Published online Sep 14, 2014. doi: 10.3748/wjg.v20.i34.12118
Revised: January 23, 2014
Accepted: May 29, 2014
Published online: September 14, 2014
Processing time: 264 Days and 19.6 Hours
Pancreatic cancer is the 4th leading cause of cancer-related death in Western countries. Considering the low incidence of pancreatic cancer, population-based screening is not feasible. However, the existence of a group of individuals with an increased risk to develop pancreatic cancer has been well established. In particular, individuals suffering from a somatic or genetic condition associated with an increased relative risk of more than 5- to 10-fold seem to be suitable for enrollment in a surveillance program for prevention or early detection of pancreatic cancer. The aim of such a program is to reduce pancreatic cancer mortality through early or preemptive surgery. Considering the risk associated with pancreatic surgery, the concept of preemptive surgery cannot consist of a prophylactic removal of the pancreas in high-risk healthy individuals, but must instead aim at treating precancerous lesions such as intraductal papillary mucinous neoplasms or pancreatic intraepithelial neoplasms, or early cancer. Currently, results from clinical trials do not convincingly demonstrate the efficacy of this approach in terms of identification of precancerous lesions, nor do they define the outcome of the surgical treatment of these lesions. For this reason, surveillance programs for individuals at risk of pancreatic cancer are thus far generally limited to the setting of a clinical trial. However, the acquisition of a deeper understanding of this complex area, together with the increasing request for screening and treatment by individuals at risk, will usher pancreatologists into a new era of preemptive pancreatic surgery. Along with the growing demand to treat individuals with precancerous lesions, the need for low-risk investigation, low-morbidity operation and a minimally invasive approach becomes increasingly pressing. All of these considerations are reasons for preemptive pancreatic surgery programs to be undertaken in specialized centers only.
Core tip: Pancreatic cancer is the 4th leading cause of cancer-related death in Western countries. Considering the low incidence of pancreatic cancer, population-based screening is not feasible. This review analyzes the possibility to identify a population at risk for pancreatic cancer and the strategies for clinical screening and prevention.
- Citation: Chiaro MD, Segersvärd R, Lohr M, Verbeke C. Early detection and prevention of pancreatic cancer: Is it really possible today? World J Gastroenterol 2014; 20(34): 12118-12131
- URL: https://www.wjgnet.com/1007-9327/full/v20/i34/12118.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i34.12118
Pancreatic cancer (PC) is the fourth leading cause of cancer-related death in Western countries. It ranks amongst the most lethal cancers and has a mortality rate that nearly equals the incidence rate and an overall 5-years survival of approximately 5%[1-3].
During the last decades there has been an overall reduction in cancer-related mortality in Western countries, in particular for lung, breast, colorectal and prostate cancer[1,4]. In contrast, mortality rates increased for PC, and the prediction for the year 2013 shows the same trend[4].
The success in reducing cancer mortality for some of the human solid tumors is not only related to the discovery of new therapeutic agents, but to a significant extent it has also been the result of the development of early detection and prevention programs. Born from this concept is a new surgical approach for patients at risk of cancer: preemptive surgery. Preemptive surgery can be defined as the prophylactic removal of an organ at high risk for malignant transformation or the resection of a precancerous lesion or an “early” malignant neoplasm in an individual with a predisposition to cancer[5]. Today, preemptive surgery is recognized as a useful approach for the management of various premalignant lesions or conditions and for the prevention of cancer in high-risk individuals. For patients with high-grade dysplasia in Barrett’s esophagus, esophagectomy is a therapeutic option to prevent esophageal cancer from developing[6-10]. Total gastrectomy can be performed to prevent gastric cancer in the rare case of familial gastric cancer[11-13]. Bile duct cyst resection or “early” liver transplantation in primary sclerosing cholangitis is proposed to reduce the incidence of cholangiocarcinoma[14,15]. Total thyroidectomy may be recommended for individuals with multiple endocrine neoplasia type 2/familial medullary carcinoma[16]. Preemptive bilateral mastectomy and possibly bilateral oophorectomy in female carriers of BRCA1 or BRCA2 mutations reduce the risk of ovarian cancer and breast cancer by more than 90%[17,18]. Patients with hereditary non-polyposis colorectal cancer or familial adenomatous polyposis can be advised to undergo prophylactic total proctocolectomy to prevent colon cancer development[19]. Furthermore, a population-wide screening program to detect premalignant lesions or early cancer is already implemented for different tumor types such as colorectal, breast and lung cancer[20-24].
Historically, PC was not considered a disease suitable for a preventive or early detection program given the aggressive biology and rapid progression of the tumor and the presumed low prevalence of high-risk individuals. Today, newly acquired knowledge regarding the biology and natural history of PC has changed this view. Even if PC is biologically aggressive at the time of diagnosis, it follows a multistep carcinogenesis process, which is very similar to that of other cancers (e.g., colon cancer) and consists essentially of a steady progression through increasing grades of dysplasia[25]. Since this process takes about a decade before the neoplastic lesion becomes an invasive cancer[26], there is a considerable window of opportunity to potentially detect the tumor at an early (pre-)invasive stage[27].
Today it has also been recognized that some of the neoplastic precursor lesions, such as intraductal papillary mucinous neoplasm (IPMN) or pancreatic intraepithelial neoplasia (PanIN), can be detected at an early stage using currently available imaging techniques[28,29].
Even if population-based screening for PC is not considered cost-effective given the relatively low incidence of the disease[25], specific screening programs for subgroups of high-risk individuals are currently evaluated by the scientific community regarding the detection of precursor lesions or early cancers and the impact on PC-related mortality[29]. In this paper we analyze the conditions that are associated with an increased risk of PC, the groups of individuals potentially suitable for screening programs, the target lesions for screening, and the potential treatment for these conditions.
A wide range of conditions are associated with an increased risk of PC. Overall, these can be divided in two distinct groups, i.e., hereditary and non-hereditary conditions.
These non-hereditary conditions are frequently associated with potentially correctable life-style factors and habits. Although they are well-established risk factors for PC, the associated increase in risk is too low to justify screening of the affected individuals. Smoking, obesity, alcohol abuse and exposure to toxic substances (Table 1) are potentially all suitable for primary prevention. Further non-genetic conditions associated with an increased risk of PC are diabetes type 1 and 2, chronic pancreatitis and a history of peptic ulcer (Table 1)[30-36]. Only the risk associated with chronic pancreatitis seems to be sufficiently high to justify screening of affected individuals.
| Risk factors | Estimated overall risk | Ref. |
| Smoking | 1.75 | [37,38] |
| Overweight | 1.12 per increased 5 kg/m2 | [39-41] |
| Alchohol abuse | 1.2 | [42,43] |
| Type 1 diabetes | 2.0 | [44] |
| New onset type 2 diabetes | 2.0 | [45] |
| Chronic pancreatitis | 14.0 | [46] |
| Exposure to nickel | 1.9 | [47] |
| Previous gastric ulcer | 1.8 | [48] |
The majority of patients within this group suffer from a pancreatic cystic neoplasm, in particular IPMN or mucinous cystic neoplasia (MCN), which bear an established risk of malignant transformation. Furthermore, recent data indicate that patients who underwent solid organ transplantation also have an increased risk of PC.
Pancreatic cystic neoplasms (PCN) are common diseases with an estimated prevalence in the general population of approximately 20%[37,38]. A wide range of different cystic tumors has been described[39], but IPMN and MCN seem to be the most prone to undergo malignant transformation. Furthermore, the incidence of IPMN appears to be high in individuals with a familial risk for PC[40] and, conversely, a positive family history for PC is a risk factor for IPMN development[41]. The risk of cancer in IPMN patients ranges from 24% in IPMN involving the branch ducts to over 60% in lesions involving the main pancreatic duct[42]. For this reason, follow-up or preventive surgery of these neoplasms is recommended[39,42].
Individuals who underwent organ transplantation have recently been identified as being at risk of developing PC. Large cohort studies of transplanted patients in the United States have convincingly demonstrated that the immunosuppressive regimen used for solid organ transplantation is associated with a risk of PC, which significantly exceeds that of the general population[43]. Furthermore, following transplantation, patients seem to be at an increased risk to develop pancreatic neoplastic precursor lesions such as IPMN[44].
Three major groups of hereditary conditions can be considered associated with an increased risk of PC: a strong family history of PC, called familial pancreatic cancer (FPC), hereditary neoplastic and hereditary non-neoplastic syndromes in which PC is one of the phenotypic manifestations[45].
Even if a susceptibility gene for FPC has not been identified yet, the distribution of PC in some families meets the criteria for autosomal dominant transmission with reduced penetrance. In members of such kindreds, the risk to develop PC increases with the number of affected family members. The relative risk ranges from 4.5-fold in case of a single affected first-degree relative to 32-fold if 3 or more first-degree relatives are affected[46]. The definition of FPC is not well established yet, but an individual can be considered at risk if there are at least two first-degree relatives affected by PC, or if three or more relatives are affected regardless of the degree of relationship[47,48] (Table 2). According to some prospective observational studies based on screening programs[49], individuals at risk are found to have an increased incidence of neoplastic precursor lesions (PanIN and IPMN).
| Syndrome | Gene | Relative risk | Risk at age of 70 |
| Familial pancreatic cancer | Unknown | ||
| 1 or more first degree relative(s) | 9 | 4% | |
| 1 first degree relative | 4.5 | 2% | |
| 2 first degree relatives | 6.4 | 3% | |
| 3 or more first degree relatives | 32 | 16% | |
| Peutz Jeghers syndrome | LKB1/STK11 | 132 | 30%-60% |
| Hereditary pancreatitis | PRSS1 | 50-70 | 40% |
| Familial atypical multiple mole melanoma | CDKN2A/p16 | 34-39 | 17% |
| Breast and ovarian cancer syndrome | BRCA1/BRCA2 | 2.3-10 | 1%-5% |
| Cystic fibrosis | CFTR | 5.3 | < 5% |
| Hereditary non-polyposis colon cancer | MSH2, MLH1, MSH6, PMS, PMS2 | 4.7 | < 5% |
| Familial adenomatous polyposis | APC | 4.5 | 2% |
This syndrome, caused by mutations of the BRCA1 or BRCA2 genes, is associated with a 2.3- to 10-fold increased risk of PC[50,51] (Table 2). The risk to develop PC appears to be higher in individuals of Ashkenazi Jewish descent[52]. BRCA 2 mutations are also identified in about 13%-17% of the families diagnosed with FPC who do not meet the inclusion criteria of the breast and ovarian cancer syndrome[53,54].
Familial atypical multiple mole melanoma (FAMMM) is associated with a mutation of the CDKN2A gene. The syndrome is also associated with extra-cutaneous tumors, and PC is present in 25% of individuals who carry this mutation[55]. The relative risk to develop PC for individuals with FAMMM is 34- to 39-fold higher than in the general population[56,57] (Table 2). In a recent report from the German Familial Pancreatic Cancer surveillance program, patients with FAMMM were found more prone than patients with FPC to develop PC directly, i.e., without the development of clinically detectable precursor lesions[58].
Peutz-Jeghers syndrome (PJS) is an autosomal dominant hereditary disease that causes increased susceptibility for the development of various tumor entities. In particular, the risk of gastrointestinal tumors such as esophageal, small bowel, colorectal and pancreatic cancer is increased. PJS is caused by a mutation of the STK11 gene[59]. The risk to develop PC is 132-fold higher in these patients compared to the general population[60] (Table 2).
Of the various types of hereditary pancreatitis (HP) described, only HP associated with a mutation of the PRSS1 gene seems to bear a significantly increased risk of PC. Patients suffering from this condition usually present with early-onset chronic pancreatitis and a family history of chronic pancreatitis associated with PC. The relative risk to develop PC is 50-70[61,62] (Table 2).
An up to 5-fold relative risk is associated with other hereditary syndromes. For hereditary non-polyposis colon cancer (HNPCC) or Lynch Syndrome II[63-65] the risk is approximately 4.7, for cystic fibrosis it is 5.3[66], 3 for ataxia telangiectasia[67] and 4.5 for familial adenomatous polyposis (FAP)[68,69]. Especially FAP patients are to be considered for preemptive pancreatic surgery, because premalignant lesions of the duodenum generally require the same surgical approach.
For several decades PanIN and IPMN have been established as precursor lesions of PC. However, it is only recently that their biology and significance are unfolding.
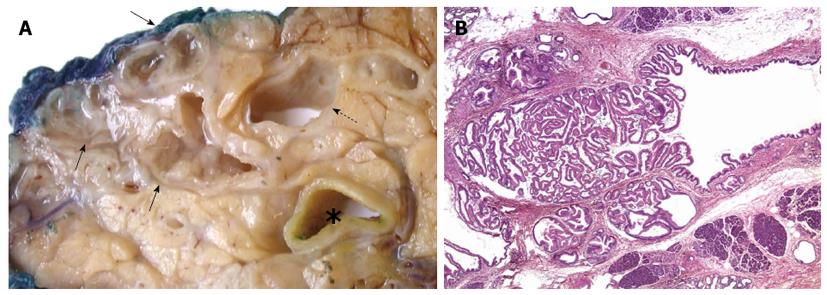
IPMN is, as the name indicates, a neoplastic proliferation within the pancreatic duct system that is characterized by a variable degree of papillary architecture and mucin production[70]. The tumour cell proliferation and mucin secretion cause duct dilatation, which is the major macroscopic and radiological feature of this tumour entity (Figure 1). Based on which part of the pancreatic duct system is involved, IPMNs are divided into main-duct, branch-duct or mixed-duct type. The neoplastic epithelium can be of gastric, intestinal, pancreatobiliary or oncocytic type[71]. Over time, IPMNs can develop increasingly dysplastic features (graded as low, intermediate and high) and eventually transform into invasive adenocarcinoma of tubular, colloid or the rare oncocytic type. Whereas the first cancer type is morphologically and prognostically identical to conventional PC, the latter two are more indolent. Interestingly, the various features of IPMN are interrelated, as outlined in Table 3. Recent studies indicate a more complex relationship between IPMN and invasive PC. The latter may not only develop through direct malignant transformation of the IPMN proper, but seems also to occur more frequently concomitant with (but topographically separate from) an IPMN, in particular branch-duct IPMN of gastric epithelial type[72-74].
| Epithelial subtype | ||||
| Gastric | Intestinal | Pancreatobiliary | Oncocytic | |
| Location | Branch duct > main duct | Main duct > branch duct | Branch duct > main duct | Branch duct > main duct |
| Dysplasia | LGD/IGD | IGD/HGD | HGD | HGD |
| Present with invasive carcinoma | 15% | 30%-60% | 60%-75% | 25% |
| Type of invasive carcinoma | Conventional (tubular) | Colloid or conventional (tubular) | Conventional (tubular) | Oncocytic or conventional (tubular) |
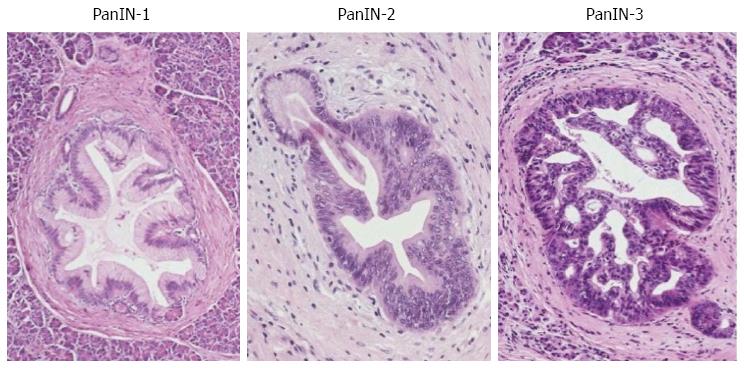
PanIN is also characterized by a neoplastic intraductal proliferation, but in contrast to IPMN, the neoplastic epithelium is flat to low-papillary, and mucin secretion is not a prominent feature[70]. The epithelial type is mainly gastric, although intestinal, oncocytic and other variants can occasionally occur[72]. Three grades of dysplasia (PanIN-1, PanIN-2, PanIN-3) are usually distinguished (Figure 2). The lower grades (PanIN-1, -2) are a common finding in otherwise healthy pancreas after the age of 40[75,76] or in chronic pancreatitis[77-79]. In contrast, PanIN-3 is rare in the normal pancreas or chronic pancreatitis[76,80], but appears most commonly in pancreas with invasive ductal adenocarcinoma[81]. Both PanIN and IPMN are more common and more often multifocal in individuals with a strong family history of PC than in patients with sporadic disease, and the precursor lesions are of a higher grade in the former group[51,82,83].
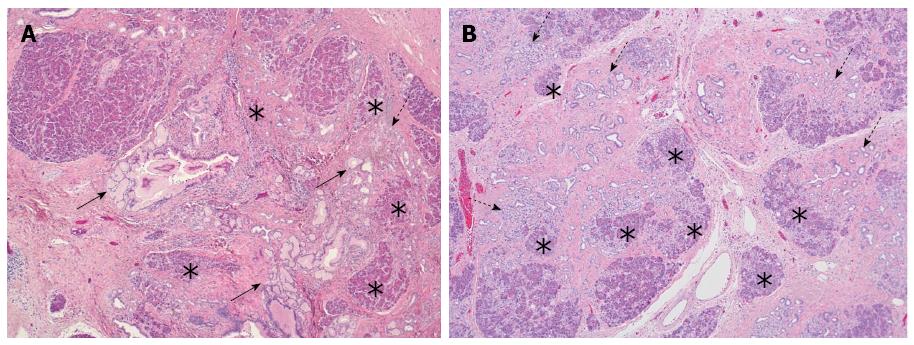
While a certain morphological overlap exists between branch-duct IPMN of gastric type and PanIN[84,85], the main difference between PanIN and IPMN is the fact that the latter represents a macroscopically identifiable lesion[86], while PanINs are too small to be visualized by naked-eye inspection or imaging. However, it has been recently suggested that PanIN is associated by parenchymal changes[51,82,87], which may be detected by EUS[88,89]. These parenchymal changes are characterized by a combination of acinar cell loss, proliferation of small ductular structures and fibrosis, and have been coined as “lobulocentric atrophy” (LCA) (Figure 3A). The initial enthusiasm about the possibility to identify PanIN by means of detecting LCA has been dampened by recent novel developments regarding the causal relationship between both lesions. While it was first assumed that LCA is caused by the duct-obstructive effect of PanIN lesions[51,82,87], and the association between both seemed more or less obligatory, recent morphological and molecular evidence indicates that PanIN is one of the possible outcomes of LCA or the process that is often associated with LCA, so-called acinar-to-ductal metaplasia[90-92]. In the light of these recent discoveries, the use of LCA as a target for pancreatic screening of high-risk individuals requires more circumspect consideration. First, because PanIN is not the cause of LCA, the association between both lesions is not 100%[93]. LCA is in fact a common finding in the ageing pancreas or in the context of various conditions[87,90] and may be present with or without associated PanIN (Figure 3B). Conversely, PanIN may well occur in the absence of LCA, i.e. remain undetectable on EUS examination. Second, there is so far no indication that the presence of LCA correlates with the grade of PanIN. In other words, EUS detection of LCA would still not provide sufficient information for patient management, as high-grade PanIN may be an indication for preventive surgery, whereas low-grade PanIN is not. Third, the accuracy with which fine needle aspiration (FNA) would be able to assess the PanIN-lesion presumed to be associated with the focus of LCA identified on EUS has not been evaluated. While a focus of LCA may be of varying size depending on whether a single pancreatic lobule or several neighbouring lobules are affected, the associated PanIN-lesion(s) may be present only focally and could thus be missed by EUS-guided FNA. From these considerations, it appears that sampling bias may represent a limitation to the successful identification of PanIN-lesions when screening individuals with an increased risk of PC.
While various modalities are available to screen patients at risk of PC, it is currently not well defined who should be screened and how this could be done.
Among individuals with a “non-hereditary” risk of PC, patients suffering from chronic pancreatitis or PCN are currently enrolled for clinical screening.
Patients with chronic pancreatitis are usually entered into a screening program to follow the evolution of the disease and detect PC at an early stage[93]. Recently, a specific algorithm based on patient history and laboratory tests has been developed to identify those chronic pancreatitis patients that have developed early PC[94]. Traditionally, screening of these patients has been based on imaging by MRI and CT scan. The use of EUS, alone or in combination with MRI, seems to offer a high accuracy in this particular patient group. The role of FNA during EUS is not conclusively defined[95], and diffusion-weighted MRI does not seem to facilitate the distinction between PC and chronic pancreatitis[96]. In the group of patients with PCN, MRI, CT scan and EUS, alone or in combination, are the most effective screening modalities. However, in view of the possible need for a prolonged screening program, CT scan is not recommended due to the risks associated with radiation exposure[39,42]. In particular for branch-duct IPMN (BD-IPMN), the surveillance strategy seems to be effective, as evidenced by the average detection rate of cancerization during follow-up, which lies between 0% and 11%[97-103] (Table 4).
Over the years, various surveillance programs have been developed for individuals with a “hereditary risk” of PC. Recently, MRI and EUS have become the most commonly used investigational modalities, whereas in the past CT scan and ERCP have also been used in this field[29]. Within this particular group, individuals affected by FAMMM deserve special mentioning. Indeed, in these individuals the development of PC follows a different pathway that is not preceded by PanIN and IPMN lesions[58]. Therefore, EUS should probably be preferred to, or used in combination with, MRI. Other methods of investigation that are potentially useful for the diagnosis of IPMN include pancreatoscopy and confocal laser microscopy. However, these methods are still experimental and cannot be used routinely for individuals with a hereditary risk of PC[104,105].
At present, the results of screening programs for PC are inconclusive. Most of the prospective studies performed so far report a highly variable detection rate of pancreatic findings, the yield ranging from 1% to 50% (Table 5)[106-115]. The significant divergence in detection rate is not only due to the use of different screening modalities but results also from differences in the definition of the concept “yield”. Some studies declared the identification of “early cancer” (T1N0M0) or high-grade dysplastic precursor lesions as the goal of screening, whereas others also included IPMN with low- or intermediate-grade dysplasia or PanIN of any grade of dysplasia. Some of the surveillance protocols attempted at detecting PanIN lesions by EUS, based on their association with lobulocentric atrophy. However, this histological change in the pancreas is not specific for PanIN, the ability to recognize it is very operator-dependent, and the progression and natural history of this type of lesion is not well known in individuals at risk of PC[29,116]. Consensus has not yet been reached regarding the timing and inclusion criteria for a surveillance program. National and international guidelines suggest that every individual with a 5- to 10-fold relative risk should be considered for surveillance[28,29]. A further point of dissensus is the age at which an individual should be enrolled for screening. For patients at risk of FPC, age 40 or 50 has been proposed for the commencement of screening. However, an earlier age has been suggested for individuals at risk who smoke[29]. For patients with HP (PRSS1 mutation carriers), starting surveillance at the age of 40 has been recommended, considering the younger age of onset of PC in this particular patient group[29]. While no recommendations have been made regarding the age at which an individual could be discharged from a screening program, it seems appropriate that this should be determined by the individual’s fitness for surgery. The exact timing of the screening procedures also lacks clear definition. In general, a yearly control is performed in patients without any finding on previous investigations. In case changes were detected that do not represent an indication for surgery, follow-up at 3- to 6-monthly intervals is generally recommended[29].
| Ref. | Year | Syndrome(s) | Patients (n) | Yield |
| Brentnall et al[106] | 1999 | FPC | 14 | 50% |
| Kimmey et al[107] | 2002 | FPC | 46 | 26% |
| Poley et al[108] | 2009 | FPC, PJS, BRCA, p16, p53, HP | 44 | 23% |
| Langer et al[109] | 2009 | FPC, BRCA | 76 | 1.3% |
| Verna et al[110] | 2010 | FPC, BRCA, p16 | 51 | 12% |
| Ludwig et al[111] | 2011 | FPC, BRCA | 109 | 8.3% |
| Vasen et al[112] | 2011 | P16 | 79 | 18% |
| Al-Sukhni et al[113] | 2011 | FPC, BRCA, PJS, p16, HP | 262 | 7.3% |
| Schneider et al[114] | 2011 | FPC, BRCA, PALB2 | 72 | 15% |
| Canto et al[115] | 2012 | FPC, BRCA, PJS | 216 | 43% |
Unfortunately, primary prevention for individuals at risk of PC is currently not available. Removal of the pancreas based exclusively on a statistical risk is not recommended[29]. In some individuals, advice regarding a healthier life style can be given, for example cessation of smoking, a diet rich in fruits and vegetables, regular exercise, and weight reduction or, if indicated, increased vitamin D intake (> 600 IU)[117].
Chronic pancreatitis: In patients with sporadic chronic pancreatitis, surgery is a second line option for the treatment of local complications and symptoms, if a conservative approach has failed[118]. Even if a number of different surgical procedures for chronic pancreatitis have been proposed in the literature[119,120], a radical pancreatic resection should be performed whenever a suspicion of malignancy arises[121]. However, in selected cases, as for example of HP, some authors suggest early removal of the gland (total pancreatectomy) combined with auto-islet transplantation[122]. The rational of this approach, which cannot be considered the gold standard, is to treat the symptoms (mostly pain), eliminate the risk of cancer, and prevent the development of diabetes following total pancreatectomy. Today, this approach is also possible with a minimally invasive technique[123].
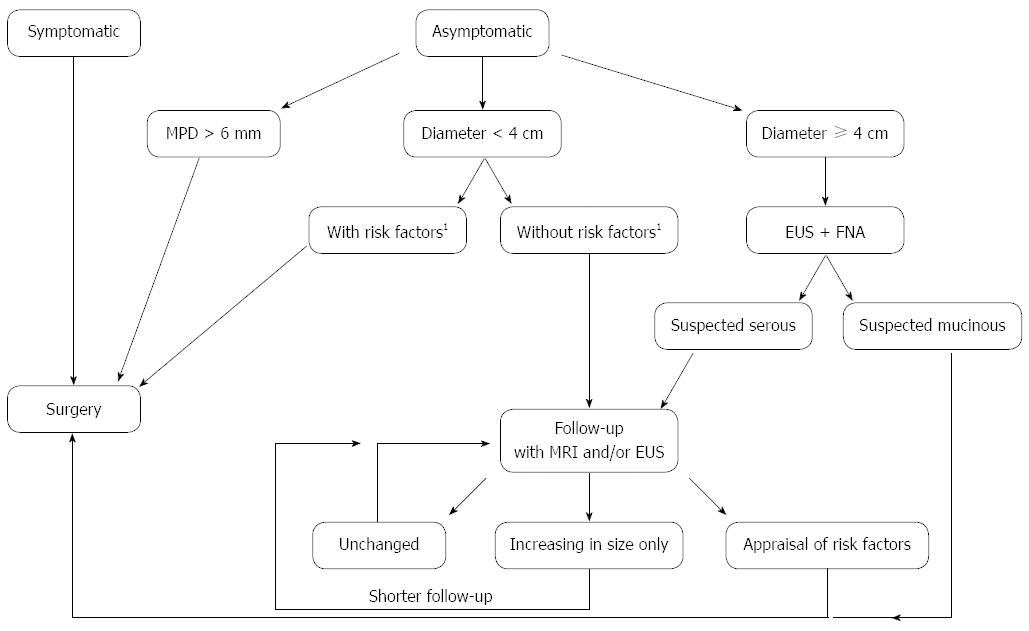
Pancreatic cystic neoplasms: The indications for surgery in patients with IPMN or MCN are spelled out in European and international guidelines[39,42]. For main-duct and mixed-type IPMN, surgical resection is always indicated because of the increased cancer risk. The extent of pancreatic resection should be planned based on the findings on preoperative imaging. In case of dilatation of the entire main pancreatic duct without signs of malignancy in the tail region, a pancreatoduodenectomy with frozen section of the pancreatic margin is recommended. Extension of the resection is indicated if intraoperative examination shows high-grade dysplasia. Low-grade dysplasia is not considered an indication, whereas intermediate-grade dysplasia represents a grey area in which extended resection is not strongly recommended and a judicious decision depends mainly on clinical considerations specific for the individual patient. Importantly, however, extended resection for high-grade dysplasia at the margin is not strongly recommended, if invasive carcinoma is present in the pancreatic head, the reason being that the cancer will determine the patient’s outcome. Therefore, intraoperative frozen section examination may be useful if there is radiological suspicion of malignancy. Total pancreatectomy should not be considered based only of the extent of the duct dilatation, because the latter can be related to mere duct obstruction[39]. Regarding the indication for resection of BD-IPMN, recent guidelines[39] recommend a surgical approach in patients with signs or symptoms of malignancy (dilatation of the main pancreatic duct up to 6 mm, mural nodules, rapid increase in size, elevated levels of CA19.9) or a lesion measuring up to 4 cm in maximum diameter[39]. In case of multifocal disease, only the lesion with these particular features should be resected (partial pancreatectomy). A radical resection should be performed when malignant transformation is suspected. An algorithm that combines the current European and international guidelines is proposed in Figure 4. When signs of malignancy are not present but the diagnosis is not entirely clear or the patient presents with certain clinical risk factors, parenchyma-sparing resection has been suggested by some[124].
Transplanted patients with premalignant lesions: Even though specific guidelines regarding screening for pancreatic disease do not exist for transplanted patients, the increased risk of PC[43] and premalignant pancreatic lesions[44] seems to justify focused attention to this group of patients. Published in the literature is only a single study that analyzed the clinical history of premalignant lesions in transplanted patients and found no difference in terms of progression time compared to non-transplanted patients[125]. However, considering the short patient follow-up in this study and the natural history of the lesions under scrutiny, the data seem insufficient as a basis for the development of a strategy. A surveillance protocol for transplanted patients and the option of early parenchyma-sparing resection have been recently suggested[126].
At present, a surgical strategy for patients with FPC or other hereditary syndromes has not been well defined. In contrast to previous practice, when aggressive approaches such as total pancreatectomy and pancreas transplantation were proposed for patients with a positive family history and findings “suggestive” of dysplasia[127], a more conservative philosophy currently prevails. Even when national and international guidelines recognize PanIN lesions as potential targets for screening[29], they also underline the difficulty to obtain a correct diagnosis for these lesions and therefore the unlikely suitability of PanINs as targets for a clinical surveillance program.
The surgical results from international studies vary considerably (Table 6). The reasons for this discrepancy are differences in inclusion criteria, screening modalities and, most importantly, differences in indications for surgery[88,106-115].
| Ref. | Year | Resected | PanIN1 | PanIN2 | PanIN3 | Pancreas cancer | Other bening lesions | Other malignant lesions | Benign IPMN | Malignant or high-grade displasia IPMN | Malignant lesions or high-grade dysplasia1 |
| Brentnall et al[106] | 2006 | 7 | - | 2 | 2 | - | - | 2 | 1 | 42.8% | |
| Poley et al[108] | 2009 | 3 | - | - | - | 3 | - | - | - | 100% | |
| Verna et al[110] | 2010 | 5 | - | - | - | 1 | - | - | 4 | - | 20% |
| Ludwig et al[111] | 2011 | 6 | 1 | 1 | 1 | 1 | - | - | 2 | - | 16.7% |
| Vasen et al[112] | 2011 | 7 | - | - | - | 7 | - | - | - | - | 100% |
| Al-Sukhni et al[113] | 2011 | 4 | - | - | - | 1 | - | 1 | 2 | - | 50% |
| Schneider et al[114] | 2011 | 9 | 1 | 1 | 1 | 1 | 3 | - | 2 | - | 22.2% |
| Canto et al[115] | 2012 | 5 | - | - | 1 | - | - | - | 3 | 1 | 40% |
| Total | 46 | 2 | 4 | 5 | 14 | 3 | 1 | 15 | 2 | 47.8% |
Current international guidelines recommend surgery for patients at risk with defined solid lesions, cystic tumors that meet criteria for resection even in a population that is not high-risk, and histologically proven PanIN-3 lesions[29]. In high-risk individuals, the indication for surgery for cystic lesions can be adjusted according to the family history, the age and the patient’s perception of the problem, as suggested by the European guidelines for cystic tumors of the pancreas[39]. The surgical treatment of patients at risk of PC should be undertaken in high-volume centers with specialization in this field[29].
The group of hereditary and non-hereditary conditions that are associated with an increased risk for PC has been well defined[30-36,42,43,46,50,51,56,57,60,62]. Even though there is general agreement that individuals with a relative risk of over 5-10 times that of the general population should be considered for enrollment in a clinical surveillance program[29], consensus regarding the latter is currently lacking. As a result, data from clinical trials in this particular area are conflicting[29]. In addition, a more fundamental reason lies at the root of divergent observations and results, namely the lack of knowledge about possible differences in natural history of the premalignant lesions that develop in the various hereditary and non-hereditary conditions of individuals at risk[28,29,42]. However, in every-day clinical practice, the relentless stream of patients with premalignant lesions of the pancreas or individuals with a genetic risk of PC who seek medical advice, represents a significant clinical burden. On one hand, there is a clear need from the pancreatologist’s point of view to offer concrete advice, while on the other hand there is the pressing need to increase our knowledge about the natural history and biology of the lesions under scrutiny. For some of the conditions associated with an increased risk of PC, guidelines and protocols that provide the possibility to standardize treatment and give advice to patients, have already been established (e.g., for cystic tumors or chronic pancreatitis)[41,44]. New discoveries for example regarding cystic tumors of the pancreas are very promising as they may allow prediction of the future evolution of the cystic tumors and thus enable the clinician to decide for surgical or non-surgical treatment[125]. For patients with a hereditary syndrome, the situation is more complex. In several countries, a surveillance program can be rolled out only in the context of a clinical trial, because the cost-effectiveness of surveillance programs for this particular group of high-risk individuals has not been demonstrated yet. Furthermore, only very limited data are available regarding the screening of other groups of patients at risk, such as transplanted patients[128]. Despite the apparently long road that still lies ahead, the recent progress that has been achieved regarding the understanding and management of premalignant lesions of the pancreas, and the role that pre-emptive surgery has acquired in cancer syndromes other than those affecting the pancreas, render a similar development for pancreatic tumors more than likely. Along with the steadily increasing number of patients that will be treated for premalignant lesions, a growing demand for technical perfection and minimally invasive approaches appears unavoidable[129]. At the same time, due to increasing patients’ expectations, the need for a more evidence-based approach, and stricter cost-effectiveness regimes, the pancreatic team will be under increasing pressure to minimize diagnostic error and surgical risk and to optimize the use of limited resources in the health care system. For this reason, surveillance and treatment of individuals at increased risk of PC should be limited to high-volume and specialized centers with a specific clinical and research interest in preemptive pancreatic surgery.
P- Reviewer: Nageshwar Reddy D, Zhang LH, Zheng L S- Editor: Gou SX L- Editor: A E- Editor: Ma S
| 1. | American Cancer Society. [Accessed September 1, 2011] Cancer facts and figures. 2011. Available from: http://www.cancer.org/acs/groups/content/epidemiologysurveilance/documents/document/ acspc-029771.pdf. |
| 2. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25509] [Article Influence: 1822.1] [Reference Citation Analysis (7)] |
| 3. | Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3032] [Cited by in RCA: 3122] [Article Influence: 223.0] [Reference Citation Analysis (0)] |
| 4. | Malvezzi M, Bertuccio P, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2013. Ann Oncol. 2013;24:792-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 225] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 5. | Sharma RR, London MJ, Magenta LL, Posner MC, Roggin KK. Preemptive surgery for premalignant foregut lesions. J Gastrointest Surg. 2009;13:1874-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Heitmiller RF. Prophylactic esophagectomy in Barrett esophagus with high-grade dysplasia. Langenbecks Arch Surg. 2003;388:83-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Edwards MJ, Gable DR, Lentsch AB, Richardson JD. The rationale for esophagectomy as the optimal therapy for Barrett’s esophagus with high-grade dysplasia. Ann Surg. 1996;223:585-59; discussion 589-91. [PubMed] |
| 8. | Chang LC, Oelschlager BK, Quiroga E, Parra JD, Mulligan M, Wood DE, Pellegrini CA. Long-term outcome of esophagectomy for high-grade dysplasia or cancer found during surveillance for Barrett‘s esophagus. J Gastrointest Surg. 2006;10:341-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Rice TW. Pro: esophagectomy is the treatment of choice for high-grade dysplasia in Barrett’s esophagus. Am J Gastroenterol. 2006;101:2177-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Sujendran V, Sica G, Warren B, Maynard N. Oesophagectomy remains the gold standard for treatment of high-grade dysplasia in Barrett’s oesophagus. Eur J Cardiothorac Surg. 2005;28:763-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Lewis FR, Mellinger JD, Hayashi A, Lorelli D, Monaghan KG, Carneiro F, Huntsman DG, Jackson CE, Caldas C. Prophylactic total gastrectomy for familial gastric cancer. Surgery. 2001;130:612-67; discussion 617-9. [PubMed] |
| 12. | Lynch H, Caldas C, Wirtzfeld D, Vaccaro C, Rubenstein W, Weissman S, Kaurah P, Boyd N, Fitzgerald R, Huntsman D. Hereditary diffuse gastric cancer: Natural history, pathology, screening limitations, and prophylactic total gastrectomy in CDH1 mutation carriers. J Clin Oncol. 2007;25:4500. |
| 13. | Newman EA, Mulholland MW. Prophylactic gastrectomy for hereditary diffuse gastric cancer syndrome. J Am Coll Surg. 2006;202:612-617. [PubMed] |
| 14. | Patt CH, Thuluvath PJ. Liver transplanatation for primary sclerosing cholangitis: screening for biliary malignancy and the role of preemptive transplantation. Curr Opin Organ Transplant. 2002;7:129-136. [DOI] [Full Text] |
| 15. | Benjamin IS. Biliary cystic disease: the risk of cancer. J Hepatobiliary Pancreat Surg. 2003;10:335-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Rebbeck TR, Friebel T, Lynch HT, Neuhausen SL, van ‘t Veer L, Garber JE, Evans GR, Narod SA, Isaacs C, Matloff E. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol. 2004;22:1055-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 813] [Cited by in RCA: 792] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 18. | Haber D. Prophylactic oophorectomy to reduce the risk of ovarian and breast cancer in carriers of BRCA mutations. N Engl J Med. 2002;346:1660-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Vasen HF, van Duijvendijk P, Buskens E, Bülow C, Björk J, Järvinen HJ, Bülow S. Decision analysis in the surgical treatment of patients with familial adenomatous polyposis: a Dutch-Scandinavian collaborative study including 659 patients. Gut. 2001;49:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Garborg K, Holme Ø, Løberg M, Kalager M, Adami HO, Bretthauer M. Current status of screening for colorectal cancer. Ann Oncol. 2013;24:1963-1972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 21. | Manser CN, Bachmann LM, Brunner J, Hunold F, Bauerfeind P, Marbet UA. Colonoscopy screening markedly reduces the occurrence of colon carcinomas and carcinoma-related death: a closed cohort study. Gastrointest Endosc. 2012;76:110-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 22. | Nickson C, Mason KE, English DR, Kavanagh AM. Mammographic screening and breast cancer mortality: a case-control study and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012;21:1479-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Broeders M, Moss S, Nyström L, Njor S, Jonsson H, Paap E, Massat N, Duffy S, Lynge E, Paci E. The impact of mammographic screening on breast cancer mortality in Europe: a review of observational studies. J Med Screen. 2012;19 Suppl 1:14-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 293] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 24. | Tanne JH. Low dose CT screening for lung cancer could save 12 000 US lives a year, researchers claim. BMJ. 2013;346:f1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Maitra A, Kern SE, Hruban RH. Molecular pathogenesis of pancreatic cancer. Best Pract Res Clin Gastroenterol. 2006;20:211-226. [PubMed] |
| 26. | Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, Kamiyama M, Hruban RH, Eshleman JR, Nowak MA. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114-1117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2041] [Cited by in RCA: 1930] [Article Influence: 128.7] [Reference Citation Analysis (0)] |
| 27. | Shin EJ, Canto MI. Pancreatic cancer screening. Gastroenterol Clin North Am. 2012;41:143-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 28. | Del Chiaro M, Zerbi A, Capurso G, Zamboni G, Maisonneuve P, Presciuttini S, Arcidiacono PG, Calculli L, Falconi M. Familial pancreatic cancer in Italy. Risk assessment, screening programs and clinical approach: a position paper from the Italian Registry. Dig Liver Dis. 2010;42:597-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Canto MI, Harinck F, Hruban RH, Offerhaus GJ, Poley JW, Kamel I, Nio Y, Schulick RS, Bassi C, Kluijt I. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut. 2013;62:339-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 546] [Cited by in RCA: 563] [Article Influence: 46.9] [Reference Citation Analysis (1)] |
| 30. | Iodice S, Gandini S, Maisonneuve P, Lowenfels AB. Tobacco and the risk of pancreatic cancer: a review and meta-analysis. Langenbecks Arch Surg. 2008;393:535-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 368] [Article Influence: 21.6] [Reference Citation Analysis (1)] |
| 31. | Mulder I, Hoogenveen RT, van Genugten ML, Lankisch PG, Lowenfels AB, de Hollander AE, Bueno-de-Mesquita HB. Smoking cessation would substantially reduce the future incidence of pancreatic cancer in the European Union. Eur J Gastroenterol Hepatol. 2002;14:1343-1353. [PubMed] |
| 32. | Giovannucci E, Michaud D. The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology. 2007;132:2208-2225. [PubMed] |
| 33. | Rulyak SJ, Lowenfels AB, Maisonneuve P, Brentnall TA. Risk factors for the development of pancreatic cancer in familial pancreatic cancer kindreds. Gastroenterology. 2003;124:1292-1299. [PubMed] |
| 34. | Hassan MM, Bondy ML, Wolff RA, Abbruzzese JL, Vauthey JN, Pisters PW, Evans DB, Khan R, Chou TH, Lenzi R. Risk factors for pancreatic cancer: case-control study. Am J Gastroenterol. 2007;102:2696-2707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 228] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 35. | Genkinger JM, Spiegelman D, Anderson KE, Bergkvist L, Bernstein L, van den Brandt PA, English DR, Freudenheim JL, Fuchs CS, Giles GG. Alcohol intake and pancreatic cancer risk: a pooled analysis of fourteen cohort studies. Cancer Epidemiol Biomarkers Prev. 2009;18:765-776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 127] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 36. | Ojajärvi IA, Partanen TJ, Ahlbom A, Boffetta P, Hakulinen T, Jourenkova N, Kauppinen TP, Kogevinas M, Porta M, Vainio HU. Occupational exposures and pancreatic cancer: a meta-analysis. Occup Environ Med. 2000;57:316-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 111] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 37. | Laffan TA, Horton KM, Klein AP, Berlanstein B, Siegelman SS, Kawamoto S, Johnson PT, Fishman EK, Hruban RH. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 724] [Cited by in RCA: 654] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 38. | Zhang XM, Mitchell DG, Dohke M, Holland GA, Parker L. Pancreatic cysts: depiction on single-shot fast spin-echo MR images. Radiology. 2002;223:547-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 277] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 39. | Del Chiaro M, Verbeke C, Salvia R, Klöppel G, Werner J, McKay C, Friess H, Manfredi R, Van Cutsem E, Löhr M. European experts consensus statement on cystic tumours of the pancreas. Dig Liver Dis. 2013;45:703-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 331] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 40. | Shi C, Klein AP, Goggins M, Maitra A, Canto M, Ali S, Schulick R, Palmisano E, Hruban RH. Increased Prevalence of Precursor Lesions in Familial Pancreatic Cancer Patients. Clin Cancer Res. 2009;15:7737-7743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 163] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 41. | Capurso G, Boccia S, Salvia R, Del Chiaro M, Frulloni L, Arcidiacono PG, Zerbi A, Manta R, Fabbri C, Ventrucci M. Risk factors for intraductal papillary mucinous neoplasm (IPMN) of the pancreas: a multicentre case-control study. Am J Gastroenterol. 2013;108:1003-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 42. | Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM, Shimizu M, Wolfgang CL, Yamaguchi K, Yamao K; International Association of Pancreatology. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 1610] [Article Influence: 123.8] [Reference Citation Analysis (0)] |
| 43. | Engels EA, Pfeiffer RM, Fraumeni JF, Kasiske BL, Israni AK, Snyder JJ, Wolfe RA, Goodrich NP, Bayakly AR, Clarke CA. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306:1891-1901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1114] [Cited by in RCA: 1085] [Article Influence: 77.5] [Reference Citation Analysis (0)] |
| 44. | Girometti R, Intini S, Brondani G, Como G, Londero F, Bresadola F, Zuiani C, Bazzocchi M. Incidental pancreatic cysts on 3D turbo spin echo magnetic resonance cholangiopancreatography: prevalence and relation with clinical and imaging features. Abdom Imaging. 2011;36:196-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 45. | Del Chiaro M, Boggi U, Presciuttini S, Bertacca L, Croce C, Mosca I, Mosca F. Genetics of pancreatic cancer: where are we now? Where are we going? JOP. 2005;6:60-67. [PubMed] |
| 46. | Klein AP, Brune KA, Petersen GM, Goggins M, Tersmette AC, Offerhaus GJ, Griffin C, Cameron JL, Yeo CJ, Kern S. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res. 2004;64:2634-2638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 462] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 47. | Tersmette AC, Petersen GM, Offerhaus GJ, Falatko FC, Brune KA, Goggins M, Rozenblum E, Wilentz RE, Yeo CJ, Cameron JL. Increased risk of incident pancreatic cancer among first-degree relatives of patients with familial pancreatic cancer. Clin Cancer Res. 2001;7:738-744. [PubMed] |
| 48. | Lynch HT, Brand RE, Deters CA, Shaw TG, Lynch JF. Hereditary pancreatic cancer. Pancreatology. 2001;1:466-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 49. | Brune K, Abe T, Canto M, O’Malley L, Klein AP, Maitra A, Volkan Adsay N, Fishman EK, Cameron JL, Yeo CJ. Multifocal neoplastic precursor lesions associated with lobular atrophy of the pancreas in patients having a strong family history of pancreatic cancer. Am J Surg Pathol. 2006;30:1067-1076. [PubMed] |
| 50. | Naderi A, Couch FJ. BRCA2 and pancreatic cancer. Int J Gastrointest Cancer. 2002;31:99-106. [PubMed] |
| 51. | Thompson D, Easton DF; Breast Cancer Linkage Consortium. Cancer Incidence in BRCA1 mutation carriers. J Natl Cancer Inst. 2002;94:1358-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 788] [Cited by in RCA: 764] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 52. | Goggins M, Schutte M, Lu J, Moskaluk CA, Weinstein CL, Petersen GM, Yeo CJ, Jackson CE, Lynch HT, Hruban RH. Germline BRCA2 gene mutations in patients with apparently sporadic pancreatic carcinomas. Cancer Res. 1996;56:5360-5364. [PubMed] |
| 53. | Murphy KM, Brune KA, Griffin C, Sollenberger JE, Petersen GM, Bansal R, Hruban RH, Kern SE. Evaluation of candidate genes MAP2K4, MADH4, ACVR1B, and BRCA2 in familial pancreatic cancer: deleterious BRCA2 mutations in 17%. Cancer Res. 2002;62:3789-3793. [PubMed] |
| 54. | Hahn SA, Greenhalf B, Ellis I, Sina-Frey M, Rieder H, Korte B, Gerdes B, Kress R, Ziegler A, Raeburn JA. BRCA2 germline mutations in familial pancreatic carcinoma. J Natl Cancer Inst. 2003;95:214-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 336] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 55. | Goldstein AM, Fraser MC, Struewing JP, Hussussian CJ, Ranade K, Zametkin DP, Fontaine LS, Organic SM, Dracopoli NC, Clark WH. Increased risk of pancreatic cancer in melanoma-prone kindreds with p16INK4 mutations. N Engl J Med. 1995;333:970-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 432] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 56. | Vasen HF, Gruis NA, Frants RR, van Der Velden PA, Hille ET, Bergman W. Risk of developing pancreatic cancer in families with familial atypical multiple mole melanoma associated with a specific 19 deletion of p16 (p16-Leiden). Int J Cancer. 2000;87:809-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 57. | Borg A, Sandberg T, Nilsson K, Johannsson O, Klinker M, Måsbäck A, Westerdahl J, Olsson H, Ingvar C. High frequency of multiple melanomas and breast and pancreas carcinomas in CDKN2A mutation-positive melanoma families. J Natl Cancer Inst. 2000;92:1260-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 233] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 58. | Potjer TP, Schot I, Langer P, Heverhagen JT, Wasser MN, Slater EP, Klöppel G, Morreau HM, Bonsing BA, de Vos Tot Nederveen Cappel WH. Variation in precursor lesions of pancreatic cancer among high-risk groups. Clin Cancer Res. 2013;19:442-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 59. | Su GH, Hruban RH, Bansal RK, Bova GS, Tang DJ, Shekher MC, Westerman AM, Entius MM, Goggins M, Yeo CJ. Germline and somatic mutations of the STK11/LKB1 Peutz-Jeghers gene in pancreatic and biliary cancers. Am J Pathol. 1999;154:1835-1840. [PubMed] |
| 60. | Giardiello FM, Brensinger JD, Tersmette AC, Goodman SN, Petersen GM, Booker SV, Cruz-Correa M, Offerhaus JA. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology. 2000;119:1447-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 961] [Cited by in RCA: 867] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 61. | Lowenfels AB, Maisonneuve P, DiMagno EP, Elitsur Y, Gates LK, Perrault J, Whitcomb DC. Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group. J Natl Cancer Inst. 1997;89:442-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 609] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 62. | Howes N, Lerch MM, Greenhalf W, Stocken DD, Ellis I, Simon P, Truninger K, Ammann R, Cavallini G, Charnley RM. Clinical and genetic characteristics of hereditary pancreatitis in Europe. Clin Gastroenterol Hepatol. 2004;2:252-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 373] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 63. | Lynch HT, Voorhees GJ, Lanspa SJ, McGreevy PS, Lynch JF. Pancreatic carcinoma and hereditary nonpolyposis colorectal cancer: a family study. Br J Cancer. 1985;52:271-273. [PubMed] |
| 64. | Aarnio M, Sankila R, Pukkala E, Salovaara R, Aaltonen LA, de la Chapelle A, Peltomäki P, Mecklin JP, Järvinen HJ. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer. 1999;81:214-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 65. | Watson P, Vasen HF, Mecklin JP, Bernstein I, Aarnio M, Järvinen HJ, Myrhøj T, Sunde L, Wijnen JT, Lynch HT. The risk of extra-colonic, extra-endometrial cancer in the Lynch syndrome. Int J Cancer. 2008;123:444-449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 454] [Cited by in RCA: 423] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 66. | Maisonneuve P, Marshall BC, Lowenfels AB. Risk of pancreatic cancer in patients with cystic fibrosis. Gut. 2007;56:1327-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 67. | Geoffroy-Perez B, Janin N, Ossian K, Laugé A, Croquette MF, Griscelli C, Debré M, Bressac-de-Paillerets B, Aurias A, Stoppa-Lyonnet D. Cancer risk in heterozygotes for ataxia-telangiectasia. Int J Cancer. 2001;93:288-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 95] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 68. | Elkharwily A, Gottlieb K. The pancreas in familial adenomatous polyposis. JOP. 2008;9:9-18. [PubMed] |
| 69. | Giardiello FM, Offerhaus GJ, Lee DH, Krush AJ, Tersmette AC, Booker SV, Kelley NC, Hamilton SR. Increased risk of thyroid and pancreatic carcinoma in familial adenomatous polyposis. Gut. 1993;34:1394-1396. [PubMed] |
| 70. | Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumors of the digestive system. Lyon: IARC 2010; 280, chapter 12. |
| 71. | Verbeke CS. Intraductal papillary-mucinous neoplasia of the pancreas: Histopathology and molecular biology. World J Gastrointest Surg. 2010;2:306-313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 72. | Campbell F, Verbeke CS. Pathology of the pancreas - a practical approach. London: Springer 2013; 215, chapter 17. |
| 73. | Ideno N, Ohtsuka T, Kono H, Fujiwara K, Oda Y, Aishima S, Ito T, Ishigami K, Tokunaga S, Ohuchida K. Intraductal papillary mucinous neoplasms of the pancreas with distinct pancreatic ductal adenocarcinomas are frequently of gastric subtype. Ann Surg. 2013;258:141-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 74. | Mino-Kenudson M, Fernández-del Castillo C, Baba Y, Valsangkar NP, Liss AS, Hsu M, Correa-Gallego C, Ingkakul T, Perez Johnston R, Turner BG. Prognosis of invasive intraductal papillary mucinous neoplasm depends on histological and precursor epithelial subtypes. Gut. 2011;60:1712-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 204] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 75. | Lüttges J, Reinecke-Lüthge A, Möllmann B, Menke MA, Clemens A, Klimpfinger M, Sipos B, Klöppel G. Duct changes and K-ras mutations in the disease-free pancreas: analysis of type, age relation and spatial distribution. Virchows Arch. 1999;435:461-468. [PubMed] |
| 76. | Andea A, Sarkar F, Adsay VN. Clinicopathological correlates of pancreatic intraepithelial neoplasia: a comparative analysis of 82 cases with and 152 cases without pancreatic ductal adenocarcinoma. Mod Pathol. 2003;16:996-1006. [PubMed] |
| 77. | Volkholz H, Stolte M, Becker V. Epithelial dysplasias in chronic pancreatitis. Virchows Arch A Pathol Anat Histol. 1982;396:331-349. [PubMed] |
| 78. | Yanagisawa A, Ohtake K, Ohashi K, Hori M, Kitagawa T, Sugano H, Kato Y. Frequent c-Ki-ras oncogene activation in mucous cell hyperplasias of pancreas suffering from chronic inflammation. Cancer Res. 1993;53:953-956. [PubMed] |
| 79. | Lüttges J, Diederichs A, Menke MA, Vogel I, Kremer B, Klöppel G. Ductal lesions in patients with chronic pancreatitis show K-ras mutations in a frequency similar to that in the normal pancreas and lack nuclear immunoreactivity for p53. Cancer. 2000;88:2495-2504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 80. | Tabata T, Fujimori T, Maeda S, Yamamoto M, Saitoh Y. The role of Ras mutation in pancreatic cancer, precancerous lesions, and chronic pancreatitis. Int J Pancreatol. 1993;14:237-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 81. | Lüttges J, Galehdari H, Bröcker V, Schwarte-Waldhoff I, Henne-Bruns D, Klöppel G, Schmiegel W, Hahn SA. Allelic loss is often the first hit in the biallelic inactivation of the p53 and DPC4 genes during pancreatic carcinogenesis. Am J Pathol. 2001;158:1677-1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 167] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 82. | Meckler KA, Brentnall TA, Haggitt RC, Crispin D, Byrd DR, Kimmey MB, Bronner MP. Familial fibrocystic pancreatic atrophy with endocrine cell hyperplasia and pancreatic carcinoma. Am J Surg Pathol. 2001;25:1047-1053. [PubMed] |
| 83. | Shi C, Hruban RH, Klein AP. Familial pancreatic cancer. Arch Pathol Lab Med. 2009;133:365-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 84. | Longnecker DS, Adsay NV, Fernandez-del Castillo C, Hruban RH, Kasugai T, Klimstra DS, Klöppel G, Lüttges J, Memoli VA, Tosteson TD. Histopathological diagnosis of pancreatic intraepithelial neoplasia and intraductal papillary-mucinous neoplasms: interobserver agreement. Pancreas. 2005;31:344-349. [PubMed] |
| 85. | Andrejevic-Blant S, Kosmahl M, Sipos B, Klöppel G. Pancreatic intraductal papillary-mucinous neoplasms: a new and evolving entity. Virchows Arch. 2007;451:863-869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 86. | Hruban RH, Takaori K, Klimstra DS, Adsay NV, Albores-Saavedra J, Biankin AV, Biankin SA, Compton C, Fukushima N, Furukawa T. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28:977-987. [PubMed] |
| 87. | Detlefsen S, Sipos B, Feyerabend B, Klöppel G. Pancreatic fibrosis associated with age and ductal papillary hyperplasia. Virchows Arch. 2005;447:800-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 88. | Canto MI, Goggins M, Hruban RH, Petersen GM, Giardiello FM, Yeo C, Fishman EK, Brune K, Axilbund J, Griffin C. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol. 2006;4:766-81; quiz 665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 367] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 89. | Maire F, Couvelard A, Palazzo L, Aubert A, Vullierme MP, Rebours V, Hammel P, Sauvanet A, Levy P, Ruszniewski P. Pancreatic intraepithelial neoplasia in patients with intraductal papillary mucinous neoplasms: the interest of endoscopic ultrasonography. Pancreas. 2013;42:1262-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 90. | Esposito I, Seiler C, Bergmann F, Kleeff J, Friess H, Schirmacher P. Hypothetical progression model of pancreatic cancer with origin in the centroacinar-acinar compartment. Pancreas. 2007;35:212-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 91. | Aichler M, Seiler C, Tost M, Siveke J, Mazur PK, Da Silva-Buttkus P, Bartsch DK, Langer P, Chiblak S, Dürr A. Origin of pancreatic ductal adenocarcinoma from atypical flat lesions: a comparative study in transgenic mice and human tissues. J Pathol. 2012;226:723-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 92. | Morris JP, Wang SC, Hebrok M. KRAS, Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma. Nat Rev Cancer. 2010;10:683-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 453] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 93. | Dítĕ P, Novotný I, Precechtĕlová M, Růzicka M, Záková A, Hermanová M, Trna J, Nechutová H. Incidence of pancreatic carcinoma in patients with chronic pancreatitis. Hepatogastroenterology. 2010;57:957-960. [PubMed] |
| 94. | Rückert F, Brussig T, Kuhn M, Kersting S, Bunk A, Hunger M, Saeger HD, Niedergethmann M, Post S, Grützmann R. Malignancy in chronic pancreatitis: analysis of diagnostic procedures and proposal of a clinical algorithm. Pancreatology. 2013;13:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 95. | Pungpapong S, Wallace MB, Woodward TA, Noh KW, Raimondo M. Accuracy of endoscopic ultrasonography and magnetic resonance cholangiopancreatography for the diagnosis of chronic pancreatitis: a prospective comparison study. J Clin Gastroenterol. 2007;41:88-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 96. | Sandrasegaran K, Nutakki K, Tahir B, Dhanabal A, Tann M, Cote GA. Use of diffusion-weighted MRI to differentiate chronic pancreatitis from pancreatic cancer. AJR Am J Roentgenol. 2013;201:1002-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 97. | Lafemina J, Katabi N, Klimstra D, Correa-Gallego C, Gaujoux S, Kingham TP, Dematteo RP, Fong Y, D’Angelica MI, Jarnagin WR. Malignant progression in IPMN: a cohort analysis of patients initially selected for resection or observation. Ann Surg Oncol. 2013;20:440-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 98. | Sahora K, Mino-Kenudson M, Brugge W, Thayer SP, Ferrone CR, Sahani D, Pitman MB, Warshaw AL, Lillemoe KD, Fernandez-del Castillo CF. Branch duct intraductal papillary mucinous neoplasms: does cyst size change the tip of the scale? A critical analysis of the revised international consensus guidelines in a large single-institutional series. Ann Surg. 2013;258:466-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 225] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 99. | Khannoussi W, Vullierme MP, Rebours V, Maire F, Hentic O, Aubert A, Sauvanet A, Dokmak S, Couvelard A, Hammel P. The long term risk of malignancy in patients with branch duct intraductal papillary mucinous neoplasms of the pancreas. Pancreatology. 2012;12:198-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 100. | Arlix A, Bournet B, Otal P, Canevet G, Thevenot A, Kirzin S, Carrere N, Suc B, Moreau J, Escourrou J. Long-term clinical and imaging follow-up of nonoperated branch duct form of intraductal papillary mucinous neoplasms of the pancreas. Pancreas. 2012;41:295-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 101. | Maguchi H, Tanno S, Mizuno N, Hanada K, Kobayashi G, Hatori T, Sadakari Y, Yamaguchi T, Tobita K, Doi R. Natural history of branch duct intraductal papillary mucinous neoplasms of the pancreas: a multicenter study in Japan. Pancreas. 2011;40:364-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 169] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 102. | Salvia R, Partelli S, Crippa S, Landoni L, Capelli P, Manfredi R, Bassi C, Pederzoli P. Intraductal papillary mucinous neoplasms of the pancreas with multifocal involvement of branch ducts. Am J Surg. 2009;198:709-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 103. | Tanno S, Nakano Y, Nishikawa T, Nakamura K, Sasajima J, Minoguchi M, Mizukami Y, Yanagawa N, Fujii T, Obara T. Natural history of branch duct intraductal papillary-mucinous neoplasms of the pancreas without mural nodules: long-term follow-up results. Gut. 2008;57:339-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 174] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 104. | Nagayoshi Y, Aso T, Ohtsuka T, Kono H, Ideno N, Igarashi H, Takahata S, Oda Y, Ito T, Tanaka M. Peroral pancreatoscopy using the SpyGlass system for the assessment of intraductal papillary mucinous neoplasm of the pancreas. J Hepatobiliary Pancreat Sci. 2014;21:410-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 105. | Konda VJ, Aslanian HR, Wallace MB, Siddiqui UD, Hart J, Waxman I. First assessment of needle-based confocal laser endomicroscopy during EUS-FNA procedures of the pancreas (with videos). Gastrointest Endosc. 2011;74:1049-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 106. | Brentnall TA, Bronner MP, Byrd DR, Haggitt RC, Kimmey MB. Early diagnosis and treatment of pancreatic dysplasia in patients with a family history of pancreatic cancer. Ann Intern Med. 1999;131:247-255. [PubMed] |
| 107. | Kimmey MB, Bronner MP, Byrd DR, Haggitt RC, Kimmey MB. Screening and surveillance for hereditary pancreatic cancer. Gastrointest Endosc. 2002;56:S82-86. |
| 108. | Poley JW, Kluijt I, Gouma DJ, Harinck F, Wagner A, Aalfs C, van Eijck CH, Cats A, Kuipers EJ, Nio Y. The yield of first-time endoscopic ultrasonography in screening individuals at a high risk of developing pancreatic cancer. Am J Gastroenterol. 2009;104:2175-2181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 187] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 109. | Langer P, Kann PH, Fendrich V, Habbe N, Schneider M, Sina M, Slater EP, Heverhagen JT, Gress TM, Rothmund M. Five years of prospective screening of high-risk individuals from families with familial pancreatic cancer. Gut. 2009;58:1410-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 172] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 110. | Verna EC, Hwang C, Stevens PD, Rotterdam H, Stavropoulos SN, Sy CD, Prince MA, Chung WK, Fine RL, Chabot JA. Pancreatic cancer screening in a prospective cohort of high-risk patients: a comprehensive strategy of imaging and genetics. Clin Cancer Res. 2010;16:5028-5037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 161] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 111. | Ludwig E, Olson SH, Bayuga S, Simon J, Schattner MA, Gerdes H, Allen PJ, Jarnagin WR, Kurtz RC. Feasibility and yield of screening in relatives from familial pancreatic cancer families. Am J Gastroenterol. 2011;106:946-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 112. | Vasen HF, Wasser M, van Mil A, Tollenaar RA, Konstantinovski M, Gruis NA, Bergman W, Hes FJ, Hommes DW, Offerhaus GJ. Magnetic resonance imaging surveillance detects early-stage pancreatic cancer in carriers of a p16-Leiden mutation. Gastroenterology. 2011;140:850-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 121] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 113. | Al-Sukhni W, Borgida A, Rothenmund H, Holter S, Semotiuk K, Grant R, Wilson S, Moore M, Narod S, Jhaveri K. Screening for pancreatic cancer in a high-risk cohort: an eight-year experience. J Gastrointest Surg. 2012;16:771-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 114] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 114. | Schneider R, Slater EP, Sina M, Habbe N, Fendrich V, Matthäi E, Langer P, Bartsch DK. German national case collection for familial pancreatic cancer (FaPaCa): ten years experience. Fam Cancer. 2011;10:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 115. | Canto MI, Hruban RH, Fishman EK, Kamel IR, Schulick R, Zhang Z, Topazian M, Takahashi N, Fletcher J, Petersen G. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology. 2012;142:796-804; quiz e14-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 485] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 116. | Terhune PG, Phifer DM, Tosteson TD, Longnecker DS. K-ras mutation in focal proliferative lesions of human pancreas. Cancer Epidemiol Biomarkers Prev. 1998;7:515-521. [PubMed] |
| 117. | Brand RE, Lerch MM, Rubinstein WS, Neoptolemos JP, Whitcomb DC, Hruban RH, Brentnall TA, Lynch HT, Canto MI. Advances in counselling and surveillance of patients at risk for pancreatic cancer. Gut. 2007;56:1460-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 217] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 118. | D'Haese JG, Ceyhan GO, Demir IE, Tieftrunk E, Friess H. Treatment options in painful chronic pancreatitis: a systematic review. HPB (Oxford). 2014;16:512-521. [PubMed] |
| 119. | Beger HG, Büchler M, Bittner RR, Oettinger W, Roscher R. Duodenum-preserving resection of the head of the pancreas in severe chronic pancreatitis. Early and late results. Ann Surg. 1989;209:273-278. [PubMed] |
| 120. | Frey CF, Mayer KL. Comparison of local resection of the head of the pancreas combined with longitudinal pancreaticojejunostomy (frey procedure) and duodenum-preserving resection of the pancreatic head (beger procedure). World J Surg. 2003;27:1217-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 121. | Sasson AR, Gulizia JM, Galva A, Anderson J, Thompson J. Pancreaticoduodenectomy for suspected malignancy: have advancements in radiographic imaging improved results? Am J Surg. 2006;192:888-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 122. | Walsh RM, Saavedra JR, Lentz G, Guerron AD, Scheman J, Stevens T, Trucco M, Bottino R, Hatipoglu B. Improved quality of life following total pancreatectomy and auto-islet transplantation for chronic pancreatitis. J Gastrointest Surg. 2012;16:1469-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 123. | Marquez S, Marquez TT, Ikramuddin S, Kandaswamy R, Antanavicius G, Freeman ML, Hering BJ, Sutherland DE. Laparoscopic and da Vinci robot-assisted total pancreaticoduodenectomy and intraportal islet autotransplantation: case report of a definitive minimally invasive treatment of chronic pancreatitis. Pancreas. 2010;39:1109-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 124. | Fritz S, Klauss M, Bergmann F, Hackert T, Hartwig W, Strobel O, Bundy BD, Büchler MW, Werner J. Small (Sendai negative) branch-duct IPMNs: not harmless. Ann Surg. 2012;256:313-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 159] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 125. | Wu J, Jiao Y, Dal Molin M, Maitra A, de Wilde RF, Wood LD, Eshleman JR, Goggins MG, Wolfgang CL, Canto MI. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc Natl Acad Sci USA. 2011;108:21188-21193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 478] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 126. | Del Chiaro M, Albiin N, Segersvärd R. Enucleation of branch duct-IPMN in a transplant patient. Pancreatology. 2013;13:312-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 127. | Charpentier KP, Brentnall TA, Bronner MP, Byrd D, Marsh C. A new indication for pancreas transplantation: high grade pancreatic dysplasia. Clin Transplant. 2004;18:105-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 128. | Gill KR, Pelaez-Luna M, Keaveny A, Woodward TA, Wallace MB, Chari ST, Smyrk TC, Takahashi N, Clain JE, Levy MJ. Branch duct intraductal papillary mucinous neoplasm of the pancreas in solid organ transplant recipients. Am J Gastroenterol. 2009;104:1256-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |