatic gastrointestinal carcinomas. J Clin Oncol. 2005;23:999-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 132] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 211. | Gilly FN, Beaujard A, Bienvenu J, Trillet Lenoir V, Glehen O, Thouvenot D, Malcus C, Favrot M, Dumontet C, Lombard-Bohas C. Gene therapy with Adv-IL-2 in unresectable digestive cancer: phase I-II study, intermediate report. Hepatogastroenterology. 1999;46 Suppl 1:1268-1273. [PubMed] |
| 212. | Le DT, Brockstedt DG, Nir-Paz R, Hampl J, Mathur S, Nemunaitis J, Sterman DH, Hassan R, Lutz E, Moyer B. A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: phase I studies of safety and immune induction. Clin Cancer Res. 2012;18:858-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 271] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 213. | Galanis E, Carlson SK, Foster NR, Lowe V, Quevedo F, McWilliams RR, Grothey A, Jatoi A, Alberts SR, Rubin J. Phase I trial of a pathotropic retroviral vector expressing a cytocidal cyclin G1 construct (Rexin-G) in patients with advanced pancreatic cancer. Mol Ther. 2008;16:979-984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 214. | Kaufman HL, Kim-Schulze S, Manson K, DeRaffele G, Mitcham J, Seo KS, Kim DW, Marshall J. Poxvirus-based vaccine therapy for patients with advanced pancreatic cancer. J Transl Med. 2007;5:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 215. | Lutz E, Yeo CJ, Lillemoe KD, Biedrzycki B, Kobrin B, Herman J, Sugar E, Piantadosi S, Cameron JL, Solt S. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann Surg. 2011;253:328-335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 296] [Article Influence: 21.1] [Reference Citation Analysis (0)] |