Published online May 14, 2014. doi: 10.3748/wjg.v20.i18.5212
Revised: January 11, 2014
Accepted: January 19, 2014
Published online: May 14, 2014
Helicobacter pylori (H. pylori) infection is the main pathogenic factor for upper digestive tract organic diseases. In addition to direct cytotoxic and proinflammatory effects, H. pylori infection may also induce abnormalities indirectly by affecting the brain-gut axis, similar to other microorganisms present in the alimentary tract. The brain-gut axis integrates the central, peripheral, enteric and autonomic nervous systems, as well as the endocrine and immunological systems, with gastrointestinal functions and environmental stimuli, including gastric and intestinal microbiota. The bidirectional relationship between H. pylori infection and the brain-gut axis influences both the contagion process and the host’s neuroendocrine-immunological reaction to it, resulting in alterations in cognitive functions, food intake and appetite, immunological response, and modification of symptom sensitivity thresholds. Furthermore, disturbances in the upper and lower digestive tract permeability, motility and secretion can occur, mainly as a form of irritable bowel syndrome. Many of these abnormalities disappear following H. pylori eradication. H. pylori may have direct neurotoxic effects that lead to alteration of the brain-gut axis through the activation of neurogenic inflammatory processes, or by microelement deficiency secondary to functional and morphological changes in the digestive tract. In digestive tissue, H. pylori can alter signaling in the brain-gut axis by mast cells, the main brain-gut axis effector, as H. pylori infection is associated with decreased mast cell infiltration in the digestive tract. Nevertheless, unequivocal data concerning the direct and immediate effect of H. pylori infection on the brain-gut axis are still lacking. Therefore, further studies evaluating the clinical importance of these host-bacteria interactions will improve our understanding of H. pylori infection pathophysiology and suggest new therapeutic approaches.
Core tip: This manuscript is the first review concerning the interplay between Helicobacter pylori (H. pylori) infection and the brain-gut axis. Data from relevant publications, including both human and animal studies, were grouped and discussed according to the pathophysiological mechanisms of interactions between H. pylori and host reaction.
-
Citation: Budzyński J, Kłopocka M. Brain-gut axis in the pathogenesis of
Helicobacter pylori infection. World J Gastroenterol 2014; 20(18): 5212-5225 - URL: https://www.wjgnet.com/1007-9327/full/v20/i18/5212.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i18.5212
Upper digestive tract diseases, including both functional dyspepsia and dyspeptic symptoms in the course of gastritis or peptic ulcers, were considered for many years to be psychosomatic[1,2]. However, Helicobacter pylori (H. pylori) infection has been recognized as the main risk factor for many upper digestive tract disorders and complications, including life-threatening bleeding from the digestive tract, gastritis, non-ulcer dyspepsia, mucosa-associated lymphoid tissue (MALT) lymphoma and gastric cancer[3,4], and a risk factor for stress-induced peptic ulcers[2,5-8]. As a result, the role of psychosomatic factors in the pathogenesis of these diseases has since lost favor[1,9]. However, the presence of H. pylori-negative upper digestive tract abnormalities, such as peptic ulcers[10,11], and the fact that not all H. pylori-infected patients develop ulcers, with only 10%-15% of them presenting dyspeptic symptoms[3], suggest a role for other individual factors, including nervous system imbalance, as an indispensable cofactor in gastritis or ulcer disease pathogenesis[12]. These arguments imply that H. pylori infection may induce changes in the function and morphology of the digestive tract both directly through cytotoxin (CagA, VacA) release and inflammatory process activation, and indirectly, via the brain-gut axis[13]. This review presents and discusses the interaction between H. pylori infection and the brain-gut axis and the clinical implications for digestive tract function.
The brain-gut axis is anatomically based in the central (CNS), peripheral (PNS, enteric nervous system, or “little brain”), and autonomic nervous systems (ANS), and modulates gastrointestinal function via the regulation of the gastrointestinal immune system, mucosal inflammation and intestinal microbiota in response to stress, emotions and environmental influences[6,14,15]. This circuitry acts bidirectionally, playing a role both in upper (peptic ulcer, functional dyspepsia) and lower [irritable bowel syndrome (IBS), inflammatory bowel disease (IBD)] digestive tract homeostasis, appetite and weight control[6,15-17], modulation of the gut-associated immune system and in the coordination of the gastrointestinal tract with the overall physical and emotional state of the organism[18]. However, so many interactions and functions work not only via the nervous system, but also through the endocrine, immunological and metabolic pathways (neuroendocrine-immune crosstalk)[6,15-22]. The main endocrine role is played by the hypothalamus-pituitary-adrenal (HPA) axis, along with the highly significant neuroendocrine-immune response modulator corticotropin releasing factor (CRF). CRF regulates inflammation, gut permeability, visceral hypersensitivity, gut motility and HPA axis activity, and is recognized as the major endocrine system response to stress[6,14,21-25].
Intestinal bacteria also play an important and proven role in the above-described relationships (“brain-gut-enteric microbiota axis,” or “microbiome-gut-brain axis”), whose composition and function are related to nervous system activity and, in turn, communicate with the brain-gut axis through endocrine, immunological and neural messages[6,18,20,26-28]. Such bidirectional interactions are suspected on the basis of the following observations: (1) stress and mental disorders have a harmful effect on digestive tract function and intestinal bacterial flora[5,6,29]; (2) patients with digestive tract disorders have a high prevalence of neuropsychological comorbidities, such as depression, anxiety and alexithymia[13]; (3) changes in cognitive function occur after diet-induced gut-dysbiosis[18] or with alimentary tract diseases (e.g., hepatic encephalopathy) which may be altered after antibiotics, probiotics or laxative administration[15,30]; (4) patients with major depression show altered intestinal fermentation profiles[31]; (5) individuals with autism have abnormal microbiota and experience shorter antibiotic effects[32-34]; (6) some patients with digestive tract disorders demonstrate an ANS imbalance[6,14,15,21,22,29,35-39]; and (7) digestive tract stimulation and/or infection can produce abnormalities in brain-imaging techniques that show the activation (evoked potentials) or deactivation of specific brain regions, such as functional magnetic resonance imaging, positron emission tomography, magnetoencephalography and electroencephalography[40].
As a gastric mucosa-colonizing microorganism, H. pylori may contribute to the bidirectional nature of the brain-gut axis as evidenced by: (1) the overlapping of upper and lower digestive tract functional symptoms in H. pylori infected patients[18,41-43] and the effect of H. pylori infection on IBS symptoms[43] (but see Breckan et al[44]) and pancreatic juice secretion[45]; (2) the protection against IBD appearance by H. pylori infection[17,46] resulting from changes induced in brain-gut axis function (neuroendocrine-immune crosstalk)[6,15,21,22,26,47,48]; (3) the association of H. pylori infection with ANS-related extra-digestive diseases, such as atherosclerosis or cardiac arrhythmia[3,35]; (4) the improved physical and psychological health-related quality of life and sexual relationships after digestive tract symptom alleviation and H. pylori eradication[49,50]; (5) the proposed association between H. pylori infection and the development of axonal type Guillain-Barré neuropathy, multiple sclerosis and epilepsy[51-53], and case reports of gastric MALT lymphoma followed by primary CNS lymphoma[54]; and (6) the modulation of ANS balance by H. pylori infection[35]. Furthermore, H. pylori can potentially regulate esophageal motility[55], gastric emptying, gastric accommodation of ingested food, gastric acid secretion, mucosal blood flow, hypersensitivity to chemo- and mechano-stimulants[56], food intake[6,19], digestive tract endocrine and immune functions and the composition of gut microbiota[6,18,21,22], by influencing the release of various neurotransmitters, including acetylocholine (vagal nerve, parasympathetic part), noradrenaline, adrenaline and sympathetic dopamine, as well as the neuropeptides leptin, ghrelin, calcitonin gene-related peptide (CGRP), nitric oxide, neuropeptide Y, substance P (SP), somatostatin (STS) and cholecystokinin (CCK). Moreover, H. pylori infection is associated with the up-regulation of toll-like receptors and cytokine overproduction, especially tumor necrosis factor (TNF)-alpha, interleukin (IL)-1, IL-6, and IL-8[57-59], thereby indirectly influencing the brain-gut axis. These immune-mediators may stimulate mast cells (MC) in the gastric mucosa, as well as the hypothalamus and brain stem (via neuroendocrine-immuno crosstalk)[6,22], thereby activating the sympathetic ANS and pituitary-suprarenal axis, resulting in increased cortisol and adrenalin secretion[6,21,22,60,61].
H. pylori infection occurs via the oral route and, like other contagions, depends on the balance between bacteria virulence (urease, AmiE, AmiF, hydrogenase and arginase, BabA, SabA and ureaseA), host immunity and environmental factors[62]. The brain-gut axis may modulate the local immunological resistance related to gastric and gut-associated lymphoid tissue (neuroendocrine-immune crosstalk). However, these interactions are bidirectional and seem to be more complicated with regard to H. pylori infection. For example, nervous system disorders and the lack of MC in the gastric mucosa may predispose an individual to H. pylori infection, while at the same time, acute H. pylori infection may change both CNS and ANS activity[2,22,29,37,63,64]. Autonomic neuropathy facilitates gastric mucosal colonization and hinders H. pylori eradication via the slowing of gastric emptying, changes in gastric mucosal blood flow and immunosuppression of relative hyper-sympatheticotonia[2,37,60,63,64]. In an animal model, Guo et al[63] found that psychological stress increased H. pylori colonization and gastric mucosal injury. Studies by Gulcelik et al[65] and Gentile et al[37], demonstrated a higher prevalence of H. pylori in type 2 diabetics with cardiovascular autonomic neuropathy, but not with other common diabetic complications, such as nephropathy and retinopathy. Diabetic patients also commonly experience dyspeptic symptoms as a result of disturbances in gastric emptying, gastric wall accommodation and visceral hypersensitivity. These abnormalities, particularly efferent and vagal dysfunction, may be secondary to ANS activity[66,67]. Testoni et al[68] also showed a significantly greater prevalence of H. pylori infection in patients without evidence of gastric phase III of the migrating motor complex. Collectively, these data indicate that the coexistence of ANS dysfunction and H. pylori infection is a necessary condition for H. pylori invasion, as well as for chronic gastritis and peptic ulcer formation[6,14,15,21,22,29,35-39] (but see Chang et al[69]).
While it is known that acute intestinal infection during psychological or physical stress may evoke gastrointestinal symptoms and lead to increased mucosal MC infiltration[19], there is little information available concerning the effect of acute H. pylori infection on brain-gut axis function. The main mechanism for post-infectious IBS is infiltration and long-term maintenance of MCs in the digestive tract[6,16,21,22]. MC number and activity are increased in the gastric mucosa of patients with functional dyspepsia, independent of inflammation[70], as well as in subjects with H. pylori-associated gastritis[71-75]. MCs are actively involved in the pathogenesis of H. pylori-infected gastritis acting as important effectors of the brain-gut axis by translating stress signals into the release of a wide range of neurotransmitters and proinflammatory cytokines capable of altering gastric nerve and muscle function[6,70]. Furthermore, MCs may act via gastritis maintenance and reparation of injured tissue. MCs can initiate and promote edema by secreting multiple, degranulated chemotactic factors that induce the infiltration of inflammatory cells[73], as well as promote tissue turnover by stimulating the degradation of pericellular matrices and the growth of cells in their vicinity. In chronic H. pylori infection, these processes continue until the bacteria are eradicated. In this way, both acute and chronic H. pylori infection could contribute to the pathogenesis of functional dyspepsia and gastritis symptoms[9,70], especially in patients who have acquired excessive responsiveness to stress, such as a result of environmental influences during early life, genetic abnormalities, residual inflammation after gastrointestinal infections, psychophysiological abnormalities, abnormal secretion of gastric acid, diet and lifestyle[6,76]. Moreover, as with every acute infection, H. pylori may evoke flu-like symptoms and neuroendocrine alterations. Oral administration of Campylobacter jejuni or Citrobacter rodentium leads to the activation of vagal sensory neurons and brain regions associated with viscerosensory pathways and the central autonomic network in mice[77]. These local stomach and gut infections were associated with increased anxiety-like behavior, reminiscent of the sickness syndrome typically seen following systemic administration of bacteria or bacterial products, such as lipopolysaccharides. Furthermore, H. pylori-infected mice show an upregulation of SP expression, which may increase visceral mechanosensitivity in the spinal cord[56].
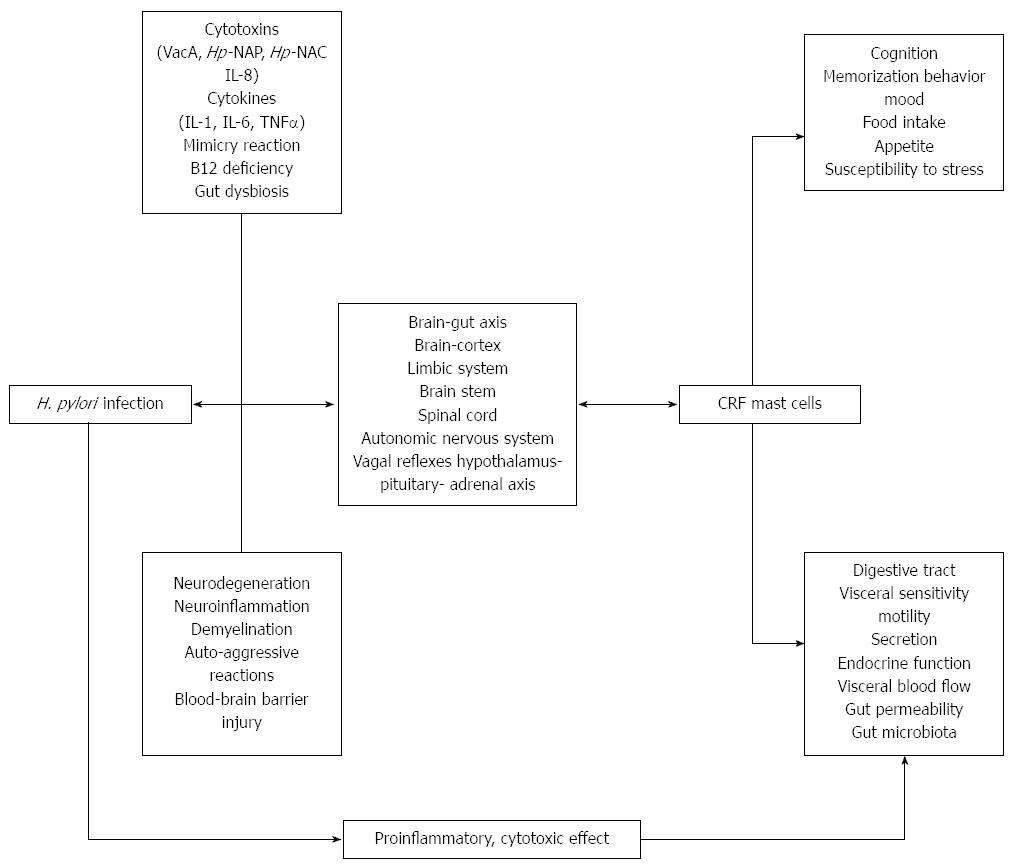
The pathophysiological interactions between H. pylori and the brain-gut axis likely involve (Figure 1): (1) axon injury or stimulation (neuroinflammation) by bacterial cytotoxins (VacA)[60,72,78], neutrophil-attractant chemokine IL-8[79], and/or neutrophil-activating protein (H. pylori-NAP)[75,79]; (2) axon damage or stimulation by autoimmunological reactions due to mimicry of VacA, bacterial aquaporin (AQP), H. pylori-NAP and human antigens[51,53,79,80]; (3) H. pylori-induced production of free radicals[80-82], cytotoxins and cytokines[57-61,78], which may also result in blood-brain barrier disruption; (4) changes in neurotransmitter secretion in gastric mucosa and spinal cord[56,72,75,81,83-85]; (5) neuron injury resulting from gastric mucosa atrophy and a decrease in vitamin B12 absorption[86]; and (6) changes in stomach and intestinal microbiota[6,87-89].
Reports concerning chronic H. pylori infection show abnormalities in neuroendocrine activity and in the digestive tract related to H. pylori virulence, and disappearance following eradicative therapy. Li et al[83] showed greater neuronal expressions of c-fos, vasoactive intestinal peptide (VIP) and CGRP in the stomach and spinal cord of H. pylori-infected animals, which they proposed could form a basis for the appearance of functional dyspeptic symptoms in H. pylori-infected patients, as these neuropeptides are involved in the sensitization of afferent neuronal pathways[84]. Similarly, Bercík et al[56] showed a decrease in acetylcholine release by electric field stimulation and a higher density of SP-, CGRP-, and VIP-immunoreactive nerves in the stomach and spinal cord as well as an increase in pro-opiomelanocortin (POMC) secretion[19] in H. pylori-infected mice. Eradication of H. pylori-normalized functional and morphological abnormalities, except for the increased density of gastric SP- and CGRP-immunoreactive nerves, which may be the basis for neuromuscular changes that produce dysmotility and hyperalgesia. This observation may have many clinical consequences, as POMC is a precursor for several important biologically active substances, including N-terminal peptide of pro-opiomelanocortin (or pro-γ-MSH), γ-melanotropin, adrenocorticotropic hormone, α-melanocyte-stimulating hormone, corticotropin-like intermediate peptide, β-lipotropin, γ-lipotropin, β-melanotropin, β-endorphin and met-enkephalin.
Neuronal and non-neuronal levels of SP, VIP, CGRP and neprilysin activity were examined in freshly frozen biopsies taken from patients with chronic gastritis or ulcers in a study by Erin et al[85] showing that neuronal SP, mucosal VIP and neprilysin levels decreased significantly in normal appearing mucosa in patients with gastritis, while levels of mucosal SP increased in areas of gastritis and ulcer. In this study the presence of H. pylori was associated with further decreases in SP levels. Hydrolysis of SP by neprilysin is known to have gastroprotective effects, therefore the decreased levels of VIP, SP and neprilysin were considered by the authors as a predisposition to cellular damage. In contrast, Dömötör et al[90] concluded that capsaicin-sensitive afferent nerves with gastroprotective properties do not participate in the harmful effects of H. pylori as they found that although the expression of the capsaicin receptor, CGRP and SP were significantly higher in the mucosa of patients with chronic gastritis, there were no significant differences between H. pylori-positive and H. pylori-negative cases.
A proportion of H. pylori effects on the brain-gut axis may be secondary to central and peripheral nerve demyelination and blood-brain barrier disruption[79]. The cross-mimicry between VacA and H. pylori-NAP is recognized as the most important immunological reaction[79], which induces autoreactive T cells and initiates or worsens gastric autoimmunity leading to atrophic gastritis and gastric cancer[91,92]. Cross-reactivity of anti-VacA antibodies with ion channels in Schwann cell plasmalemma could potentially induce demyelination, since cross-mimicry between H. pylori VacA and the human Na+/K+-ATPaseA subunit was identified by sequence homology. Cross-mimicry between H. pylori-NAP and/or anti-bacterial AQP antibodies and neural tissues is also possible. Whereas blood-brain barrier disruption may be secondary to the effects of H. pylori-induced inflammatory mediators, it can lead to increased brain vessel permeability for immunomediators, non-specific antibodies, AQP4-specific antibodies and inflammatory cells (e.g., lymphocytes), which can evoke many pathological processes in the CNS, including demyelination, local edema and neuroinflammation. These processes are recognized as important for associations between H. pylori infection and the pathogenesis of demyelination, multiple sclerosis and neuromyelitis optica[51,52,79].
Intermediate effects of chronic H. pylori infection on brain-gut axis function have been clinically observed as: (1) the alteration of feeding patterns[15,19]; (2) cognitive and memory dysfunction[18,19,27,82,93], increased vulnerability to stress[15,19,27] and anxiety- and depressive-like behaviors[19]; (3) alterations in endocrine functions of the stomach, including the production of SP, VIP, CCK, STS, gastrin and ghrelin[81,94]; (4) changes in visceral ANS balance and the action of vagal visceral reflexes[95]; (5) alterations in gastrointestinal motility[21]; (6) increased visceral perception (chemo- and mechano-hypersensitivity)[67,96]; (7) changes in gastrointestinal secretion[21,45]; (8) increased intestinal permeability[23,61]; (9) intestinal microbiota[87-89], with indirect effects on the brain-gut axis[27,28,97-99]; (10) alterations in immunological reactivity, resulting in decreased prevalence of food allergies and inflammatory bowel diseases[46,100]; and (11) the overlapping of gastrointestinal disorders from upper and lower parts of the digestive tract[43]. Moreover, H. pylori eradication has also been shown to normalize some of these alterations[55,95,100-103].
Clinical practice shows appetite improvement and increased body weight following H. pylori eradication[104,105]. Furthermore, corresponding changes in plasma and/or the gastric mucosal levels of hormones regulating appetite and/or energy expenditure were identified, such as ghrelin and leptin, two hormones primarily produced, stored and secreted by the gastric mucosa[106]. An animal model of H. pylori infection also showed abnormal feeding behavior observed as an increase in eating frequency and a decrease in the quantity of food consumed per meal[56]. These changes in feeding behavior were accompanied by elevated plasma ghrelin, postprandial CCK and higher TNF-alpha, along with reduced POMC mRNA in the arcuate nucleus of the brain. However, eradication therapy had no effect on eating behavior in this study, despite the normalization of gastric emptying and sensitivity.
A study of dyspeptic patients by Ulasoglu et al[55] found decreased ghrelin and increased obestatin blood levels after H. pylori eradication, which were greatest in overweight and male patients. However, Francois et al[102] reported a postprandial increase in ghrelin and leptin levels, and an increase in body mass index (BMI) in subjects 7-18 mo after successful H. pylori eradication treatment. Restored tissue levels of ghrelin and an improved appetite were also achieved after gastric ulcer healing and H. pylori eradication in studies by Roper et al[106], Jang et al[107] and Osawa et al[108]. Similar results were also obtained by Yang et al[101], who found lower serum ghrelin levels, reduced body weight and height and lower BMI and body weight gain in H. pylori-infected children, with restored ghrelin levels and increased growth observed one year after H. pylori eradication. Increased plasma and gastric mucosal ghrelin levels after H. pylori eradication were also shown by Deng et al[103] in 50 children with H. pylori-associated functional dyspepsia. However, a study reported by Choe et al[109] failed to show a relationship between H. pylori infection and stomach mucosal ghrelin levels, with no effect of eradication therapy. It should be noted that a greater BMI and corpus predominant gastritis are recognized as independent risk factors for eradication failure[110].
The participation of the brain-gut axis in associations between bacterial flora in the bowel and some behavior patterns, and cognitive and mental disorders as well as their improvement following antibiotic, pre- and/or probiotic administration, have been reported previously, both in animals[19,24,25,93,97-99] and in humans[6,14,27,28]. It is also possible that psychiatric disorders in H. pylori-infected subjects result from disease overlap, with comorbidity in 40% of patients with functional dyspepsia, of which depression is the most prevalent (24%)[111].
Bacterial infection of germ-free mice evokes anxiety-like behavior, which is relieved after introducing normal gut microbiota[19,24,25,97-99]. A corresponding increase in brain-derived neurotrophic factor (BDNF) in the hippocampus and amygdala was observed that also diminished after antibiotic therapy in these mice[19]. BDNF is involved in the neurobiology of depression[112], and is also considered a biomarker for gastric hypersensitivity and an overactive bladder[113,114]. Moreover, stress-induced memory dysfunction in mice after bacterial infection was reported by Gareau et al[93].
In a large, cross-sectional study of United States adults by Beydoun et al[115], H. pylori-seropositivity was associated with poor cognition based on declining verbal memory test performance. H. pylori, as with other urease-positive bacteria that produce ammonia, is also recognized as a cause of liver-like encephalopathy[116]. In a study by Roubaud Baudron et al[117] who followed-up individuals aged 65 years or older for 20 years, H. pylori infection was recognized as a significant risk factor for the development of dementia as a result of enhanced neurodegenerative processes, a hypothesis that has been suggested by Deretzi et al[80]. Moreover, a study by Muhsen et al[118] indicated that H. pylori might also negatively affect early cognitive development. In our own study, depressive and alexithymic alcoholics had less intensive H. pylori antral colonization than subjects with lower Beck Depression Inventory and Toronto Alexithymia Scale scores[29]. In a study by Ozdemir et al[119], a significant association was observed between schizophrenia and diminished risk of duodenal and gastric but not gastrojejunal ulcers. The authors concluded that schizophrenia and attendant neurobiological mechanisms (e.g., variability in dopamine pathways) may therefore modify the risk of gastric and duodenal ulcers as both central and peripheral dopamine pathways influence ulcer pathogenesis, and dopamine agonists preclude antagonists from augmenting stress - and chemically - induced gastrointestinal ulcers in preclinical models.
Probiotics reduce the adverse effects, prevent complications and improve the outcome of H. pylori-eradicative therapy[120]. Furthermore, Bifidobacterium bifidum YIT 4007 in fermented milk suppresses H. pylori growth[100]. However, research concerning the effect of probiotic supplementation on brain-gut axis function and mental disorders in subjects with H. pylori is lacking, as is data regarding the adverse effect of eradicative therapy on intestinal microbiota for a sub-form of dysbiosis in the stomach[89]. Myllyluoma et al[88] showed that the triple standard H. pylori-eradicative therapy (clarithromycin, amoxicillin and lansoprazole) significantly changed the quantities of the predominant bacterial groups both in H. pylori-positive and H. pylori-negative patients, with disturbances observed up to nine weeks after treatment completion. In their study, a combination of probiotics containing Lactobacillus rhamnosus GG, L. rhamnosus LC705, Propionibacterium freudenreichii spp., Shermanii JS and Bifidobacterium breve Bb99 only slightly counteracted the effects of anti-H. pylori treatment, seen as significantly fewer alterations in the total numbers of aerobes and lactobacilli/enterococci. However, it was found that probiotics in animal models reduced vagal-dependent activation of GABA receptors in response to physical or psychological stress[6,19]. Moreover, probiotics prevented stress-induced memory deficits in mice, reduced BDNF and c-fos expression in the hippocampus, as well as attenuated corticosterone release in response to stress[27,93,97-99].
The ANS is the most important part of the brain-gut axis, containing afferent and efferent pathways regulating saliva and gastric juice secretion, mucosal blood flow, inflammatory process activity, digestive tract motility and visceral pain threshold (visceral sensitivity)[15,21,22,35,56]. Disturbances in ANS balance are observed in patients presenting with H. pylori infection and functional and organic digestive tract disorders[10,14,15,21,22,29,35-39]. Our study found that among patients diagnosed as a result of atypical chest pain, subjects infected with H. pylori had increased ANS activity, an effect that was confirmed by multi-factorial analysis[35]. Moreover, our study showed negative relationships between H. pylori infection and esophageal sensitivity to acid, the number of acid gastroesophageal refluxes, and time spent at a gastric pH < 4, as well as positive relationships between H. pylori and the efficacy of esophageal peristalsis, and the amplitude and duration of esophageal contractions. We therefore concluded that gastric mucosal colonization by H. pylori affects ANS activity and can evoke disturbances in esophageal and gastric function. Vagal nerve predominance, as diagnosed by heart rate variability analyses, was also recognized as a risk factor for duodenal ulcer development[2,38-39,121]. Lugon et al[122] reported blunted sympathetic reactivity and an exacerbated vagal response to feeding in H. pylori-positive subjects. However, Katoh et al[36] have shown that H. pylori eradication in duodenal ulcer patients does not change nocturnal sympatheticotonia or parasympatheticotonia, which may be the cause for persistently increased gastric acid secretion, gastric mucosal vasoconstriction and ulcer recurrence[38,121], though the time course between eradication and ANS evaluation may have been too short to reveal statistically significant differences. Indeed, Stanghellini et al[123] suggest there is long-term recovery of H. pylori-induced neuroplastic changes in the ANS and highlight the importance of an incomplete resolution of gastritis and the presence of a persistent production of inflammatory mediators even after successful H. pylori eradication. However, Thor and Blaut[124] suggest that H. pylori eradication restores the parasympatheticotonic imbalance via a ghrelin-leptin interplay. Shahabi et al[125] further suggest that H. pylori infection of the gastric mucosa may induce a T-helper-1-like immune response and production of proinflammatory cytokines, which can inhibit local, and increase systemic, sympathetic and cholinergic tone. Such ANS changes can inhibit inflammation in the esophagus and stomach.
It is known that the brain-gut axis, including the CNS, PNS and ANS, modulates visceral reflex activity. This activity is responsible for the local regulation of digestive tract function, such as the gastric and intestinal phase of gastric or pancreatic juice secretion, esophageal, gastric and jejunal motility and visceral sensitivity. Furthermore, visceral reflexes regulate the remote effect of gastrointestinal stimulation, including the cardio-esophageal or gastro-cardiac reflexes responsible for coronary artery spasm in response to esophageal acidification or distension. In return, coronary angioplasty can induce esophageal motility abnormalities, a decrease in the pain threshold and esophageal and gastric hyperalgesia, via cardio-esophageal reflex activation[67,95]. As these effects were only observed in 56% of subjects with non-cardiac chest pain[95], reciprocal modulation by the brain-gut axis may have had further influence along with individual reactions to stress[6,21,22]. Thus, more research is needed concerning the effect of H. pylori infection on visceral reflexes.
Visceral hypersensitivity is a symptom of functional digestive tract disorders that may be modulated by H. pylori infection and the brain-gut axis[76]. The brain-gut axis plays an important role in the tonic or phasic up- or downregulation of afferent and efferent nerve sensitivities[18,21,22]. Visceral afferent pathways (enteric, spinal and vagal) transmit signals from the stomach to the CNS. As mentioned above, these afferents may also modulate emotion, pain, satiety and immunity in response to emotional influences or environmental demands. Visceral hypersensitivity may also be affected by hypersympatheticotonia. However, both hypervagotonia and hypersympatheticotonia may change efferent nerve activity due to modulation of vagal visceral reflex activity and the patient’s digestive tract response to visceral or environmental stimuli[67].
H. pylori infection may additionally affect brain-gut axis function and visceral sensitivity by neurotransmitter and hormone release and activation of inflammatory processes[61], as well as induce digestive tract dysmotility and trigger neuroplastic changes in the afferent neural pathways leading to visceral hyperalgesia[123]. A reduction in central antinociceptive control systems, visceral pain modulators both in digestive tract disorders and extra-gastric syndromes, may also play a pathophysiological role in H. pylori infection.
Bercík et al[56] found that an increase in visceral perception (mechanosensitivity) in H. pylori-infected mice was related to an increase in vagal SP expression. Similarly, Mönnikes et al[126] showed that discomfort and pain thresholds on gastric distension were lower in patients with functional dyspepsia that were H. pylori-positive. These patients also showed higher antral mucosal levels of CGRP and SP, which negatively correlated with the levels of discomfort and pain thresholds, demonstrating the role of the brain-gut axis in hypersensitivity and SP and CGRP involvement in the sensitization of afferent neuronal pathways, which result from chronic inflammation[84,126]. Similar inflammation-related changes in sensory-motor function have been observed in subjects with intestinal mucosal inflammation[9,11,123]. The role of H. pylori infection in the symptoms of functional dyspepsia was also confirmed by a Cochrane review, which reported that eradication of H. pylori had a small but statistically significant long-term effect on symptom relief when compared with a placebo, lasting at least 12 mo after one week of eradication therapy, expressed more in patients from Asian rather than Western countries[92,127]. However, Mearin et al[128] found that H. pylori-infected patients with functional dyspepsia presented no distinctive symptoms compared to their H. pylori-negative counterparts. Furthermore, additional placebo-controlled trials and a meta-analysis did not show improvement in dyspeptic symptoms with H. pylori eradication[129-131]. Discrepancies regarding symptomatic response to H. pylori eradication may relate to commonly occurring comorbid psychiatric disorders, which can affect treatment outcome[111]. H. pylori infection is also considered a cause of visceral hypersensitivity in patients with IBS, as individuals reporting typical abdominal discomfort after rectal barostat testing were more frequently H. pylori positive[42,43].
Disturbances in ANS activity, particularly hyperparasympatheticotonia, should also be recognized as a factor responsible for the clinical manifestation of lowered pain thresholds. H. pylori infection may increase sympathetic ANS activity via induction of gastritis and overproduction of systemic inflammatory mediators through a pathogen-burden mechanism[61]. Increased ANS activity is related to symptom thresholds in patients with peptic ulcers[38-39,121], in acid-sensitive and insensitive patients with non-cardiac chest pain[67], in patients with non-specific disorders of esophageal motility[132], as well as in individuals with functional dyspepsia[9,21,42,66,76,94,126,133] and IBS[134]. Such a pathway is also considered as one of the causes of pain in H. pylori-infected patients with fibromyalgia[96].
Digestive tract motor disorders are a source of functional symptoms originating from the upper and lower parts of the digestive tract[76]. Typical gastric motility abnormalities involve impaired fundic accommodation and reduced compliance to meals, impaired initial distribution of a meal, antral hypomotility, altered myoelectric activity and gastric emptying and disturbed gastro- and duodeno-jejunal motor coordination[133]. Delayed gastric emptying has been described in about 30%-80% of patients with functional dyspepsia as a result of antral or fundal hypomotility, uncoordinated antro-pyloro-duodenal activity or inhibitory feedback of the intestine[135]. Symptoms of motility disorders may be evoked through the activation of chemo- (delayed clearance) and mechano-receptors (digestive tract tube contraction or extension due to hypertensive spasm or dilatation proximal to contraction), via activation of viscerosomatic reflexes, and somatic receptors localized in the distended abdominal wall in the course of bloating, especially in individuals with hypersensitivity to stress. Furthermore, symptoms relating to reflux of gastric content into the esophagus, or other causes of delayed gastric emptying and/or its accommodation impairment, may appear in patients with gastroparesis[136].
The effect of H. pylori infection on digestive tract motility may result from its direct local action, or an intermediate effect via the above-described ANS and endocrine effects (i.e., due to alteration in gastrin, STS, CCK and plasma and mucosal ghrelin levels)[137-140]. However, there are no recent studies on the effect of H. pylori infection and its eradication on esophageal and gastric motility. Chiloiro et al[140] showed higher gastrin and lower CCK plasma levels in H. pylori-infected patients with functional dyspepsia, but did not find a difference in gastric emptying. Similarly, Schenk et al[137] showed that severe hypergastrinemia during omeprazole maintenance therapy for gastroesophageal reflux was associated with the duration of therapy and H. pylori infection, but not with abnormalities of gastric emptying or vagal nerve integrity. Currently, there are no reports concerning the effect of H. pylori-induced hypergastrinemia secondary to hypochloremia in the course of H. pylori infection-related gastric corpus mucosa atrophy. However, an in vitro study showed that eradication of H. pylori did not affect muscle contractility and acetylcholine release but augmented antral relaxation after nerve electrical field stimulation[56].
A study by our group found positive relationships between H. pylori infection and the efficacy of esophageal peristalsis and the amplitude and duration of esophageal contractions in patients with atypical chest pain[35]. However, Tsai et al[141] and Zerbib et al[142] did not find any differences in the patterns of esophageal motility and transient lower esophageal sphincter relaxations between H. pylori-positive and H. pylori-negative subjects. Similarly, Tanaka et al[143] did not observe changes in gastric and esophageal function after H. pylori eradication, besides an increase in gastric acidity at night, suggesting that H. pylori does not produce esophageal-motility-related chest pain, although it induced a more active inflammatory process in esophageal mucosa[142].
The presence of H. pylori infection also did not significantly affect gastric emptying rates for solids and liquids, discomfort sensitivity thresholds, or meal-induced gastric relaxation in dyspeptic patients[144]. H. pylori-positive and -negative patients in a study by Mearin et al[128] also suffered from similar severity and frequency of dyspeptic symptoms and global symptom scores, and presented no differences in gastric compliance. However, postprandial antral motility in H. pylori-positive patients was significantly decreased, though without an increase in their perception of gastric distension. A report by Thumshirn et al[145] showed that impaired gastric accommodation is frequent in non-ulcer dyspepsia, but unrelated to H. pylori infection and vagal efferent dysfunction. Additionally, a placebo-controlled double-blind study showed that H. pylori eradication in patients with functional dyspepsia had no impact on gastric emptying as estimated using a standardized scintigraphic double-tracer[146]. Furthermore, a decreased prevalence of normal electrogastrography (EGG) patterns was found in both H. pylori-positive and -negative patients with functional dyspepsia compared to healthy controls, though only subjects with delayed gastric emptying achieved symptom improvement and normalization of the EGG pattern after H. pylori eradication[135]. In contrast, a study by Simrén et al[20] indicated that H. pylori infection was independently associated with the presence of unsuppressed phasic fundic contractility after a meal, which induced a transient increase in gastric wall tension that was observed in 15% of subjects. Lin et al[147] found abnormal gastric myoelectrical activity in approximately 40% of H. pylori-infected patients with functional dyspepsia, which was normalized in some following H. pylori eradication, with similar observations reported by Thor et al[148]. In a double-blind study performed in patients with duodenal ulcers, Konturek et al[139] showed that H. pylori infection was accompanied by enhanced gastric emptying and reduced luminal release of STS, although a CCK antagonist had no effect in H. pylori-infected subjects. The authors therefore concluded that, since gastric motor function and STS secretion were normalized after H. pylori eradication, CCK and STS contribute to the normalization of gastric emptying following H. pylori eradication in patients with duodenal ulcer.
Current data demonstrate contrary outcomes of the effect of H. pylori-infection on digestive tract motor function, and further studies are therefore needed to address these discrepancies as well as the involvement of the brain-gut axis. Indeed, this has been suggested by Manes et al[149] who highlighted the low quality of the studies performed, methodological problems relating to the small numbers of patients, differences in methodological approaches and the well-known difficulties in studying both gastrointestinal motility and functional dyspepsia.
The known phrase, “no acid - no ulcer” emphasizes the important role of gastric acid secretion in the pathogenesis of upper digestive tract symptoms and injuries. As H. pylori infection is recognized as the main ulcerogenic factor, it is an important pathomechanism of direct and immediate harmful H. pylori action via the brain-gut axis. The effect of H. pylori infection on gastric acid secretion depends on the location of the gastritis. In localization of the antral inflammatory process, acid hypersecretion was observed, leading to stomach mucosal atrophy and hypochlorhydria in patients with corporal gastritis or pan-gastritis[141]. After H. pylori eradication, intragastric pH in the postprandial period was significantly lower, whereas baseline pH remained unchanged[150]. These changes were expressed particularly in patients with the proinflammatory IL-1B genotype, which is associated with an increased risk of gastric mucosa atrophy, lower gastric acidity and gastric carcinoma[151].
Chronic gastritis also affects a variety of endocrine functions of the stomach, including the production of STS, gastrin and ghrelin[94]. As gastric parietal cells are regulated by hormones and neurotransmitters, such as gastrin, histamine and acetylocholine, analysis of the effect of H. pylori-infection on the brain-gut axis is reasonable. H. pylori infection leads to a decrease in gastric acid secretion due to mucosal atrophy and vagal nerve dysfunction[81], which may result in alteration of both gastric motility and secretion, as well as food intake.
Abnormal vagal nerve activity should be considered as a potential mediator of H. pylori-induced changes in secretion of gastric acid, gastrin and ghrelin. However, there are no recent studies investigating the effect of H. pylori infection and eradication on gastric acidity. Lucini et al[39] examined the association between ANS activity and gastric acid secretion and found that sham-fed patients with duodenal ulcers had greater vagal and lower sympathetic nerve activities using heart rate variability (HRV) analyses. In these patients, the sham feeding to basal acid output ratio value correlated with markers of ANS activity. A study by Nada et al[121] reported high sympatho-vagal tone at night in patients with peptic ulcers, which can result in gastric artery spasms and excess secretion of gastric acid. In the same study, HRV markers of vagal nerve activity and gastric juice secretion increased in adult mongrel dogs following an intravenous injection of insulin.
H. pylori-positive patients with duodenal ulcers showed increased plasma gastrin concentrations in a study by Iijima et al[152], while H. pylori-positive patients with gastric ulcers showed lower concentrations compared to H. pylori-negative counterparts. In both groups, the gastrin plasma levels changed after H. pylori eradication, decreasing in individuals with duodenal ulcers, and increasing in those with gastric ulcers. Ghrelin is also secreted by gastric mucosa cells, and not only regulates food intake, but also exhibits an effect on gastroprotection and increases esophageal and gastric motility and gastric acid secretion[6,14,124,148,153].
H. pylori infection, the main pathogenic factor of upper digestive tract organic diseases, displays not only local cytotoxic and proinflammatory effects, but may also exert systemic action via modulation of the brain-gut axis. These relationships are bidirectional and influence the contagion process and the host neuro-endocrine immunological reaction, including alterations in cognitive functions, modification of symptom sensitivity thresholds and digestive tract secretion and motility. The effect on the brain-gut axis by this microorganism may arise from a direct neurotoxic effect, activation of inflammatory processes in nerves and microelement deficiency. However, many of the investigations concerning the interplay between H. pylori and the effects on brain-gut axis activation are dated. Therefore, further studies are necessary to evaluate the clinical importance of these host-bacteria interactions. Results from such studies will improve our understanding of H. pylori infection pathophysiology and suggest new therapeutic approaches.
P- Reviewers: D’Elios MM, Shi HY, Yan SL S- Editor: Gou SX L- Editor: A E- Editor: Ma S
| 1. | Melmed RN, Gelpin Y. Duodenal ulcer: the helicobacterization of a psychosomatic disease? Isr J Med Sci. 1996;32:211-216. [PubMed] [Cited in This Article: ] |
| 2. | Moriya M, Uehara A, Okumura T, Miyamoto M, Kohgo Y. Stress-induced hemorrhagic gastric ulcer after successful Helicobacter pylori eradication: two case reports. J Med Case Rep. 2011;5:252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646-664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1541] [Cited by in F6Publishing: 1494] [Article Influence: 124.5] [Reference Citation Analysis (3)] |
| 4. | Stenström B, Mendis A, Marshall B. Helicobacter pylori--the latest in diagnosis and treatment. Aust Fam Physician. 2008;37:608-612. [PubMed] [Cited in This Article: ] |
| 5. | Aoyama N, Shinoda Y, Matsushima Y, Shirasaka D, Kinoshita Y, Kasuga M, Chiba T. Helicobacter pylori-negative peptic ulcer in Japan: which contributes most to peptic ulcer development, Helicobacter pylori, NSAIDS or stress? J Gastroenterol. 2000;35 Suppl 12:33-37. [PubMed] [Cited in This Article: ] |
| 6. | Konturek PC, Brzozowski T, Konturek SJ. Stress and the gut: pathophysiology, clinical consequences, diagnostic approach and treatment options. J Physiol Pharmacol. 2011;62:591-599. [PubMed] [Cited in This Article: ] |
| 7. | Svoboda P, Kantorova I, Scheer P, Doubek J, Ochmann J, Rehorkova D, Bosakova H. Helicobacter pylori is not a risk factor for stress ulcer bleeding in polytraumatized patients. Hepatogastroenterology. 2004;51:476-480. [PubMed] [Cited in This Article: ] |
| 8. | van der Voort PH, van der Hulst RW, Zandstra DF, Geraedts AA, van der Ende A, Tytgat GN. Prevalence of Helicobacter pylori infection in stress-induced gastric mucosal injury. Intensive Care Med. 2001;27:68-73. [PubMed] [Cited in This Article: ] |
| 9. | Keohane J, Quigley EM. Functional dyspepsia: the role of visceral hypersensitivity in its pathogenesis. World J Gastroenterol. 2006;12:2672-2676. [PubMed] [Cited in This Article: ] |
| 10. | Charpignon C, Lesgourgues B, Pariente A, Nahon S, Pelaquier A, Gatineau-Sailliant G, Roucayrol AM, Courillon-Mallet A. Peptic ulcer disease: one in five is related to neither Helicobacter pylori nor aspirin/NSAID intake. Aliment Pharmacol Ther. 2013;38:946-954. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Jones MP. The role of psychosocial factors in peptic ulcer disease: beyond Helicobacter pylori and NSAIDs. J Psychosom Res. 2006;60:407-412. [PubMed] [Cited in This Article: ] |
| 12. | Chen TS, Chang FY. Clinical characteristics of Helicobacter pylori-negative duodenal ulcer disease. Hepatogastroenterology. 2008;55:1615-1618. [PubMed] [Cited in This Article: ] |
| 13. | Goodwin RD, Talley NJ, Hotopf M, Cowles RA, Galea S, Jacobi F. A link between physician-diagnosed ulcer and anxiety disorders among adults. Ann Epidemiol. 2013;23:189-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Caso JR, Leza JC, Menchén L. The effects of physical and psychological stress on the gastro-intestinal tract: lessons from animal models. Curr Mol Med. 2008;8:299-312. [PubMed] [Cited in This Article: ] |
| 15. | Collins SM, Denou E, Verdu EF, Bercik P. The putative role of the intestinal microbiota in the irritable bowel syndrome. Dig Liver Dis. 2009;41:850-853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Mach T. The brain-gut axis in irritable bowel syndrome--clinical aspects. Med Sci Monit. 2004;10:RA125-RA131. [PubMed] [Cited in This Article: ] |
| 17. | Luther J, Dave M, Higgins PD, Kao JY. Association between Helicobacter pylori infection and inflammatory bowel disease: a meta-analysis and systematic review of the literature. Inflamm Bowel Dis. 2010;16:1077-1084. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 18. | Mayer EA, Tillisch K, Bradesi S. Review article: modulation of the brain-gut axis as a therapeutic approach in gastrointestinal disease. Aliment Pharmacol Ther. 2006;24:919-933. [PubMed] [Cited in This Article: ] |
| 19. | Bercik P, Verdú EF, Foster JA, Lu J, Scharringa A, Kean I, Wang L, Blennerhassett P, Collins SM. Role of gut-brain axis in persistent abnormal feeding behavior in mice following eradication of Helicobacter pylori infection. Am J Physiol Regul Integr Comp Physiol. 2009;296:R587-R594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 49] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 20. | Simrén M, Vos R, Janssens J, Tack J. Unsuppressed postprandial phasic contractility in the proximal stomach in functional dyspepsia: relevance to symptoms. Am J Gastroenterol. 2003;98:2169-2175. [PubMed] [Cited in This Article: ] |
| 21. | Wood JD. Neuropathophysiology of functional gastrointestinal disorders. World J Gastroenterol. 2007;13:1313-1332. [PubMed] [Cited in This Article: ] |
| 22. | Ziemssen T, Kern S. Psychoneuroimmunology--cross-talk between the immune and nervous systems. J Neurol. 2007;254 Suppl 2:II8-I11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Fukuda Y, Bamba H, Okui M, Tamura K, Tanida N, Satomi M, Shimoyama T, Nishigami T. Helicobacter pylori infection increases mucosal permeability of the stomach and intestine. Digestion. 2001;63 Suppl 1:93-96. [PubMed] [Cited in This Article: ] |
| 24. | Neufeld KA, Kang N, Bienenstock J, Foster JA. Effects of intestinal microbiota on anxiety-like behavior. Commun Integr Biol. 2011;4:492-494. [PubMed] [Cited in This Article: ] |
| 25. | Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil. 2011;23:255-64, e119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 887] [Cited by in F6Publishing: 859] [Article Influence: 66.1] [Reference Citation Analysis (0)] |
| 26. | Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6:306-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 760] [Cited by in F6Publishing: 814] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 27. | Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701-712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2403] [Cited by in F6Publishing: 2574] [Article Influence: 214.5] [Reference Citation Analysis (0)] |
| 28. | Neary NM, Small CJ, Bloom SR. Gut and mind. Gut. 2003;52:918-921. [PubMed] [Cited in This Article: ] |
| 29. | Swiatkowski M, Budzyński J, Kłopocka M, Ziółkowski M, Bujak R, Sinkiewicz W. Parameters of the functional and morphological status of the upper digestive tract in alcohol-dependent male patients with depression and alexithymia in the context of autonomic nervous system activity and nitric oxide plasma level. Med Sci Monit. 2004;10:CR68-CR74. [PubMed] [Cited in This Article: ] |
| 30. | Bajaj JS, Hylemon PB, Ridlon JM, Heuman DM, Daita K, White MB, Monteith P, Noble NA, Sikaroodi M, Gillevet PM. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am J Physiol Gastrointest Liver Physiol. 2012;303:G675-G685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 354] [Cited by in F6Publishing: 375] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 31. | Ledochowski M, Widner B, Bair H, Probst T, Fuchs D. Fructose- and sorbitol-reduced diet improves mood and gastrointestinal disturbances in fructose malabsorbers. Scand J Gastroenterol. 2000;35:1048-1052. [PubMed] [Cited in This Article: ] |
| 32. | Sandler RH, Finegold SM, Bolte ER, Buchanan CP, Maxwell AP, Väisänen ML, Nelson MN, Wexler HM. Short-term benefit from oral vancomycin treatment of regressive-onset autism. J Child Neurol. 2000;15:429-435. [PubMed] [Cited in This Article: ] |
| 33. | Parracho HM, Bingham MO, Gibson GR, McCartney AL. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J Med Microbiol. 2005;54:987-991. [PubMed] [Cited in This Article: ] |
| 34. | Finegold SM, Downes J, Summanen PH. Microbiology of regressive autism. Anaerobe. 2012;18:260-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 154] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 35. | Budzyński J, Kłopocka M, Bujak R, Swiatkowski M, Pulkowski G, Sinkiewicz W. Autonomic nervous function in Helicobacter pylori-infected patients with atypical chest pain studied by analysis of heart rate variability. Eur J Gastroenterol Hepatol. 2004;16:451-457. [PubMed] [Cited in This Article: ] |
| 36. | Katoh K, Nomura M, Nakaya Y, Iga A, Nada T, Hiasa A, Ochi Y, Kawaguchi R, Uemura N, Honda H. Autonomic nervous activity before and after eradication of Helicobacter pylori in patients with chronic duodenal ulcer. Aliment Pharmacol Ther. 2002;16 Suppl 2:180-186. [PubMed] [Cited in This Article: ] |
| 37. | Gentile S, Turco S, Oliviero B, Torella R. The role of autonomic neuropathy as a risk factor of Helicobacter pylori infection in dyspeptic patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 1998;42:41-48. [PubMed] [Cited in This Article: ] |
| 38. | Nomura M, Yukinaka M, Miyajima H, Nada T, Kondo Y, Okahisa T, Shibata H, Okamura S, Honda H, Shimizu I. Is autonomic dysfunction a necessary condition for chronic peptic ulcer formation? Aliment Pharmacol Ther. 2000;14 Suppl 1:82-86. [PubMed] [Cited in This Article: ] |
| 39. | Lucini D, Cerchiello M, Basilisco G, Cainelli M, Bianchi PA, Fiorelli G, Malliani A, Pagani M. Autonomic control of heart period in duodenal ulcer patients insights from spectral analysis of heart rate variability. Auton Neurosci. 2000;84:122-129. [PubMed] [Cited in This Article: ] |
| 40. | Sharma A, Lelic D, Brock C, Paine P, Aziz Q. New technologies to investigate the brain-gut axis. World J Gastroenterol. 2009;15:182-191. [PubMed] [Cited in This Article: ] |
| 41. | Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480-1491. [PubMed] [Cited in This Article: ] |
| 42. | Su YC, Wang WM, Wang SY, Lu SN, Chen LT, Wu DC, Chen CY, Jan CM, Horowitz M. The association between Helicobacter pylori infection and functional dyspepsia in patients with irritable bowel syndrome. Am J Gastroenterol. 2000;95:1900-1905. [PubMed] [Cited in This Article: ] |
| 43. | Gerards C, Leodolter A, Glasbrenner B, Malfertheiner P. H. pylori infection and visceral hypersensitivity in patients with irritable bowel syndrome. Dig Dis. 2001;19:170-173. [PubMed] [Cited in This Article: ] |
| 44. | Breckan RK, Asfeldt AM, Straume B, Florholmen J, Paulssen EJ. Prevalence, comorbidity, and risk factors for functional bowel symptoms: a population-based survey in Northern Norway. Scand J Gastroenterol. 2012;47:1274-1282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Domínguez-Muñoz JE, Malfertheiner P. Effect of Helicobacter pylori infection on gastrointestinal motility, pancreatic secretion and hormone release in asymptomatic humans. Scand J Gastroenterol. 2001;36:1141-1147. [PubMed] [Cited in This Article: ] |
| 46. | Xiang Z, Chen YP, Ye YF, Ma KF, Chen SH, Zheng L, Yang YD, Jin X. Helicobacter pylori and Crohn’s disease: a retrospective single-center study from China. World J Gastroenterol. 2013;19:4576-4581. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 21] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 47. | Grundy D, Al-Chaer ED, Aziz Q, Collins SM, Ke M, Taché Y, Wood JD. Fundamentals of neurogastroenterology: basic science. Gastroenterology. 2006;130:1391-1411. [PubMed] [Cited in This Article: ] |
| 48. | Marques AH, Silverman MN, Sternberg EM. Evaluation of stress systems by applying noninvasive methodologies: measurements of neuroimmune biomarkers in the sweat, heart rate variability and salivary cortisol. Neuroimmunomodulation. 2010;17:205-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 49. | Budzyński J, Pulkowski G, Suppan K, Fabisiak J, Majer M, Kłopocka M, Galus-Pulkowska B, Wasielewski M. Improvement in health-related quality of life after therapy with omeprazole in patients with coronary artery disease and recurrent angina-like chest pain. A double-blind, placebo-controlled trial of the SF-36 survey. Health Qual Life Outcomes. 2011;9:77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 50. | Wilhelmsen I. Quality of life and Helicobacter pylori eradication. Scand J Gastroenterol Suppl. 1996;221:18-20. [PubMed] [Cited in This Article: ] |
| 51. | Kountouras J, Deretzi G, Zavos C, Tsiptsios D, Gavalas E, Vardaka E, Polyzos SA, Klonizakis P, Kyriakou P. Helicobacter pylori infection may trigger Guillain-Barré syndrome, Fisher syndrome and Bickerstaff brainstem encephalitis. J Neurol Sci. 2011;305:167-18; author reply 169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 52. | Kountouras J, Zavos C, Deretzi G, Polyzos SA, Katsinelos P, Anastasiadou K, Stergiopoulos C, Pilpilidis I, Klonizakis P, Boura P. Helicobacter pylori might be a potential therapeutic target in epilepsy. Med Hypotheses. 2011;76:763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 53. | Deretzi G, Kountouras J, Grigoriadis N, Zavos C, Chatzigeorgiou S, Koutlas E, Tsiptsios I. From the “little brain” gastrointestinal infection to the “big brain” neuroinflammation: a proposed fast axonal transport pathway involved in multiple sclerosis. Med Hypotheses. 2009;73:781-787. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 54. | Eom KS, Park MR, Choi KH, Kim TY. Gastric mucosa-associated lymphoid tissue lymphoma followed by primary central nervous system lymphoma. J Korean Neurosurg Soc. 2012;51:377-379. [PubMed] [Cited in This Article: ] |
| 55. | Ulasoglu C, Isbilen B, Doganay L, Ozen F, Kiziltas S, Tuncer I. Effect of Helicobacter pylori eradication on serum ghrelin and obestatin levels. World J Gastroenterol. 2013;19:2388-2394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 13] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 56. | Bercík P, De Giorgio R, Blennerhassett P, Verdú EF, Barbara G, Collins SM. Immune-mediated neural dysfunction in a murine model of chronic Helicobacter pylori infection. Gastroenterology. 2002;123:1205-1215. [PubMed] [Cited in This Article: ] |
| 57. | Lagunes-Servin H, Torres J, Maldonado-Bernal C, Pérez-Rodríguez M, Huerta-Yépez S, Madrazo de la Garza A, Muñoz-Pérez L, Flores-Luna L, Ramón-García G, Camorlinga-Ponce M. Toll-like receptors and cytokines are upregulated during Helicobacter pylori infection in children. Helicobacter. 2013;18:423-432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 58. | Rahimi HR, Rasouli M, Jamshidzadeh A, Farshad S, Firoozi MS, Taghavi AR, Kiany S. New immunological investigations on Helicobacter pylori-induced gastric ulcer in patients. Microbiol Immunol. 2013;57:455-462. [PubMed] [Cited in This Article: ] |
| 59. | Romero-Adrián TB, Leal-Montiel J, Monsalve-Castillo F, Mengual-Moreno E, McGregor EG, Perini L, Antúnez A. Helicobacter pylori: bacterial factors and the role of cytokines in the immune response. Curr Microbiol. 2010;60:143-155. [PubMed] [Cited in This Article: ] |
| 60. | Vizi ES, Elenkov IJ. Nonsynaptic noradrenaline release in neuro-immune responses. Acta Biol Hung. 2002;53:229-244. [PubMed] [Cited in This Article: ] |
| 61. | Peuhkuri K, Vapaatalo H, Korpela R. Even low-grade inflammation impacts on small intestinal function. World J Gastroenterol. 2010;16:1057-1062. [PubMed] [Cited in This Article: ] |
| 62. | Molnar B, Galamb O, Sipos F, Leiszter K, Tulassay Z. Molecular pathogenesis of Helicobacter pylori infection: the role of bacterial virulence factors. Dig Dis. 2010;28:604-608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 63. | Guo G, Jia KR, Shi Y, Liu XF, Liu KY, Qi W, Guo Y, Zhang WJ, Wang T, Xiao B. Psychological stress enhances the colonization of the stomach by Helicobacter pylori in the BALB/c mouse. Stress. 2009;12:478-485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 64. | Ding H, Nedrud JG, Wershil B, Redline RW, Blanchard TG, Czinn SJ. Partial protection against Helicobacter pylori in the absence of mast cells in mice. Infect Immun. 2009;77:5543-5550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 65. | Gulcelik NE, Kaya E, Demirbas B, Culha C, Koc G, Ozkaya M, Cakal E, Serter R, Aral Y. Helicobacter pylori prevalence in diabetic patients and its relationship with dyspepsia and autonomic neuropathy. J Endocrinol Invest. 2005;28:214-217. [PubMed] [Cited in This Article: ] |
| 66. | Tack J, Caenepeel P, Fischler B, Piessevaux H, Janssens J. Symptoms associated with hypersensitivity to gastric distention in functional dyspepsia. Gastroenterology. 2001;121:526-535. [PubMed] [Cited in This Article: ] |
| 67. | Tougas G, Spaziani R, Hollerbach S, Djuric V, Pang C, Upton AR, Fallen EL, Kamath MV. Cardiac autonomic function and oesophageal acid sensitivity in patients with non-cardiac chest pain. Gut. 2001;49:706-712. [PubMed] [Cited in This Article: ] |
| 68. | Testoni PA, Bagnolo F, Bologna P, Colombo E, Bonassi U, Lella F, Buizza M. Higher prevalence of Helicobacter pylori infection in dyspeptic patients who do not have gastric phase III of the migrating motor complex. Scand J Gastroenterol. 1996;31:1063-1068. [PubMed] [Cited in This Article: ] |
| 69. | Chang CS, Chen GH, Kao CH, Wang SJ. Delayed gastric emptying does not predispose to Helicobacter pylori infection in non-ulcer dyspepsia patients. Nucl Med Commun. 1995;16:1063-1067. [PubMed] [Cited in This Article: ] |
| 70. | Hall W, Buckley M, Crotty P, O’Morain CA. Gastric mucosal mast cells are increased in Helicobacter pylori-negative functional dyspepsia. Clin Gastroenterol Hepatol. 2003;1:363-369. [PubMed] [Cited in This Article: ] |
| 71. | Sipos G, Altdorfer K, Pongor E, Chen LP, Fehér E. Neuroimmune link in the mucosa of chronic gastritis with Helicobacter pylori infection. Dig Dis Sci. 2006;51:1810-1817. [PubMed] [Cited in This Article: ] |
| 72. | Supajatura V, Ushio H, Wada A, Yahiro K, Okumura K, Ogawa H, Hirayama T, Ra C. Cutting edge: VacA, a vacuolating cytotoxin of Helicobacter pylori, directly activates mast cells for migration and production of proinflammatory cytokines. J Immunol. 2002;168:2603-2607. [PubMed] [Cited in This Article: ] |
| 73. | Nakajima S, Bamba N, Hattori T. Histological aspects and role of mast cells in Helicobacter pylori-infected gastritis. Aliment Pharmacol Ther. 2004;20 Suppl 1:165-170. [PubMed] [Cited in This Article: ] |
| 74. | Otaka M, Watanabe S. Role of mucosal mast cells in Helicobacter pylori infection. J Gastroenterol. 2002;37:70-72. [PubMed] [Cited in This Article: ] |
| 75. | Montemurro P, Nishioka H, Dundon WG, de Bernard M, Del Giudice G, Rappuoli R, Montecucco C. The neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a potent stimulant of mast cells. Eur J Immunol. 2002;32:671-676. [PubMed] [Cited in This Article: ] |
| 76. | Miwa H. Why dyspepsia can occur without organic disease: pathogenesis and management of functional dyspepsia. J Gastroenterol. 2012;47:862-871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 77. | Gaykema RP, Goehler LE, Lyte M. Brain response to cecal infection with Campylobacter jejuni: analysis with Fos immunohistochemistry. Brain Behav Immun. 2004;18:238-245. [PubMed] [Cited in This Article: ] |
| 78. | Rossetto O, de Bernard M, Pellizzari R, Vitale G, Caccin P, Schiavo G, Montecucco C. Bacterial toxins with intracellular protease activity. Clin Chim Acta. 2000;291:189-199. [PubMed] [Cited in This Article: ] |
| 79. | Kountouras J, Zavos C, Deretzi G, Gavalas E, Chatzopoulos D, Katsinelos P, Tsiaousi E, Gagalis S, Polyzos SA, Venizelos I. Potential implications of Helicobacter pylori-related neutrophil-activating protein. World J Gastroenterol. 2012;18:489-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 80. | Deretzi G, Kountouras J, Polyzos SA, Zavos C, Giartza-Taxidou E, Gavalas E, Tsiptsios I. Gastrointestinal immune system and brain dialogue implicated in neuroinflammatory and neurodegenerative diseases. Curr Mol Med. 2011;11:696-707. [PubMed] [Cited in This Article: ] |
| 81. | Suzuki H, Matsuzaki J, Hibi T. Ghrelin and oxidative stress in gastrointestinal tract. J Clin Biochem Nutr. 2011;48:122-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 82. | Suzuki H, Miura S, Suzuki M, Terada S, Nakamura M, Tsuchiya M. Gastric mucosal injury: microcirculation and Helicobacter pylori. Keio J Med. 1994;43:1-8. [PubMed] [Cited in This Article: ] |
| 83. | Li XB, Chen HM, Lu H, Zheng Q, Chen XY, Peng YS, Ge ZZ, Liu WZ. Role of Helicobacter pylori infection on neuronal expression in the stomach and spinal cord of a murine model. J Dig Dis. 2009;10:286-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 84. | Xu X, Li Z, Zou D, Yang M, Liu Z, Wang X. High expression of calcitonin gene-related peptide and substance P in esophageal mucosa of patients with non-erosive reflux disease. Dig Dis Sci. 2013;58:53-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 85. | Erin N, Türker S, Elpek O, Yıldırım B. Differential changes in Substance P, VIP as well as neprilysin levels in patients with gastritis or ulcer. Peptides. 2012;35:218-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 86. | Yağci M, Yamaç K, Acar K, Cingi E, Kitapçi M, Haznedar R. Gastric emptying in patients with vitamin B(12) deficiency. Eur J Nucl Med Mol Imaging. 2002;29:1125-1127. [PubMed] [Cited in This Article: ] |
| 87. | Aebischer T, Fischer A, Walduck A, Schlötelburg C, Lindig M, Schreiber S, Meyer TF, Bereswill S, Göbel UB. Vaccination prevents Helicobacter pylori-induced alterations of the gastric flora in mice. FEMS Immunol Med Microbiol. 2006;46:221-229. [PubMed] [Cited in This Article: ] |
| 88. | Myllyluoma E, Ahlroos T, Veijola L, Rautelin H, Tynkkynen S, Korpela R. Effects of anti-Helicobacter pylori treatment and probiotic supplementation on intestinal microbiota. Int J Antimicrob Agents. 2007;29:66-72. [PubMed] [Cited in This Article: ] |
| 89. | Engstrand L, Lindberg M. Helicobacter pylori and the gastric microbiota. Best Pract Res Clin Gastroenterol. 2013;27:39-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 90. | Dömötör A, Kereskay L, Szekeres G, Hunyady B, Szolcsányi J, Mózsik G. Participation of capsaicin-sensitive afferent nerves in the gastric mucosa of patients with Helicobacter pylori-positive or-negative chronic gastritis. Dig Dis Sci. 2007;52:411-417. [PubMed] [Cited in This Article: ] |
| 91. | D’Elios MM, Appelmelk BJ, Amedei A, Bergman MP, Del Prete G. Gastric autoimmunity: the role of Helicobacter pylori and molecular mimicry. Trends Mol Med. 2004;10:316-323. [PubMed] [Cited in This Article: ] |
| 92. | Amedei A, Munari F, Bella CD, Niccolai E, Benagiano M, Bencini L, Cianchi F, Farsi M, Emmi G, Zanotti G. Helicobacter pylori secreted peptidyl prolyl cis, trans-isomerase drives Th17 inflammation in gastric adenocarcinoma. Intern Emerg Med. 2014;9:303-309. [PubMed] [Cited in This Article: ] |
| 93. | Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ, Macqueen G, Sherman PM. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60:307-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 611] [Cited by in F6Publishing: 599] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 94. | Suzuki H, Moayyedi P. Helicobacter pylori infection in functional dyspepsia. Nat Rev Gastroenterol Hepatol. 2013;10:168-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 133] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 95. | Chauhan A, Mullins PA, Taylor G, Petch MC, Schofield PM. Cardioesophageal reflex: a mechanism for “linked angina” in patients with angiographically proven coronary artery disease. J Am Coll Cardiol. 1996;27:1621-1628. [PubMed] [Cited in This Article: ] |
| 96. | Akkaya N, Akkaya S, Polat Y, Turk M, Turk T, Turhal E, Sahin F. Helicobacter pylori seropositivity in fibromyalgia syndrome. Clin Rheumatol. 2011;30:43-49. [PubMed] [Cited in This Article: ] |
| 97. | Lee YY, Chua AS. Influence of gut microbes on the brain-gut axis (Gut 2011; 60: 307-317). J Neurogastroenterol Motil. 2011;17:427-429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 98. | Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10:735-742. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 969] [Cited by in F6Publishing: 1012] [Article Influence: 84.3] [Reference Citation Analysis (0)] |
| 99. | Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1299] [Cited by in F6Publishing: 1350] [Article Influence: 122.7] [Reference Citation Analysis (0)] |
| 100. | Yang YJ, Sheu BS. Probiotics-containing yogurts suppress Helicobacter pylori load and modify immune response and intestinal microbiota in the Helicobacter pylori-infected children. Helicobacter. 2012;17:297-304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 101. | Yang YJ, Sheu BS, Yang HB, Lu CC, Chuang CC. Eradication of Helicobacter pylori increases childhood growth and serum acylated ghrelin levels. World J Gastroenterol. 2012;18:2674-2681. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 37] [Cited by in F6Publishing: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 102. | Francois F, Roper J, Joseph N, Pei Z, Chhada A, Shak JR, de Perez AZ, Perez-Perez GI, Blaser MJ. The effect of H. pylori eradication on meal-associated changes in plasma ghrelin and leptin. BMC Gastroenterol. 2011;11:37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 103. | Deng ZH, Chu B, Xu YZ, Zhang B, Jiang LR. Influence of Helicobacter pylori infection on ghrelin levels in children. World J Gastroenterol. 2012;18:5096-5100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 104. | Tatsuguchi A, Miyake K, Gudis K, Futagami S, Tsukui T, Wada K, Kishida T, Fukuda Y, Sugisaki Y, Sakamoto C. Effect of Helicobacter pylori infection on ghrelin expression in human gastric mucosa. Am J Gastroenterol. 2004;99:2121-2127. [PubMed] [Cited in This Article: ] |
| 105. | Weigt J, Malfertheiner P. Influence of Helicobacter pylori on gastric regulation of food intake. Curr Opin Clin Nutr Metab Care. 2009;12:522-525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 106. | Roper J, Francois F, Shue PL, Mourad MS, Pei Z, Olivares de Perez AZ, Perez-Perez GI, Tseng CH, Blaser MJ. Leptin and ghrelin in relation to Helicobacter pylori status in adult males. J Clin Endocrinol Metab. 2008;93:2350-2357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 107. | Jang EJ, Park SW, Park JS, Park SJ, Hahm KB, Paik SY, Sin MK, Lee ES, Oh SW, Park CY. The influence of the eradication of Helicobacter pylori on gastric ghrelin, appetite, and body mass index in patients with peptic ulcer disease. J Gastroenterol Hepatol. 2008;23 Suppl 2:S278-S285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 108. | Osawa H, Kita H, Ohnishi H, Nakazato M, Date Y, Bowlus CL, Ishino Y, Watanabe E, Shiiya T, Ueno H. Changes in plasma ghrelin levels, gastric ghrelin production, and body weight after Helicobacter pylori cure. J Gastroenterol. 2006;41:954-961. [PubMed] [Cited in This Article: ] |
| 109. | Choe YH, Lee JH, Lee HJ, Paik KH, Jin DK, Song SY, Lee JH. Ghrelin Levels in Gastric Mucosa before and after Eradication of Helicobacter pylori. Gut Liver. 2007;1:132-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 12] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 110. | Abdullahi M, Annibale B, Capoccia D, Tari R, Lahner E, Osborn J, Leonetti F, Severi C. The eradication of Helicobacter pylori is affected by body mass index (BMI). Obes Surg. 2008;18:1450-1454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 111. | Ünal HÜ, Akin E, Aydin İ, Korkmaz M, Özel S, Selçuk H, Yilmaz U. Ongoing symptoms after eradication of Helicobacter pylori: psychiatric disorders may accompany. Turk J Gastroenterol. 2013;24:15-21. [PubMed] [Cited in This Article: ] |
| 112. | Kotan Z, Sarandöl E, Kırhan E, Ozkaya G, Kırlı S. Serum brain-derived neurotrophic factor, vascular endothelial growth factor and leptin levels in patients with a diagnosis of severe major depressive disorder with melancholic features. Ther Adv Psychopharmacol. 2012;2:65-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 113. | Winston JH, Sarna SK. Developmental origins of functional dyspepsia-like gastric hypersensitivity in rats. Gastroenterology. 2013;144:570-579.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 114. | Bhide AA, Cartwright R, Khullar V, Digesu GA. Biomarkers in overactive bladder. Int Urogynecol J. 2013;24:1065-1072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 115. | Beydoun MA, Beydoun HA, Shroff MR, Kitner-Triolo MH, Zonderman AB. Helicobacter pylori seropositivity and cognitive performance among US adults: evidence from a large national survey. Psychosom Med. 2013;75:486-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 116. | Agrawal A, Gupta A, Chandra M, Koowar S. Role of Helicobacter pylori infection in the pathogenesis of minimal hepatic encephalopathy and effect of its eradication. Indian J Gastroenterol. 2011;30:29-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 117. | Roubaud Baudron C, Letenneur L, Langlais A, Buissonnière A, Mégraud F, Dartigues JF, Salles N. Does Helicobacter pylori infection increase incidence of dementia? The Personnes Agées QUID Study. J Am Geriatr Soc. 2013;61:74-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 118. | Muhsen K, Ornoy A, Akawi A, Alpert G, Cohen D. An association between Helicobacter pylori infection and cognitive function in children at early school age: a community-based study. BMC Pediatr. 2011;11:43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 119. | Ozdemir V, Jamal MM, Osapay K, Jadus MR, Sandor Z, Hashemzadeh M, Szabo S. Cosegregation of gastrointestinal ulcers and schizophrenia in a large national inpatient discharge database: revisiting the “brain-gut axis” hypothesis in ulcer pathogenesis. J Investig Med. 2007;55:315-320. [PubMed] [Cited in This Article: ] |
| 120. | Dylag K, Hubalewska-Mazgaj M, Surmiak M, Szmyd J, Brzozowski T. Probiotics in the mechanism of protection against gut inflammation and therapy of gastrointestinal disorders. Curr Pharm Des. 2014;20:1149-1155. [PubMed] [Cited in This Article: ] |
| 121. | Nada T, Nomura M, Iga A, Kawaguchi R, Ochi Y, Saito K, Nakaya Y, Ito S. Autonomic nervous function in patients with peptic ulcer studied by spectral analysis of heart rate variability. J Med. 2001;32:333-347. [PubMed] [Cited in This Article: ] |
| 122. | Lugon JR, Moreira MD, de Almeida JM, Silva AS, Esberard EC, Bousquet-Santos K, Carvalho AG, Mendes LC, da Nobrega AC. Cardiovascular autonomic response to food ingestion in patients with gastritis: a comparison between Helicobacter pylori-positive and -negative patients. Helicobacter. 2006;11:173-180. [PubMed] [Cited in This Article: ] |
| 123. | Stanghellini V, Barbara G, de Giorgio R, Tosetti C, Cogliandro R, Cogliandro L, Salvioli B, Corinaldesi R. Review article: Helicobacter pylori, mucosal inflammation and symptom perception--new insights into an old hypothesis. Aliment Pharmacol Ther. 2001;15 Suppl 1:28-32. [PubMed] [Cited in This Article: ] |
| 124. | Thor PJ, Błaut U. Helicobacter pylori infection in pathogenesis of gastroesophageal reflux disease. J Physiol Pharmacol. 2006;57 Suppl 3:81-90. [PubMed] [Cited in This Article: ] |
| 125. | Shahabi S, Rasmi Y, Jazani NH, Hassan ZM. Protective effects of Helicobacter pylori against gastroesophageal reflux disease may be due to a neuroimmunological anti-inflammatory mechanism. Immunol Cell Biol. 2008;86:175-178. [PubMed] [Cited in This Article: ] |
| 126. | Mönnikes H, van der Voort IR, Wollenberg B, Heymann-Monnikes I, Tebbe JJ, Alt W, Arnold R, Klapp BF, Wiedenmann B, McGregor GP. Gastric perception thresholds are low and sensory neuropeptide levels high in helicobacter pylori-positive functional dyspepsia. Digestion. 2005;71:111-123. [PubMed] [Cited in This Article: ] |
| 127. | Moayyedi P, Soo S, Deeks JJ, Delaney B, Harris A, Innes M, Oakes R, Wilson S, Roalfe A, Bennett C. WITHDRAWN: Eradication of Helicobacter pylori for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2011;CD002096. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 128. | Mearin F, de Ribot X, Balboa A, Salas A, Varas MJ, Cucala M, Bartolomé R, Armengol JR, Malagelada JR. Does Helicobacter pylori infection increase gastric sensitivity in functional dyspepsia? Gut. 1995;37:47-51. [PubMed] [Cited in This Article: ] |
| 129. | Danesh J, Lawrence M, Murphy M, Roberts S, Collins R. Systematic review of the epidemiological evidence on Helicobacter pylori infection and nonulcer or uninvestigated dyspepsia. Arch Intern Med. 2000;160:1192-1198. [PubMed] [Cited in This Article: ] |
| 130. | McColl K, Murray L, El-Omar E, Dickson A, El-Nujumi A, Wirz A, Kelman A, Penny C, Knill-Jones R, Hilditch T. Symptomatic benefit from eradicating Helicobacter pylori infection in patients with nonulcer dyspepsia. N Engl J Med. 1998;339:1869-1874. [PubMed] [Cited in This Article: ] |
| 131. | Laine L, Schoenfeld P, Fennerty MB. Therapy for Helicobacter pylori in patients with nonulcer dyspepsia. A meta-analysis of randomized, controlled trials. Ann Intern Med. 2001;134:361-369. [PubMed] [Cited in This Article: ] |
| 132. | Pirtniecks A, Smith LF, Thorpe JA. Autonomic dysfunction in non-specific disorders of oesophageal motility. Eur J Cardiothorac Surg. 2000;17:101-105. [PubMed] [Cited in This Article: ] |
| 133. | Futagami S, Shimpuku M, Yin Y, Shindo T, Kodaka Y, Nagoya H, Nakazawa S, Fujimoto M, Izumi N, Ohishi N. Pathophysiology of functional dyspepsia. J Nippon Med Sch. 2011;78:280-285. [PubMed] [Cited in This Article: ] |
| 134. | Tillisch K, Mayer EA, Labus JS, Stains J, Chang L, Naliboff BD. Sex specific alterations in autonomic function among patients with irritable bowel syndrome. Gut. 2005;54:1396-1401. [PubMed] [Cited in This Article: ] |
| 135. | Miyaji H, Azuma T, Ito S, Abe Y, Ono H, Suto H, Ito Y, Yamazaki Y, Kohli Y, Kuriyama M. The effect of helicobacter pylori eradication therapy on gastric antral myoelectrical activity and gastric emptying in patients with non-ulcer dyspepsia. Aliment Pharmacol Ther. 1999;13:1473-1480. [PubMed] [Cited in This Article: ] |
| 136. | Ghoshal UC, Chourasia D. Gastroesophageal Reflux Disease and Helicobacter pylori: What May Be the Relationship? J Neurogastroenterol Motil. 2010;16:243-250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 137. | Schenk BE, Kuipers EJ, Klinkenberg-Knol EC, Bloemena E, Nelis GF, Festen HP, Jansen EH, Biemond I, Lamers CB, Meuwissen SG. Hypergastrinaemia during long-term omeprazole therapy: influences of vagal nerve function, gastric emptying and Helicobacter pylori infection. Aliment Pharmacol Ther. 1998;12:605-612. [PubMed] [Cited in This Article: ] |
| 138. | Zub-Pokrowiecka A, Rembiasz K, Konturek SJ, Budzynski A, Konturek PC, Budzynski P. Ghrelin in diseases of the gastric mucosa associated with Helicobacter pylori infection. Med Sci Monit. 2010;16:CR493-CR500. [PubMed] [Cited in This Article: ] |
| 139. | Konturek JW, Stoll R, Menzel J, Konturek M, Konturek SJ, Domschke W. Eradication of Helicobacter pylori restores the inhibitory effect of cholecystokinin on gastric motility in duodenal ulcer patients. Scand J Gastroenterol. 2001;36:241-246. [PubMed] [Cited in This Article: ] |
| 140. | Chiloiro M, Russo F, Riezzo G, Leoci C, Clemente C, Messa C, Di Leo A. Effect of Helicobacter pylori infection on gastric emptying and gastrointestinal hormones in dyspeptic and healthy subjects. Dig Dis Sci. 2001;46:46-53. [PubMed] [Cited in This Article: ] |
| 141. | Tsai SH, Chen CM, Chang CS, Chen GH. Effect of Helicobacter pylori infection on intragastric acidity in patients with reflux esophagitis. J Gastroenterol. 2004;39:821-826. [PubMed] [Cited in This Article: ] |
| 142. | Zerbib F, Bicheler V, Leray V, Joubert M, Bruley des Varannes S, Galmiche JP. H. pylori and transient lower esophageal sphincter relaxations induced by gastric distension in healthy humans. Am J Physiol Gastrointest Liver Physiol. 2001;281:G350-G356. [PubMed] [Cited in This Article: ] |
| 143. | Tanaka I, Tatsumi Y, Kodama T, Kato K, Fujita S, Mitsufuji S, Kashima K. Effect of Helicobacter pylori eradication on gastroesophageal function. J Gastroenterol Hepatol. 2004;19:251-257. [PubMed] [Cited in This Article: ] |
| 144. | Sarnelli G, Cuomo R, Janssens J, Tack J. Symptom patterns and pathophysiological mechanisms in dyspeptic patients with and without Helicobacter pylori. Dig Dis Sci. 2003;48:2229-2236. [PubMed] [Cited in This Article: ] |
| 145. | Thumshirn M, Camilleri M, Saslow SB, Williams DE, Burton DD, Hanson RB. Gastric accommodation in non-ulcer dyspepsia and the roles of Helicobacter pylori infection and vagal function. Gut. 1999;44:55-64. [PubMed] [Cited in This Article: ] |
| 146. | Koskenpato J, Korppi-Tommola T, Kairemo K, Färkkilä M. Long-term follow-up study of gastric emptying and Helicobacter pylori eradication among patients with functional dyspepsia. Dig Dis Sci. 2000;45:1763-1768. [PubMed] [Cited in This Article: ] |
| 147. | Lin Z, Chen JD, Parolisi S, Shifflett J, Peura DA, McCallum RW. Prevalence of gastric myoelectrical abnormalities in patients with nonulcer dyspepsia and H. pylori infection: resolution after H. pylori eradication. Dig Dis Sci. 2001;46:739-745. [PubMed] [Cited in This Article: ] |
| 148. | Thor P, Lorens K, Tabor S, Herman R, Konturek JW, Konturek SJ. Dysfunction in gastric myoelectric and motor activity in Helicobacter pylori positive gastritis patients with non-ulcer dyspesia. J Physiol Pharmacol. 1996;47:469-476. [PubMed] [Cited in This Article: ] |
| 149. | Manes G, Esposito P, Lioniello M, Bove A, Mosca S, Balzano A. Manometric and pH-metric features in gastro-oesophageal reflux disease patients with and without Helicobacter pylori infection. Dig Liver Dis. 2000;32:372-377. [PubMed] [Cited in This Article: ] |
| 150. | Giral A, Celikel CA, Ozdogan O, Tözün N, Ulusoy NB, Kalayci C. Impact of Helicobacter pylori eradication on the anti-secretory efficacy of lansoprazole in gastroesophageal reflux disease patients. J Gastroenterol Hepatol. 2005;20:1886-1891. [PubMed] [Cited in This Article: ] |
| 151. | Ando T, El-Omar EM, Goto Y, Nobata K, Watanabe O, Maeda O, Ishiguro K, Minami M, Hamajima N, Goto H. Interleukin 1B proinflammatory genotypes protect against gastro-oesophageal reflux disease through induction of corpus atrophy. Gut. 2006;55:158-164. [PubMed] [Cited in This Article: ] |
| 152. | Iijima K, Ohara S, Sekine H, Koike T, Kato K, Asaki S, Shimosegawa T, Toyota T. Changes in gastric acid secretion assayed by endoscopic gastrin test before and after Helicobacter pylori eradication. Gut. 2000;46:20-26. [PubMed] [Cited in This Article: ] |
| 153. | Konturek SJ, Brzozowski T, Konturek PC, Schubert ML, Pawlik WW, Padol S, Bayner J. Brain-gut and appetite regulating hormones in the control of gastric secretion and mucosal protection. J Physiol Pharmacol. 2008;59 Suppl 2:7-31. [PubMed] [Cited in This Article: ] |